
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edyta Mądry | -- | 1668 | 2022-09-14 16:32:00 | | | |
| 2 | Jessie Wu | + 6 word(s) | 1674 | 2022-09-15 06:49:21 | | |
Video Upload Options
Inflammatory Bowel Diseases, including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, relapsing inflammatory conditions of the gastrointestinal (GI) tract. Interactions between the environmental factors and commensal intestinal microflora in genetically predisposed individuals are considered the leading cause of an inappropriate immune response and as a result, the development of inflammatory disease. Iron is an indispensable nutrient for life. A lack of it leads to iron deficiency anaemia (IDA), which currently affects about 1.2 billion people worldwide. The primary means of IDA treatment is oral or parenteral iron supplementation. This can be burdened with numerous side effects such as oxidative stress, systemic and local-intestinal inflammation, dysbiosis, carcinogenic processes and gastrointestinal adverse events.
1. Anaemia as a Complication of Inflammatory Bowel Diseases
2. Iron Replacement Therapy in Inflammatory Bowel Diseases
3. Negative Consequences of Oral Iron
4. Impact of Iron on the Intestine
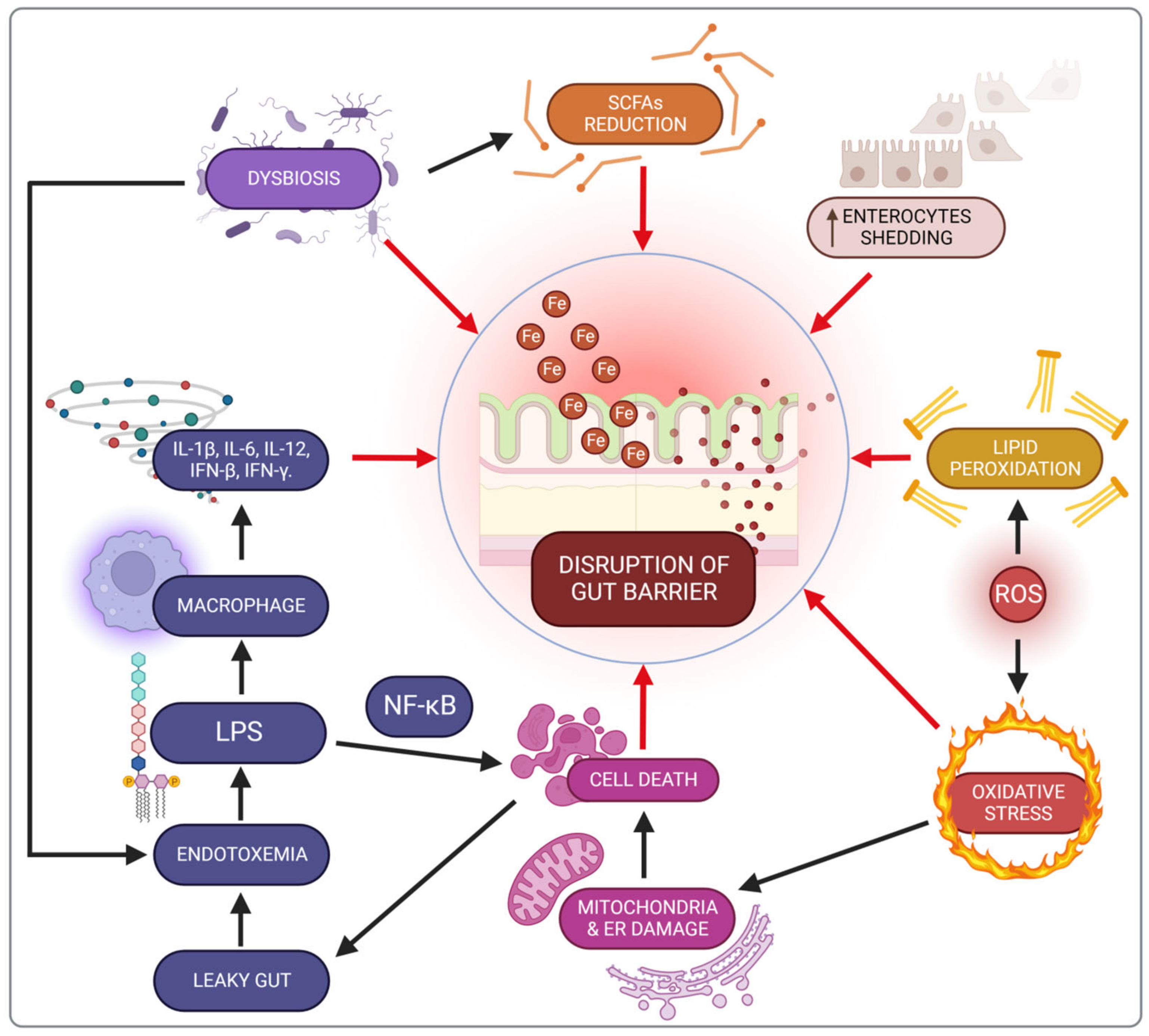
5. Impact of Iron on the Microbiota in Inflammatory Bowel Diseases Patients
References
- Nielsen, O.H.; Soendergaard, C.; Vikner, M.E.; Weiss, G. Rational Management of Iron-Deficiency Anaemia in Inflammatory Bowel Disease. Nutrients 2018, 10, 82.
- Mahadea, D.; Adamczewska, E.; Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Iron Deficiency Anemia in Inflammatory Bowel Diseases-A Narrative Review. Nutrients 2021, 13, 4008.
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023.
- Iqbal, T.; Stein, J.; Sharma, N.; Kulnigg-Dabsch, S.; Vel, S.; Gasche, C. Clinical Significance of C-Reactive Protein Levels in Predicting Responsiveness to Iron Therapy in Patients with Inflammatory Bowel Disease and Iron Deficiency Anemia. Dig. Dis. Sci. 2015, 60, 1375–1381.
- European Crohn’s and Colitis Organisatio—ECCO—Industry Exhibition. Available online: https://www.ecco-ibd.eu/exhibit-sponsor-2015/industry-exhibition-2015.html (accessed on 21 July 2022).
- D’Amico, F.; Peyrin-Biroulet, L.; Danese, S. Oral Iron for IBD Patients: Lessons Learned at Time of COVID-19 Pandemic. J. Clin. Med. 2020, 9, E1536.
- Niepel, D.; Klag, T.; Malek, N.P.; Wehkamp, J. Practical Guidance for the Management of Iron Deficiency in Patients with Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2018, 11, 1756284818769074.
- Stein, J.; Walper, A.; Klemm, W.; Farrag, K.; Aksan, A.; Dignass, A. Safety and Efficacy of Intravenous Iron Isomaltoside for Correction of Anaemia in Patients with Inflammatory Bowel Disease in Everyday Clinical Practice. Scand. J. Gastroenterol. 2018, 53, 1059–1065.
- Stein, J.; Dignass, A.U. Management of Iron Deficiency Anemia in Inflammatory Bowel Disease—A Practical Approach. Ann. Gastroenterol. 2013, 26, 104–113.
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and Intervention Measures of Iron Side Effects on the Intestine. Crit. Rev. Food Sci. Nutr. 2020, 60, 2113–2125.
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248.
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383.
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron Absorption from Oral Iron Supplements given on Consecutive versus Alternate Days and as Single Morning Doses versus Twice-Daily Split Dosing in Iron-Depleted Women: Two Open-Label, Randomised Controlled Trials. Lancet Haematol. 2017, 4, e524–e533.
- Carrier, J.C.; Aghdassi, E.; Jeejeebhoy, K.; Allard, J.P. Exacerbation of Dextran Sulfate Sodium-Induced Colitis by Dietary Iron Supplementation: Role of NF-KappaB. Int. J. Colorectal Dis. 2006, 21, 381–387.
- Werner, T.; Wagner, S.J.; Martínez, I.; Walter, J.; Chang, J.-S.; Clavel, T.; Kisling, S.; Schuemann, K.; Haller, D. Depletion of Luminal Iron Alters the Gut Microbiota and Prevents Crohn’s Disease-like Ileitis. Gut 2011, 60, 325–333.
- Piechota-Polanczyk, A.; Fichna, J. Review Article: The Role of Oxidative Stress in Pathogenesis and Treatment of Inflammatory Bowel Diseases. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620.
- Yu, L.C.-H.; Wei, S.-C.; Ni, Y.-H. Impact of Microbiota in Colorectal Carcinogenesis: Lessons from Experimental Models. Intest. Res. 2018, 16, 346–357.
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. Nihon Chikusan Gakkaiho 2020, 91, e13357.
- Li, Y.; Hansen, S.L.; Borst, L.B.; Spears, J.W.; Moeser, A.J. Dietary Iron Deficiency and Oversupplementation Increase Intestinal Permeability, Ion Transport, and Inflammation in Pigs. J. Nutr. 2016, 146, 1499–1505.
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91.
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic Effect of Iron on Human Intestinal Caco-2 Cells: Early Effect on Tight Junction Permeability with Delayed Onset of Oxidative Cytotoxic Damage. Cell. Mol. Biol. Noisy-Gd. Fr. 2003, 49, 89–99.
- Constante, M.; Fragoso, G.; Calvé, A.; Samba-Mondonga, M.; Santos, M.M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 2017, 8, 1809.
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation. Front. Immunol. 2019, 10, 2051.
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron Depletion and Repletion with Ferrous Sulfate or Electrolytic Iron Modifies the Composition and Metabolic Activity of the Gut Microbiota in Rats. J. Nutr. 2012, 142, 271–277.
- Botta, A.; Barra, N.G.; Lam, N.H.; Chow, S.; Pantopoulos, K.; Schertzer, J.D.; Sweeney, G. Iron Reshapes the Gut Microbiome and Host Metabolism. J. Lipid Atheroscler. 2021, 10, 160–183.
- Dostal, A.; Fehlbaum, S.; Chassard, C.; Zimmermann, M.B.; Lacroix, C. Low Iron Availability in Continuous in Vitro Colonic Fermentations Induces Strong Dysbiosis of the Child Gut Microbial Consortium and a Decrease in Main Metabolites. FEMS Microbiol. Ecol. 2013, 83, 161–175.
- Mahalhal, A.; Williams, J.M.; Johnson, S.; Ellaby, N.; Duckworth, C.A.; Burkitt, M.D.; Liu, X.; Hold, G.L.; Campbell, B.J.; Pritchard, D.M.; et al. Oral Iron Exacerbates Colitis and Influences the Intestinal Microbiome. PLoS ONE 2018, 13, e0202460.
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont Release from Dysbiotic Gut Microbiota Biofilms in Intestinal Inflammatory Diseases: A Role for Iron? J. Biomed. Sci. 2019, 26, 1.
- Rehman, A.; Rausch, P.; Wang, J.; Skieceviciene, J.; Kiudelis, G.; Bhagalia, K.; Amarapurkar, D.; Kupcinskas, L.; Schreiber, S.; Rosenstiel, P.; et al. Geographical Patterns of the Standing and Active Human Gut Microbiome in Health and IBD. Gut 2016, 65, 238–248.
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004.
- Lee, J.-Y.; Cevallos, S.A.; Byndloss, M.X.; Tiffany, C.R.; Olsan, E.E.; Butler, B.P.; Young, B.M.; Rogers, A.W.L.; Nguyen, H.; Kim, K.; et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis That Exacerbates Pre-Inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 273–284.e6.




