Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | M. Shahinuzzaman | -- | 2479 | 2022-09-13 06:03:04 | | | |
| 2 | Conner Chen | + 1 word(s) | 2480 | 2022-09-14 08:22:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shahinuzzaman, M.; Afroz, S.; Mohafez, H.; Jamal, M.S.; Khandaker, M.U.; Sulieman, A.; Tamam, N.; Islam, M.A. The Perovskite Solar Cell. Encyclopedia. Available online: https://encyclopedia.pub/entry/27113 (accessed on 01 March 2026).
Shahinuzzaman M, Afroz S, Mohafez H, Jamal MS, Khandaker MU, Sulieman A, et al. The Perovskite Solar Cell. Encyclopedia. Available at: https://encyclopedia.pub/entry/27113. Accessed March 01, 2026.
Shahinuzzaman, M., Sanjida Afroz, Hamidreza Mohafez, M. S. Jamal, Mayeen Uddin Khandaker, Abdelmoneim Sulieman, Nissren Tamam, Mohammad Aminul Islam. "The Perovskite Solar Cell" Encyclopedia, https://encyclopedia.pub/entry/27113 (accessed March 01, 2026).
Shahinuzzaman, M., Afroz, S., Mohafez, H., Jamal, M.S., Khandaker, M.U., Sulieman, A., Tamam, N., & Islam, M.A. (2022, September 13). The Perovskite Solar Cell. In Encyclopedia. https://encyclopedia.pub/entry/27113
Shahinuzzaman, M., et al. "The Perovskite Solar Cell." Encyclopedia. Web. 13 September, 2022.
Copy Citation
Perovskite, an organic–inorganic hybrid material, tends to be a promising light-harvesting material. PSCs (organic-inorganic perovskite solar cells) are considered a significant breakthrough in photovoltaics and have received great attention. Due to the inherent advantage of perovskite thin films that can be fabricated using simple solution techniques at low temperatures, PSCs are regarded as one of the most important low-cost and mass-production prospects.
perovskite solar cell
stability
efficiency
1. Introduction
Perovskite, an organic–inorganic hybrid material, tends to be a promising light-harvesting material. In 2009, the Miyasaka group [1] reported a perovskite solar cell (PSC) of 3.8% using a DSSC system configuration with liquid electrolyte based on MAPbI3 (MA = CH3NH3+). Park group [2] obtained a nearly doubled power conversion efficiency (PCE) of 6.5% in 2011 using a high concentration perovskite precursor solution. Since then, several efforts have been made to enhance PSC photovoltaic efficiency from various angles, including perovskite layer fabrication methods, interface engineering, cell architecture design, and development of the hole transporting materials (HTM), an electron transporting materials (ETM). A certified PCE of 22.7% has already been achieved via the above optimization [3]. Although the PCE currently available is appealing, the PSCs still have low stability (thermal, light, and moisture stability), which hinders their commercialization.
The discovery of high-efficiency and highly stable perovskite solar cells has sparked extensive research, which is still ongoing [4][5]. Particularly, organometallic semiconducting perovskite has a direct band gap with high absorption coefficients [6] that enables efficient light absorption in ultra-thin films. Furthermore, it has a long diffusion length [7][8][9], low exciton binding energy [10][11], high carrier mobility [12][13], and simple and easy preparation techniques [14] that help to get high efficiency and low-cost showing promising alternative to the conventional crystalline silicon-based solar cell. Moreover, perovskite materials can be implemented in two different cell structures, either as planer (n-i-p) or inverted (p-i-n) architecture. Moreover, both architectures could be (i) regular structures in which no mesoporous layer is employed, and (ii) mesoscopic structures where a mesoporous layer is needed. The significant improvement in efficiency already achieved in all kinds of architecture, and the stability of PSCs remain the key concerns for the researchers at present time. Many changes were made to the working electrode, the electron transport layer (ETL), and the hole transport layer (HTL) to improve their stability and charge transport properties. The hole transporting materials is a very much important factor in PSCs to achieve high efficiency and performance. It acts as the mediator to transfer positive charges (Holes) between the perovskite and counter electrode [15]. Particularly, highest efficiency (PSCs) are achieved with organic HTL such as 2,2,7,7-tetrakis-(N,N-di-pmethoxyphenylamine)-9,90-spiro-biuorene(spiro-MeOTAD) [16]. The other most commonly used organic HTMs are poly(3,4-ethylene dioxythiophene) (PEDOT) or poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) [17], poly-[[9-(1-octylnonyl)-9H-carbazole-2,7-diyl]-2,5-thiophenediyl-2,1,3-benzothiadiazole-4,7-diyl-2,5 thiophenediyl] (PCDTBT) [18][19], poly-[3-hexylthiophene-2,5-diyl] (P3HT) [20][21], 4-(diethylamino)-benzaldehyde diphenylhydrazone (DEH) [22], poly-triarylamine (PTAA) [19][23], N,N-dialkyl perylene diimide (PDI) [24], polypyrrole (PPy), polyaniline (PANI) [25], etc. From a commercial standpoint, the production of solar cells utilizing an organic hole transport layer has encountered numerous challenges, the most significant of which are material cost and stability. Particularly, high purity spiro-OMeTAD is more costly than novel metals such as gold and platinum, which are commonly used as a counter electrodes. Commercially available spiro-OMeTAD is nearly ten times more expensive than platinum and gold. On the other hand, organic HTMs are typically hygroscopic in nature and that’s why it has an impact on the PSCs’ general stability.
In contrast, several low-cost inorganic HTLs were also proposed and implemented for enhancing the stability of PSCs, among them, some of the HTMs are CuSCN [26], NiOx [27], Cu2O or CuO [28], CuI [29], CuGaO3 [30] and CuAlO2 [31], MoOx [32], CuS [33], MoS2 [34], and polymer electrolyte [35]. The above-mentioned HTMs have shown potential as they offer suitable properties for application in PSCs including the suitable band-to-band alignment with the perovskite layer, low resistivity, and low-cost solution-process ability [2]. In the case of inorganic HTM, increased demand for inorganic HTM will certainly lower the cost of large-scale manufacturing, while organic HTM will likely stay expensive due to the preparation processes and materials with very high purity required for solar cell applications. These are the primary reason why researchers have concentrated their efforts on the development of an inorganic HTM. Consecutively, the quest for the perfect HTM is a great topic yet. There is a lot of literature on various HTMs, but only a few of them show promise in terms of improving the overall efficiency and stability of the PSCs. Several approaches have evolved to utilize inorganic p-type semiconductor materials, such as NiOx, CuOx, etc., focusing on developing non-hygroscopic and highly conductive HTMs [36]. Moreover, carbon-based materials, including graphene, activated carbon, carbon black, graphite powder, carbon nanotube (CNT), etc., have been employed in the case of HTM-free PSC structures [37][38][39][40]. In particular, current approaches to HTMs are low cost, high mobility, low absorption in the visible region, ease of synthesis, and good chemical stability that could ensure high efficiency and stable PSCs. In recent years, several review works have been published on inorganic metal oxide hole-transporting materials for perovskite solar cells in different formats and among them, some are focused on fabrication way, some are on efficiency and some are focused on stability. A list of some important review articles on inorganic metal oxide-based PSCs for the year 2015–2021 is shown in Table 1.
Table 1. List of recently published review articles on inorganic HTMs for PSCs.
| No. | Title | Journal | Year | References |
|---|---|---|---|---|
| 01 | Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells | Nano Select | 2021 | [41] |
| 02 | A brief review of hole transporting materials commonly used in perovskite solar cells | Rare Metals | 2021 | [42] |
| 03 | Nickel Oxide for Perovskite Photovoltaic Cells | Advanced Photonics Research | 2021 | [43] |
| 04 | Toward efficient and stable operation of perovskite solar cells: Impact of sputtered metal oxide interlayers | Nano Select | 2021 | [44] |
| 05 | Inorganic hole transport layers in inverted perovskite solar cells: A review | Nano Select | 2021 | [45] |
| 06 | Progress, highlights, and perspectives on NiO in perovskite photovoltaics | Chemical Science | 2020 | [46] |
| 07 | A review on the classification of organic/inorganic/carbonaceous hole-transporting materials for perovskite solar cell application | Arabian Journal of Chemistry | 2020 | [47] |
| 08 | Review of current progress in inorganic hole-transport materials for perovskite solar cells | Applied Materials Today | 2019 | [48] |
| 09 | Recent progress of inorganic perovskite solar cells | Energy & Environmental Science | 2019 | [49] |
| 10 | Inorganic hole transporting materials for stable and high efficiency perovskite solar cells | The Journal of Physical Chemistry C | 2018 | [50] |
| 11 | Analysing the prospects of perovskite solar cells within the purview of recent scientific advancements | Crystals | 2018 | [51] |
| 12 | Recent progress in stability of perovskite solar cells | Journal of Semiconductors | 2017 | [52] |
| 13 | Emerging of inorganic hole transporting materials for perovskite solar cells | The Chemical Record | 2017 | [53] |
| 14 | Recent advances in the inverted planar structure of perovskite solar cells | Accounts of chemical research | 2016 | [54] |
| 15 | The progress of interface design in perovskite-based solar cells | Advanced Energy Materials | 2016 | [55] |
| 16 | Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells | Advanced Energy Materials | 2015 | [36] |
2. Perovskite Solar Cell
PSCs (organic-inorganic perovskite solar cells) are considered a significant recent breakthrough in photovoltaics and have recently received great attention [49]. The power conversion efficiency (PCE) of PSCs has already enhanced from 3.8 percent to 25.8 percent through the system engineering and materials design regarding the correct optoelectronic aspects in just 10 years [56]. Thus, PSCs are recognized as the best alternative approach for replacing the costly and market-dominant crystalline silicon solar cells [51][57][58][59][60]. Moreover, PSCs are more cost-effective than conventional inorganic semiconductor thin-film solar cells, such as CIGS and CdTe [52]. The real obstacle to commercialization, however, is maintaining long-term stability. PSCs are particularly susceptible to deterioration when exposed to moisture, oxygen, heat, and light, and they must address before they can use in practical applications. Perovskite is itself very reactive due to the presence of vacancies in its structure. This is the defect of perovskite and it can encourage ion migration through the perovskite layer. Furthermore, the organic cations which are used in PSCs are hygroscopic in nature. When the PSCs are contacted with moisture, the water molecule reacts with it and forms a weak hydrogen bond with the cation which results in the formation of a hydrated perovskite phase [52]. Oxygen, heat, and UV influence this chemical reaction and favor the instability of PSCs. For commercialization, PSCs must be able to operate without major degradation for almost 25 years in outdoor conditions [61]. PSCs have so far been claimed to have one-year stability, which is considerably less than the PV systems that are already on the market. Thus, it is evident that the stability and limited longevity of PSC PV are the main factors impeding its commercialization [62].
The basic building block of the perovskite structure, ABX3, is shown in Figure 1, where A and B are cations with different sizes (A being larger than B) and X is an anion [63]. Figure 1 represents the simplest structure made up of cubic symmetry of corner-sharing BX6 octahedra, where the B cations are in the middle of the octahedron and the X anions are at the corners [64][65]. In the gap of cuboctahedra, the A cations are located at interstices, surrounded by eight octahedral, and form a cubic Pm3m crystal structure [66]. In the case of frequently used perovskites in solar cells are organo-metal halide perovskite materials, where ‘A’ may be an organic or inorganic cation (i.e., MA+, FA+, Cs+, K+, and Rb+), while ‘B’ is a metal cation (i.e., Pb2+ or Sn2+), and ‘X’ is a halide anion (i.e., Cl, Br, I, etc.) [67][68].
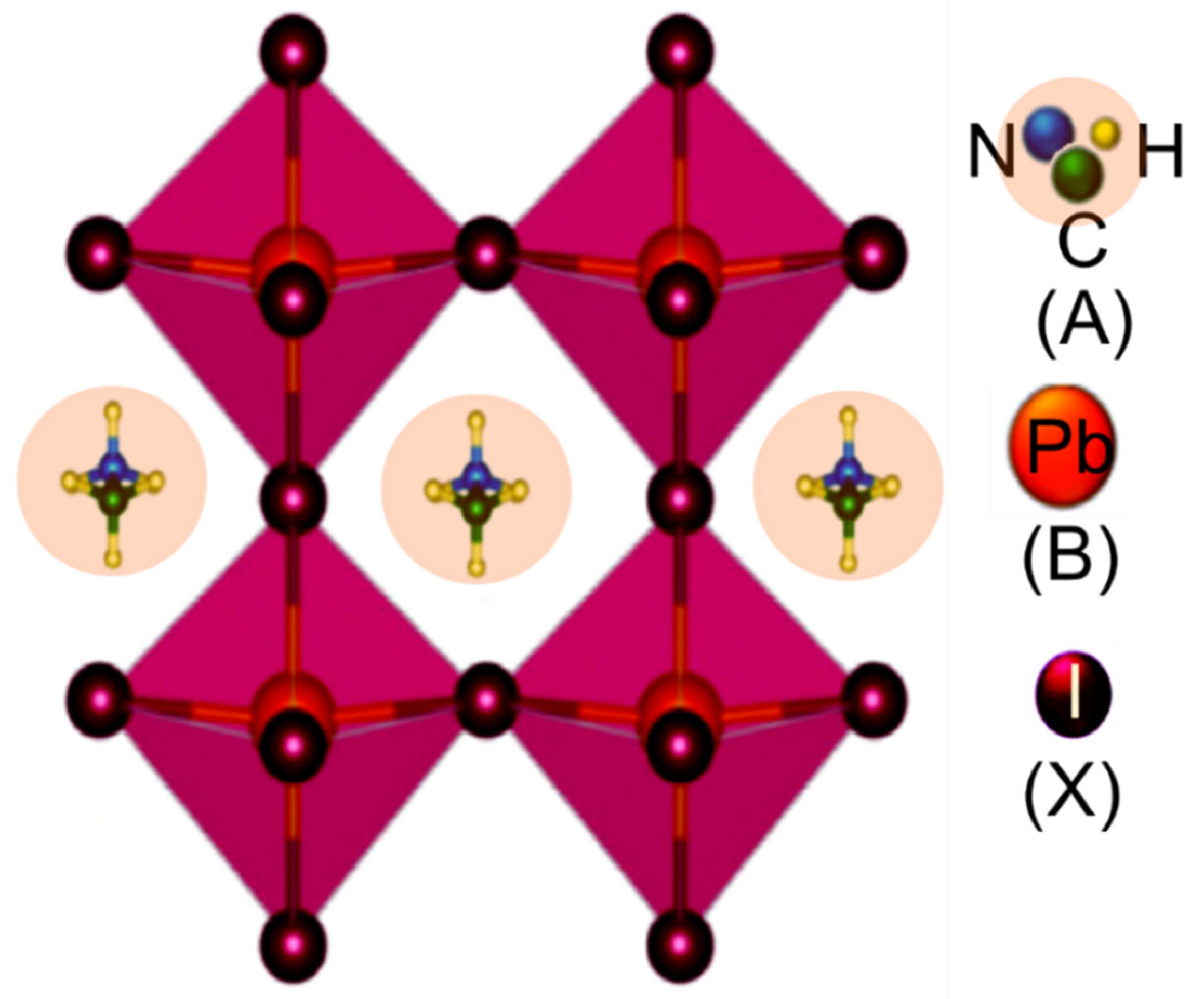
Figure 1. Crystal structure of perovskite with a general chemical formula of ABX3 (in the case of CH3NH3PbI3, A represents the CH3NH3, B represents the Pb, and X represents I).
It should be mentioned that the A, B, and X ions must satisfy this formula, t = (RA + RX)/2 (RB + RX), where RA, RB, and RX are the corresponding ionic radii and t = 1, is the tolerance factor. For most cubic perovskite structures, 0.8 t 0.9 is found quantitatively. In the case of lower symmetry, the value of “t” is very small and then the film structure will be tetragonal or orthorhombic. Alternatively, if t ≥ 1, hexagonal structures are formed, and layers of face-sharing octahedra are added to the structure [67][68]. Moreover, organometal halide perovskites have already been proven several outstanding optoelectronic properties, such as a large absorption coefficient, direct bandgap, small exciton-binding energy, ambipolar semiconducting characteristics, long charge-carrier diffusion length with high charge-carrier mobility [67][68]. Furthermore, the researcher proposed hybrid organometal perovskite material with structure ABX3−xYx, for example, MAPbI3−xClx and MAPbI3−xBrx, which has tunable optical properties. The tunable optical properties make it easier to experiment with device performance and improve PSCs’ overall performance [68]. On the other hand, perovskite films can be prepared by versatile low cost and simple film deposition methods, such as spin-coating [69][70], sequential deposition [71][72], and evaporation [73][74] techniques. Low-temperature spin coating is the simplest method to fabricate low-cost and high-efficiency PVSC devices. However, it is very challenging to form continuous perovskite films means non-fully covered perovskite films by spin-coating via the direct methyl ammonium halide and lead iodide (PbI2) mixed precursor solution [75][76]. All the above process has their own limitation and commercial viability.
Miyasaka and co-workers first reported the liquid-electrolyte-based dye-sensitized solar cells (DSCs) of PCE as a maximum of 3.8% using MAPbI3 and MAPbBr3 perovskites as light absorbers [1]. However, due to the dissolution of the perovskites in the liquid electrolyte, the system was found to be very unstable. In 2012, a significant advance was made independently by Grätzel et al. [60] and Snaith et al. [77] where the liquid electrolyte was replaced with a small-molecule-based hole-transporting material (HTM), 2,2′,7,7′-tetrakis(N,N-di-p methoxyphenylamine)-9,9′-spirobifluorene(spiro-OMeTAD). The perovskite is penetrated the mesoporous TiO2 (mp-TiO2) scaffold with an additional capping layer as shown in Figure 2a, which is covered with a thin layer of the HTM in a typical mesoscopic PSC. Finally, a metal electrode, preferably gold (Au), is deposited on the top of the HTM [61][77]. Instead of TiO2, Al2O3 insulating scaffold can also be used in this mesoscopic structure [77]. The device has been found to work well, signifying that the perovskite could serve as a light harvester as well as an electron transporter (ETM). This finding led to a planar PSC configuration without the mesoporous scaffold as shown in Figure 2b. Particularly, in planar PSCs, the perovskite is simply sandwiched between a thin layer of HTM and a compact ETM, such as TiO2, ZnO, SnO2, etc. [78]. Moreover, HTM-free PSC also reported where the perovskite works as a hole transporter as well as a light absorber [79]. Moreover, ambipolar semiconducting characteristics of the perovskite support fabricating PSC in an inverted fashion, which is typically known as inverted PSCs. Figure 2c represents the mesoscopic inverted PSCs where a p-type mesoporous matrix (such as NiO) is used to deposit the perovskite, and then, a thin layer of ETM is deposited on top of the perovskite [80]. Finally, fabrication has been completed by depositing a metal electrode, such as silver (Ag), by the thermal evaporation technique. Analogous to usual architectures, the PSCs in inverted structure can be fabricated as shown in Figure 2d, where the perovskite layer is sandwiched by an ETM, such as PCBM, and a thin HTM, such as poly(3,4-ethylene dioxythiophene):poly(styrene sulfonic acid) (PEDOT:PSS) [54].
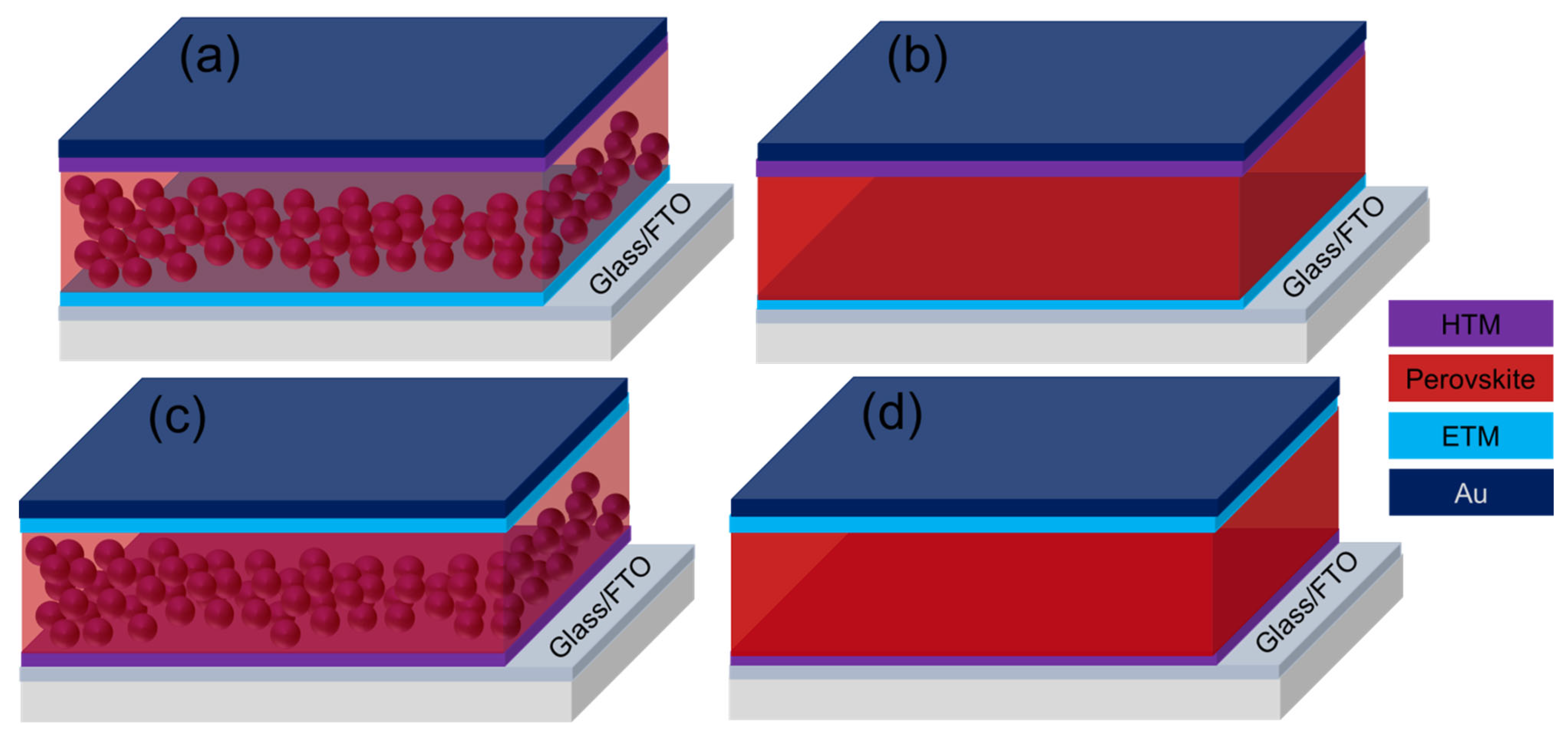
Figure 2. Device architectures of perovskite solar cells; (a) normal mesoscopic, (b) normal planar, (c) inverted mesoscopic, and (d) inverted planar structure.
As previously mentioned, PSCs use primarily two types of system structures (normal and inverted) and obviously, transparent conductive oxide (TCO) (such as ITO or FTO), HTM, perovskite layer, ETM, and contact electrodes (like Au and Ag) are the main components of both structures as shown in Figure 2a,b. The energy band diagram of a normal configuration, shown schematically in Figure 3, depicts the transporting trajectory of electrons and holes during the action. Excitons are produced and then separated into free carriers when sunlight illuminates the perovskite active layer. The generated electrons and holes can then be transported to each interface and injected into ETM and HTM, respectively. Finally, counter electrodes capture electrons and holes in ETM and HTM, respectively, transport them to an external circuit, and generate current [55][81]. Charge separation between MAPbI3 and HTM such as spiro-MeOTAD was observed in transient absorption spectroscopy, but electron injection at open-circuit conditions was not detected yet [61]. It has already been confirmed that HTM plays a crucial role in carrier separation and transport in PSCs [50] which will be discussed in their study for most of the inorganic HTMs used in PSCs.
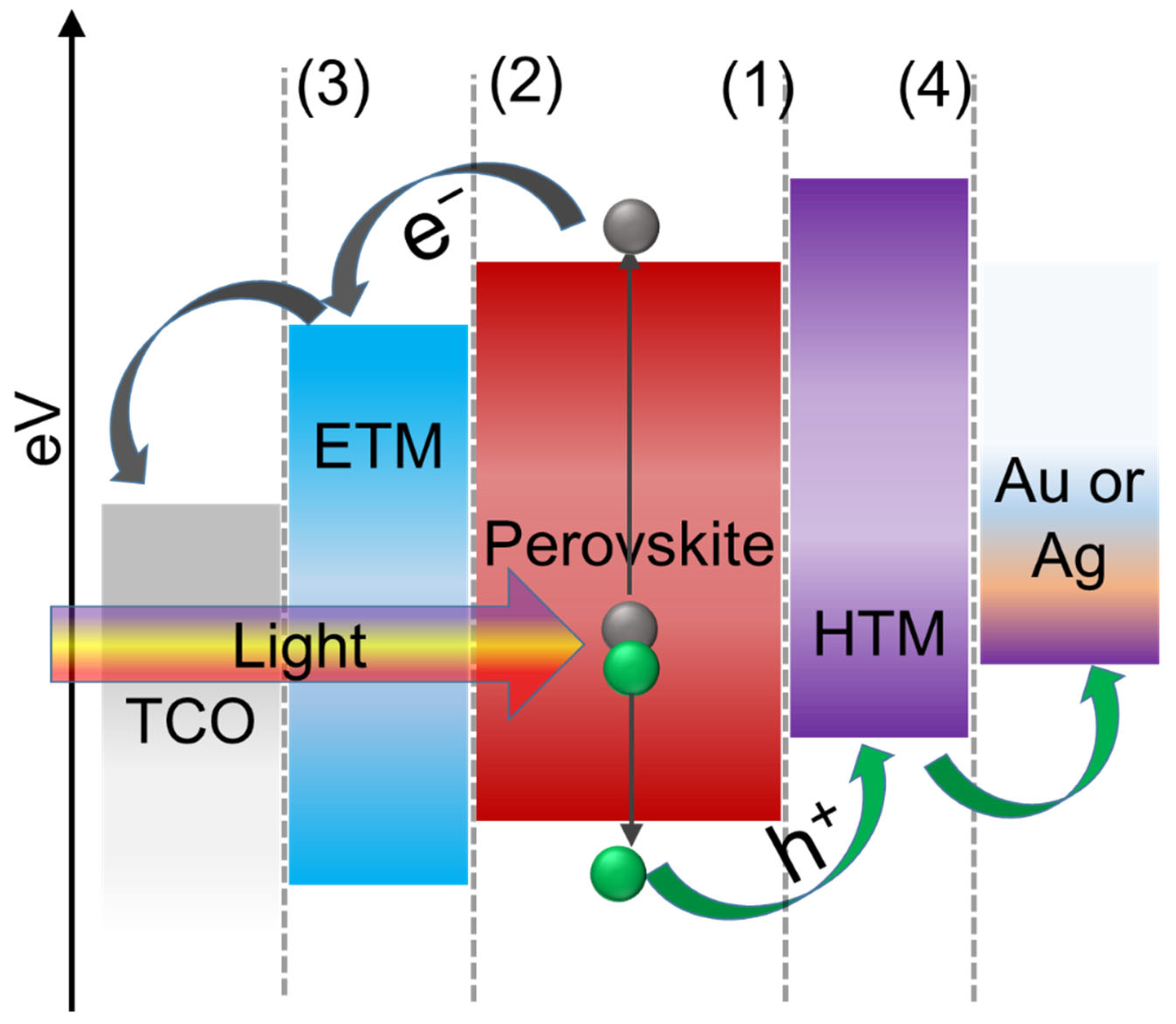
Figure 3. Energy level diagram and the carrier transport mechanism of perovskite solar cell in normal configuration (Interfaces in planar PSCs showing (1) HTL/perovskite interface, (2) perovskite/ETL interface, (3) ETL/cathode interface, and (4) HTL/anode interface).
Particularly, there are primarily four types of interfaces in the inverted and/or normal structure of PSCs as shown in Figure 3. Each of the interfaces is methodically related to interfacial carrier dynamics including charge separation, charge injection, charge transport, charge collection, and recombination processes, and consequently affects how well the device functions in the end. Charge transport, extraction, and collection in real-time operation of PSCs are usually accompanied by charge recombination, which is closely related to PCE, stability, and hysteresis. It clearly shows that interface engineering is essential for developing effective and reliable PSCs
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051.
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093.
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W. Solar cell efficiency tables (version 52). Prog. Photovolt. Res. Appl. 2018, 26, 427–436.
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997.
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319.
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photon. 2014, 8, 489–494.
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970.
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344.
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347.
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587.
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photon. 2015, 9, 106–112.
- Brenner, T.M.; Egger, D.A.; Rappe, A.M.; Kronik, L.; Hodes, G.; Cahen, D. Are mobilities in hybrid organic–inorganic halide perovskites actually “high”? J. Phys. Chem. Lett. 2015, 6, 4754–4757.
- Motta, C.; El-Mellouhi, F.; Sanvito, S. Charge carrier mobility in hybrid halide perovskites. Sci. Rep. 2015, 5, 12746.
- Park, N.-G. Methodologies for high efficiency perovskite solar cells. Nano Converg. 2016, 3, 15.
- Paek, S. Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells. Processes 2021, 9, 2249.
- Wang, S.; Yuan, W.; Meng, Y.S. Spectrum-dependent spiro-OMeTAD oxidization mechanism in perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 24791–24798.
- Chiang, C.-H.; Wu, C.-G. Bulk heterojunction perovskite–PCBM solar cells with high fill factor. Nat. Photon. 2016, 10, 196–200.
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-j.; Sarkar, A.; Nazeeruddin, M.K. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photon. 2013, 7, 486–491.
- Seo, J.; Noh, J.H.; Seok, S.I. Rational strategies for efficient perovskite solar cells. Acc. Chem. Res. 2016, 49, 562–572.
- Chen, H.-W.; Huang, T.-Y.; Chang, T.-H.; Sanehira, Y.; Kung, C.-W.; Chu, C.-W.; Ikegami, M.; Miyasaka, T.; Ho, K.-C. Efficiency enhancement of hybrid perovskite solar cells with MEH-PPV hole-transporting layers. Sci. Rep. 2016, 6, 34319.
- Xiao, J.; Shi, J.; Liu, H.; Xu, Y.; Lv, S.; Luo, Y.; Li, D.; Meng, Q.; Li, Y. Efficient CH3NH3PbI3 Perovskite Solar Cells Based on Graphdiyne (GD)-Modified P3HT Hole-Transporting Material. Adv. Energy Mater. 2015, 5, 1401943.
- Bi, D.; Yang, L.; Boschloo, G.; Hagfeldt, A.; Johansson, E.M. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskite-sensitized mesoscopic solar cells. J. Phys. Chem. Lett. 2013, 4, 1532–1536.
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573.
- Zhang, H.; Xue, L.; Han, J.; Fu, Y.Q.; Shen, Y.; Zhang, Z.; Li, Y.; Wang, M. New generation perovskite solar cells with solution-processed amino-substituted perylene diimide derivative as electron-transport layer. J. Mater. Chem. A 2016, 4, 8724–8733.
- Xiao, Y.; Han, G.; Chang, Y.; Zhou, H.; Li, M.; Li, Y. An all-solid-state perovskite-sensitized solar cell based on the dual function polyaniline as the sensitizer and p-type hole-transporting material. J. Power Sources 2014, 267, 1–8.
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771.
- Jung, J.W.; Chueh, C.C.; Jen, A.K.Y. A low-temperature, solution-processable, Cu-doped nickel oxide hole-transporting layer via the combustion method for high-performance thin-film perovskite solar cells. Adv. Mater. 2015, 27, 7874–7880.
- Sun, W.; Li, Y.; Ye, S.; Rao, H.; Yan, W.; Peng, H.; Li, Y.; Liu, Z.; Wang, S.; Chen, Z. High-performance inverted planar heterojunction perovskite solar cells based on a solution-processed CuOx hole transport layer. Nanoscale 2016, 8, 10806–10813.
- Huangfu, M.; Shen, Y.; Zhu, G.; Xu, K.; Cao, M.; Gu, F.; Wang, L. Copper iodide as inorganic hole conductor for perovskite solar cells with different thickness of mesoporous layer and hole transport layer. Appl. Surf. Sci. 2015, 357, 2234–2240.
- Zhang, H.; Wang, H.; Chen, W.; Jen, A.K.Y. CuGaO2: A promising inorganic hole-transporting material for highly efficient and stable perovskite solar cells. Adv. Mater. 2017, 29, 1604984.
- Igbari, F.; Li, M.; Hu, Y.; Wang, Z.-K.; Liao, L.-S. A room-temperature CuAlO2 hole interfacial layer for efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 1326–1335.
- Sung, H.; Ahn, N.; Jang, M.S.; Lee, J.K.; Yoon, H.; Park, N.G.; Choi, M. Transparent conductive oxide-free graphene-based perovskite solar cells with over 17% efficiency. Adv. Energy Mater. 2016, 6, 1501873.
- Rao, H.; Sun, W.; Ye, S.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Huang, C. Solution-processed CuS NPs as an inorganic hole-selective contact material for inverted planar perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 7800–7805.
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Atomically thin-layered molybdenum disulfide (MoS2) for bulk-heterojunction solar cells. ACS Appl. Mater. Interfaces 2017, 9, 3223–3245.
- Bhattacharya, B.; Singh, P.K.; Singh, R.; Khan, Z.H. Perovskite sensitized solar cell using solid polymer electrolyte. Int. J. Hydrog. Energy 2016, 41, 2847–2852.
- Yu, Z.; Sun, L. Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500213.
- Al Mamun, A.; Ava, T.T.; Zhang, K.; Baumgart, H.; Namkoong, G. New PCBM/carbon based electron transport layer for perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 17960–17966.
- Aitola, K.; Sveinbjörnsson, K.; Correa-Baena, J.-P.; Kaskela, A.; Abate, A.; Tian, Y.; Johansson, E.M.; Grätzel, M.; Kauppinen, E.I.; Hagfeldt, A. Carbon nanotube-based hybrid hole-transporting material and selective contact for high efficiency perovskite solar cells. Energy Environ. Sci. 2016, 9, 461–466.
- Cao, J.; Liu, Y.-M.; Jing, X.; Yin, J.; Li, J.; Xu, B.; Tan, Y.-Z.; Zheng, N. Well-defined thiolated nanographene as hole-transporting material for efficient and stable perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 10914–10917.
- Singh, R.; Jun, H.; Arof, A.K. Activated carbon as back contact for HTM-free mixed cation perovskite solar cell. Phase Transit. 2018, 91, 1268–1276.
- Wang, Q.; Lin, Z.; Su, J.; Hu, Z.; Chang, J.; Hao, Y. Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells. Nano Sel. 2021, 2, 1055–1080.
- Li, S.; Cao, Y.-L.; Li, W.-H.; Bo, Z.-S. A brief review of hole transporting materials commonly used in perovskite solar cells. Rare Met. 2021, 40, 2712–2729.
- Park, H.; Chaurasiya, R.; Ho Jeong, B.; Sakthivel, P.; Joon Park, H. Nickel Oxide for Perovskite Photovoltaic Cells. Adv. Photon. Res. 2021, 2, 2000178.
- Cai, L.; Zhu, F. Toward efficient and stable operation of perovskite solar cells: Impact of sputtered metal oxide interlayers. Nano Sel. 2021, 2, 1417–1436.
- Arumugam, G.M.; Karunakaran, S.K.; Liu, C.; Zhang, C.; Guo, F.; Wu, S.; Mai, Y. Inorganic hole transport layers in inverted perovskite solar cells: A review. Nano Sel. 2021, 2, 1081–1116.
- Di Girolamo, D.; Di Giacomo, F.; Matteocci, F.; Marrani, A.G.; Dini, D.; Abate, A. Progress, highlights and perspectives on NiO in perovskite photovoltaics. Chem. Sci. 2020, 11, 7746–7759.
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Ramakrishnan, V.M.; Palanisamy, S.E.; Sundaram, S.; Velauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2020, 13, 2526–2557.
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.-W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200.
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405.
- Chen, J.; Park, N.-G. Inorganic hole transporting materials for stable and high efficiency perovskite solar cells. J. Phys. Chem. C 2018, 122, 14039–14063.
- Bhat, A.; Dhamaniya, B.P.; Chhillar, P.; Korukonda, T.B.; Rawat, G.; Pathak, S.K. Analysing the Prospects of Perovskite Solar Cells within the Purview of Recent Scientific Advancements. Crystals 2018, 8, 242.
- Qin, X.; Zhao, Z.; Wang, Y.; Wu, J.; Jiang, Q.; You, J. Recent progress in stability of perovskite solar cells. J. Semicond. 2017, 38, 011002.
- Rajeswari, R.; Mrinalini, M.; Prasanthkumar, S.; Giribabu, L. Emerging of inorganic hole transporting materials for perovskite solar cells. Chem. Rec. 2017, 17, 681–699.
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165.
- Fan, R.; Huang, Y.; Wang, L.; Li, L.; Zheng, G.; Zhou, H. The progress of interface design in perovskite-based solar cells. Adv. Energy Mater. 2016, 6, 1600460.
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450.
- Ibn-Mohammed, T.; Koh, S.; Reaney, I.; Acquaye, A.; Schileo, G.; Mustapha, K.; Greenough, R. Perovskite solar cells: An integrated hybrid lifecycle assessment and review in comparison with other photovoltaic technologies. Renew. Sustain. Energy Rev. 2017, 80, 1321–1344.
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H. Optical properties and limiting photocurrent of thin-film perovskite solar cells. Energy Environ. Sci. 2015, 8, 602–609.
- Guo, T.; Lin, M.; Huang, J.; Zhou, C.; Tian, W.; Yu, H.; Jiang, X.; Ye, J.; Shi, Y.; Xiao, Y. The recent advances of magnetic nanoparticles in medicine. J. Nanomater. 2018, 2018, 7805147.
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684.
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591.
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235.
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–514.
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824.
- Bretschneider, S.A.; Weickert, J.; Dorman, J.A.; Schmidt-Mende, L. Research update: Physical and electrical characteristics of lead halide perovskites for solar cell applications. APL Mater. 2014, 2, 155204.
- Mitzi, D.B. Synthesis, structure, and properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 1999, 48, 1–121.
- Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727.
- Jiang, X.; Yu, Z.; Lai, J.; Zhang, Y.; Lei, N.; Wang, D.; Sun, L. Efficient perovskite solar cells employing a solution-processable copper phthalocyanine as a hole-transporting material. Sci. China Chem. 2017, 60, 423–430.
- Tzounis, L.; Stergiopoulos, T.; Zachariadis, A.; Gravalidis, C.; Laskarakis, A.; Logothetidis, S. Perovskite solar cells from small scale spin coating process towards roll-to-roll printing: Optical and morphological studies. Mater. Today Proc. 2017, 4, 5082–5089.
- Wang, G.; Wu, F.; Wu, R.; Chen, T.; Ding, B.F.; Song, Q.L. Crystallization process of perovskite modified by adding lead acetate in precursor solution for better morphology and higher device efficiency. Org. Electron. 2017, 43, 189–195.
- Guo, F.; He, W.; Qiu, S.; Wang, C.; Liu, X.; Forberich, K.; Brabec, C.J.; Mai, Y. Sequential Deposition of High-Quality Photovoltaic Perovskite Layers via Scalable Printing Methods. Adv. Funct. Mater. 2019, 29, 1900964.
- Li, H.; Li, S.; Wang, Y.; Sarvari, H.; Zhang, P.; Wang, M.; Chen, Z. A modified sequential deposition method for fabrication of perovskite solar cells. Sol. Energy 2016, 126, 243–251.
- Reinoso, M.Á.; Otálora, C.A.; Gordillo, G. Improvement Properties of Hybrid Halide Perovskite Thin Films Prepared by Sequential Evaporation for Planar Solar Cells. Materials 2019, 12, 1394.
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho-Baillie, A.W. Hole transport layer free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation. Adv. Energy Mater. 2016, 6, 1502202.
- Yang, M.; Li, Z.; Reese, M.O.; Reid, O.G.; Kim, D.H.; Siol, S.; Klein, T.R.; Yan, Y.; Berry, J.J.; Van Hest, M.F. Perovskite ink with wide processing window for scalable high-efficiency solar cells. Nat. Energy 2017, 2, 17038.
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038.
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647.
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733.
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399.
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P. P-type mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells. Sci. Rep. 2014, 4, 4756.
- Jung, H.S.; Park, N.G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25.
More
Information
Subjects:
Materials Science, Coatings & Films
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
14 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





