Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefan Harsanyi | -- | 5196 | 2022-09-10 18:22:11 | | | |
| 2 | Conner Chen | + 4 word(s) | 5200 | 2022-09-13 11:37:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kupcova, I.; Danisovic, L.; Grgac, I.; Harsanyi, S. Neuropeptides in Anxiety and Depression. Encyclopedia. Available online: https://encyclopedia.pub/entry/27084 (accessed on 07 February 2026).
Kupcova I, Danisovic L, Grgac I, Harsanyi S. Neuropeptides in Anxiety and Depression. Encyclopedia. Available at: https://encyclopedia.pub/entry/27084. Accessed February 07, 2026.
Kupcova, Ida, Lubos Danisovic, Ivan Grgac, Stefan Harsanyi. "Neuropeptides in Anxiety and Depression" Encyclopedia, https://encyclopedia.pub/entry/27084 (accessed February 07, 2026).
Kupcova, I., Danisovic, L., Grgac, I., & Harsanyi, S. (2022, September 10). Neuropeptides in Anxiety and Depression. In Encyclopedia. https://encyclopedia.pub/entry/27084
Kupcova, Ida, et al. "Neuropeptides in Anxiety and Depression." Encyclopedia. Web. 10 September, 2022.
Copy Citation
In modern society, there has been a rising trend of depression and anxiety. This trend heavily impacts the population’s mental health and thus contributes significantly to morbidity and, in the worst case, to suicides. Modern medicine, with many antidepressants and anxiolytics at hand, is still unable to achieve remission in many patients. The pathophysiology of depression and anxiety is still only marginally understood, which encouraged researchers to focus on neuropeptides, as they are a vast group of signaling molecules in the nervous system. Neuropeptides are involved in the regulation of many physiological functions
anxiety
depression
neuropeptides
1. Neuropeptides in Anxiety and Depression
Neuropeptides are small polypeptides of size ranging from 3 to 100 amino acids. Secretion is localized in neurons and the effect, depending on receptors, is local or distant. Affected are both neurons and other somatic cells, indicating a wide array of effects [1][2]. Some are synthesized as precursors and later cleaved into mature forms, while some have no post-translational changes [3]. Stress, pain, or emotional distress often leads to anxiety and depression. Studies of neuropeptides and their related receptors pose a high potential for a better understanding of the etiology/etiopathogenesis of these disorders, leading to better, more targeted surveillance and treatment, especially as diagnostic markers. In Table 1 summarize the neuropeptides mentioned in these contents with their related receptors and encoding genes. Data were acquired using the OMIM® database accessed on 25 May 2022 [4].
Table 1. Anxiety and depression-related neuropeptides, encoding genes, and related receptors. (Receptors of neuropeptides without any relation to anxiety or depression are not mentioned in this table.)
| Neuropeptide | Gene (Symbol) | Cytogenetic Location | Related Receptors |
|---|---|---|---|
| Oxytocin | OXYTOCIN (OXT) | 20p13 | OXTR |
| Vasopressin | ARGININE VASOPRESSIN (AVP) | 20p13 | AVPR1A; AVPR1B; AVPR2 |
| Adrenocorticotropic hormone | PROOPIOMELANOCORTIN (POMC) | 2q23.3 | MCR1; MCR2; MCR3; MCR4; MCR5 |
| Corticotropin-releasing hormone | CORTICOTROPIN-RELEASING HORMONE (CRH) | 8q13.1 | CRHR1; CRHR2 |
| Urocortin 1 | UROCORTIN (UCN) | 2p23.3 | |
| Urocortin 2 | UROCORTIN II (UCN2) | 3p21.31 | |
| Urocortin 3 | UROCORTIN III (UCN3) | 10p15.1 | |
| Pituitary adenylate cyclase-activating polypeptide | ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE 1 (ADCYAP1) |
18p11.32 | ADCYAP1R1; VIPR1 |
| Melanocyte stimulating hormone | PROOPIOMELANOCORTIN (POMC) | 2p23.3 | MC3R; MC4R |
| Melanin-concentrating hormone | PRO-MELANIN-CONCENTRATING HORMONE (PMCH) |
12q23.2 | MCH-R1; MCH-R2 |
| Beta-endorphin | PROOPIOMELANOCORTIN (POMC) | 2q23.3 | OPRM1 other μ-opioid receptors |
| Neuropeptide Y | NEUROPEPTIDE Y (NPY) | 7p15.3 | NPY1R; NPY2R; NPY5R |
| Neuropeptide S | NEUROPEPTIDE S (NPS) | 10q26.2 | NPSR1 |
| Neuropeptide FF | NEUROPEPTIDE FF-AMIDE PEPTIDE (NPFF) | 12q13.13 | NPFFR1; NPFFR2 |
| Galanin | GALANIN (GAL) | 11q13.2 | GALR1; GALR2; GALR3; GPR151 |
| Galanin-like peptide | GALANIN-LIKE PEPTIDE (GALP) | 19q13.43 | GALR1; GALR2 |
| Spexin | SPEXIN HORMONE (SPX) | 12p12.1 | GALR2; GALR3 |
| Kisspeptin | KISS1 METASTASIS SUPPRESSOR (KISS1) |
1q32.1 | KISS1R |
| Substance P | TACHYKININ 1 (TAC1) | 7q21.3 | TACR1; TACR2; TACR3 |
| Neurotensin | NEUROTENSIN (NTS) | 12q21.31 | NTSR1; NTSR2 |
| Hypocretin | HYPOCRETIN (HCRT) | 17q21.2 | HCRTR1; HCRTR2 |
| Phoenixin | SMALL INTEGRAL MEMBRANE PROTEIN-20 (SMIM20) | 4p15.2 | GPR17 |
| Relaxin 3 | RELAXIN 3 (RLN3) | 19p13.12 | RXFP3 |
| Nesfatin-1 | NUCLEOBINDIN 2 (NUCB2) | 11p15.1 | Not discovered |
| Nociceptin | PREPRONOCICEPTIN (PNOC) | 8p21.1 | OPRL-1 |
| Cholecystokinin | CHOLECYSTOKININ (CCK) | 3p22.1 | CCKAR; CCKBR |
| Calcitonin gene-related peptide | CGRP RECEPTOR COMPONENT (CGRP) | 7q11.21 | CGRPR |
| Neurokinin A | TACHYKININ 1 (TAC1) | 7q21.3 | TACR1; TACR2; TACR3 |
Signaling axes (or pathways) studied in relation to anxiety and depression are the hypothalamic–pituitary–adrenal (HPA) axis, the hypothalamic–pituitary–gonadal (HPG) axis, and the gut–brain axis (GBA). The GBA is newer, and less thoroughly studied, although reports indicate that the microbiome and gut hormones may play a role in the etiology of depression [5]. More studied are the HPA and HPG axes. Interactions of these axes are presented in Figure 1.
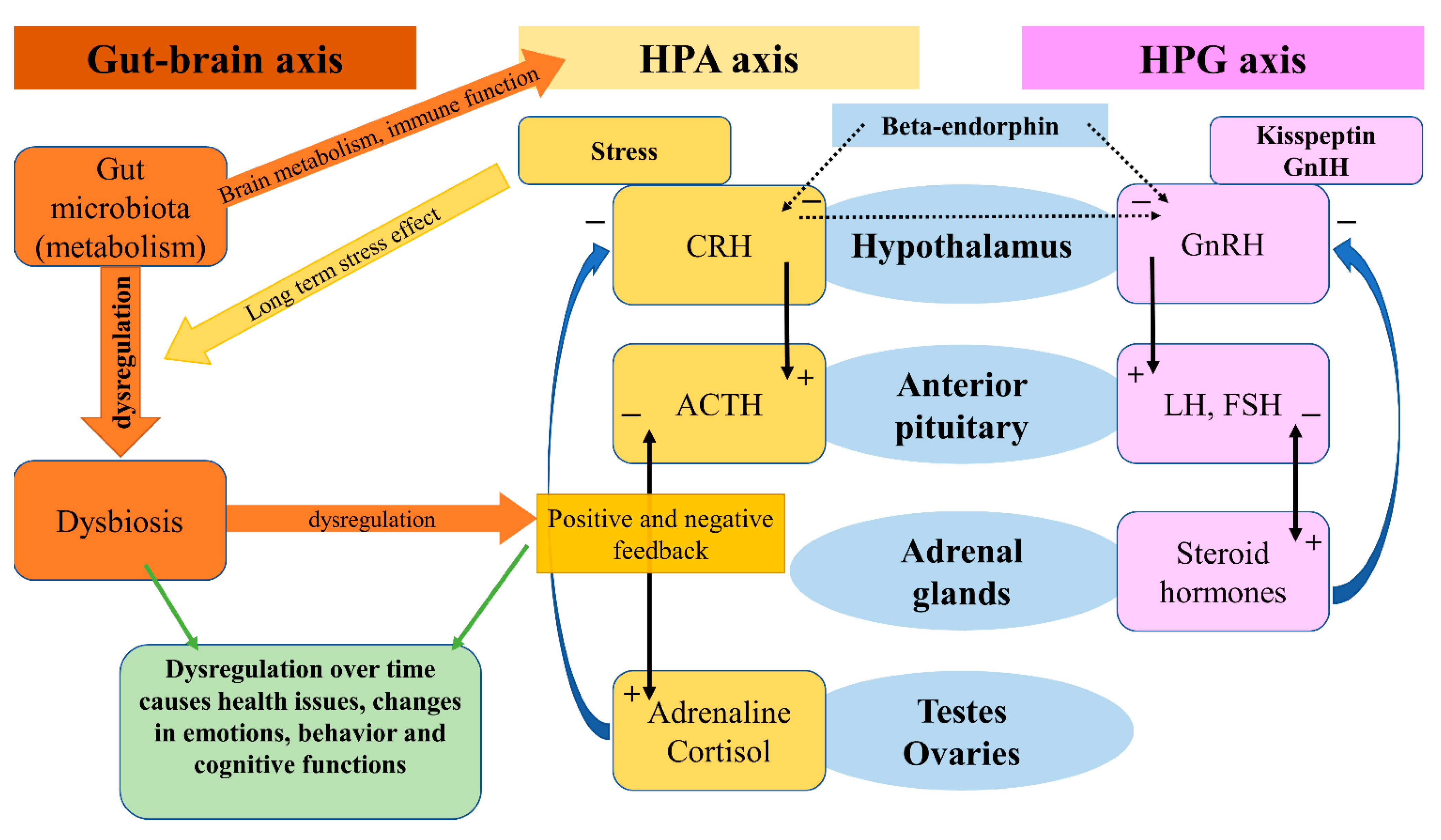
Figure 1. Gut-brain axis, HPA axis, HPG axis and their interactions.
The HPA axis affects many different systems in the mammalian body, with a modern link to neuropeptides, psychiatry, and neurology, one review will focus on this aspect [6]. The HPA axis is controlled by the limbic system depending on stimuli (stressors) received by the latter, and so inducing release of the CRH. CRH stimulates the release of ACTH (adrenocorticotropic hormone), which affects the adrenal glands and the production of glucocorticoids. Stress-adaptation responses are mediated by corticosterone in rodents and by cortisol in humans.
Circulating levels of gluco- and mineralo-corticoids are essential for negative feedback on the hypothalamus, which has been reported faulty in patients suffering from depression [7]. A systematic review and meta-analysis on the effects of aging and the HPA axis in patients with depression reported that older patients suffering from depression showed a higher degree of dysregulation in the HPA axis [8]. A bipolar disorder- (BD) focused systematic review and meta-analysis found a possible association of progressive HPA axis dysfunction with the cognitive deterioration of BD patients, which seemed rather a risk factor than a determinant, but nonetheless an important one [9]. In a recent review, an association between early-life stress in the HPA axis and anxiety has been found, followed by hyperactivity in the axis acting as a risk factor for relapses [10]. Effects of the microbiota on the HPA axis, stress response, anxiety-like behavior, endocrine abnormalities, and wider neuropsychiatric disorders have been observed, calling for further investigation [11][12][13][14]. In a systematic review, Juruena et al., based on the activity of the HPA axis, found a difference between melancholic and atypical depressive subtypes [15]. However, they attributed these findings to hypercortisolism in melancholia and rather normal, than decreased, function in atypical depression [15].
The HPG axis is represented by neurons of the gonadotropin-releasing hormone (GnRH), which in mammals is a 10-amino acid peptide. GnRH stimulates the synthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thus having an important role in the regulation of fertility and reproduction. GnRH is inhibited by the gonadotropin-inhibitory hormone (GnIH), which belongs to the RFRP family. The RFRPs (RF-amide-related peptides) act in regulatory regard to the HPG axis [16]. Studies on the GnIH have been associated with different behaviors in male rats [17]. Stress-induced increase in adrenal glucocorticoids increased the RFRPs, which contributed to the suppression of reproductive function through the HPG axis [18]. In birds, a small dose of interfering RNA targeted against the GnIH precursor mRNA caused elevation in aggressive and sexual behaviors [19]. Iwasa et al. reviewed the field of stress-induced reproductive dysfunction and found an association with the GnIH/RFRP-3 system [20]. These findings are important as stress, whether physical or emotional, is related to anxiety and depression in a reciprocal character, and one may affect or over time induce the other. The use of GnRH analogs caused upregulation in the expression of phoenixin in hypothalamic, hypophyseal, and ovarian regions, while the GPR173 expressions were downregulated in the hypothalamus and pituitary [21].
2. Oxytocin
Oxytocin (OXT) is composed of nine amino acids and was the first polypeptide hormone to be sequenced. OXT is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus [22]. It became first known for its role in lactation, parturition, and maternal behavior, yet over the last several decades it has also been implicated in memory, regulation of anxiety, mood, and social behavior [23]. In reaction to acute stress, oxytocin is released from the nucleus centralis of the amygdala, which leads to the local activation of gamma-aminobutyric acid (GABA)-ergic interneurons [24]. While the amygdala is responsible for acute stress reactions, stria terminalis is the structure involved in the transition to chronic anxious states. A portion of oxytocinergic neurons from the periventricular nucleus projects directly into this structure [25]. The anterior cingulate cortex likely facilitates learning and social adaptation [26][27]. Oxytocin receptors (OXTR) are present across the whole brain, and it seems that the research of polymorphisms and epigenetic markers for OXTR is a promising area for future research [28]. Myers et al. reported two single nucleotide polymorphisms (SNP) in the OXTR gene in depressed patients [23]. Costa et al. described a relationship between separation anxiety in adulthood and SNP rs53576 of the third intron of the OXTR gene, which seems of substance for the epigenetic regulation of this gene [29]. An allele of rs53576 is also related to increased suicide risk, according to some authors [30]. Oxytocin interacts with various neurotransmitters and neuroendocrine systems [31]. The most prominent ones are the serotonergic and GABA-ergic systems and the HPA axis. Mottolese et al. have proven the interplay between oxytocin and serotonin using a 5-HT1A receptor antagonist 2’methoxyphenyl-fluorobenzamidoethylpiperazine (MPFF). Intranasal administration of oxytocin resulted in increased levels of MPPF in nucleus dorsalis raphes, the right amygdala-hippocampus-parahippocampus complex, insula, and right medioventral prefrontal cortex, measured using PET MRI [32]. The interplay between the oxytocinergic and serotoninergic systems is further supported by the fact that MDMA (3,4-methylenedioxymethamphetamine) causes oxytocin-mediated behavioral changes via serotoninergic 5HT1A receptors [33]. Furthermore, oxytocin is also connected to the HPA axis. Part of the neurons in the paraventricular nucleus co-expresses oxytocin and CRH, a portion of CRH-releasing neurons expresses mRNA for the OXTR, and clusters of OXTR express CRH receptor CRHR2. Centrally administered oxytocin reduces the excitability of CRH neurons, inhibits their spontaneous excitability, and lowers the expression of CRH mRNA and neuroendocrine response to stress [24]. In animals, the central administration of oxytocin reduces anxiety-like behaviors and lowers plasmatic cortisol levels [34]. Furthermore, the medial prefrontal cortex contains oxytocinergic interneurons synthetizing CRH binding protein (CRHBP) and GABA. In male rodents, the stimulation of these interneurons leads to the release of CRHBP and the reduction of CRH activity and anxiety, while in female rodents CRHBP was not able to effectively inhibit CRH activity, which may be due to higher levels of CRH in paraventricular nuclei (PVN) [35]. Antagonistic activity on GABAA receptors in the PVN blocks the suppression of stress-induced CRH release and the application of oxytocin into the PVN results in a significant increase in GABA release from the PVN [34]. Meisenberg was the first to describe the antidepressant-like effect of oxytocin when oxytocin application had an effect similar to the tricyclic antidepressant imipramine in rodents—it resulted in reduced immobility during the forced swim test [33]. Later, Scantamburlo et al. reported an inverse relationship between plasma oxytocin levels and the severity of symptoms of depression and anxiety in depressed patients [36]. Jobst et al. measured plasmatic levels of oxytocin in chronically depressed patients before and after psychotherapy. In their research, increased levels of oxytocin correlated with a reduction of subjectively reported depressive symptoms according to the Beck Depression Inventory-II [37]. Lancaster et al. found that higher endogenous oxytocin levels are associated with a reduced central amygdala volume and blood oxygen level-dependent activity in response to aversive stimuli [38]. However, the relationship between oxytocin and depressive and anxiety symptoms is not as straightforward as it may seem, and the literature is full of contradictory results. For example, Parker et al. found increased oxytocin levels in depressed subjects, and more recently Tabak et al., examining the role of oxytocin in social anxiety in humans, reported increased plasma concentrations of oxytocin following the Trier Social Stress Test (TSST), but this increase was specific to women, and that social anxiety moderated the OXT increase with more socially anxious participants showing greater increases than those with lower levels of social anxiety [39]. This can be the result of the so-called oxytocin paradox, which has been described as the context-dependent feedback loop of OXT signaling based on socio-psychological settings leading to positive or adverse psychological consequences [40]. The functioning of oxytocin varies according to contextual factors and individual differences, including gender, age, and psychosocial functioning [41]. In the past years, research allowed people to better understand signaling ways affected by OXT and its complex regulation, and further multi-peptide-centered research will reveal this intricate web of regulation.
3. Vasopressin
Vasopressin, also known as the antidiuretic hormone (ADH) or arginine vasopressin (AVP), consists of 9 amino acids, belongs to nonapeptides, and is synthesized by magnocellular cells of the hypothalamic supraoptic nucleus and PVN whose axons project to the posterior pituitary [42][43]. After release into the bloodstream, it is transported predominantly to kidneys and blood vessels, where it regulates physiological functions (e.g., resorption of water, vasoconstriction, …). Vasopressin has amino acid sequences and tertiary structures similar to oxytocin, probably due to the shared origin of their encoding genes [44]. In addition, it is possible that an interplay between oxytocin and vasopressin is involved in mood and stress-response regulation [45]. It binds to four types of G-protein-coupled receptors: AVPR1A, AVPR1B, AVPR2 (for clarity reasons further as V1A, V1B, and V2, respectively), and oxytocin receptor [46]. V2 receptor binds to vasopressin in the kidney, where it participates in water homeostasis [47]. On the other hand, V1B and V1A receptor subtypes are promising sites of interest for psychiatry. The V1B subtype is highly expressed in the anterior pituitary gland, where it stimulates corticotropin release and is also localized in the amygdala, hippocampus, and hypothalamus [48]. Purba et al. already in 1996 reported increased numbers of V1B receptors in the postmortem analysis of brains of depressed patients compared to controls [49]. In depression and anxiety, vasopressin binds to V1 receptors in the brain. Hoghson et al. used V1B-30N-A potent V1B receptor antagonist in rat pups to examine the potential role of V1B in anxiety. They reported that V1B-30N had a dose-dependent anxiolytic effect in the separation-induced vocalization test in rat pups, moreover without causing sedation [46]. The interaction between vasopressin and oxytocin has already been mentioned, but vasopressin co-operates at least with one other neuromodulator system—the serotonergic. Ishizuka et al. used V1B knockout mice treated with a selective serotonin reuptake inhibitor (SSRI) and serotonin noradrenaline reuptake inhibitor (SNRI) to examine whether at least part of the SSRI´s effects might be mediated via the V1B receptor. The effects were evaluated in experiments using an elevated plus-maze (EPM) test and a hole-board (HB) test. Chronic treatment of V1bR KO mice with SSRI did not change the amount of time spent on the open arms, the number of head dips, or the number of readings, while chronic treatment with SNRI significantly increased the time spent on the open arms and the number of head dips. These results suggest that the anxiety action of 5-HT reuptake inhibitors might partly involve V1bR regulating the anxiety behaviors [48]. While the V1B receptor likely plays a role in depression and anxiety, the V1A subtype seems to be related to a different type of psychopathology. Vollegbregt et al. in their study reported an association between RS3 microsatellite repeats within the promoter region of the V1A gene and extreme childhood aggression, while no association was found between childhood aggression and RS1 repeats [50]. However, in addition to factors related to the V1 receptor, the levels of vasopressin also likely play a role in the pathophysiology of depression and anxiety. It was shown that chronically elevated plasma vasopressin levels may induce depressive symptomatology [51]. Another research project pointing to the positive correlation between vasopressin levels and depression was performed by Mlynarik et al., which showed reduced depression-like behavior in vasopressin-deficient Battleboro rats during the forced swim test [52]. The results of human studies agree with animal research. Goekoop et al. measured plasma levels of vasopressin in 89 patients with a highly anxious-retarded subtype of depression with a family history of depression. Depression with above-normal plasma AVP, as well as familial depression with above-normal plasma AVP, showed a high correlation between anxiety and retardation and this correlation was significantly higher than that found in the depressed patient control groups. The data support the delimitation of a largely familial depression with above-normal plasma AVP, vasopressinergic activation of the HPA axis, and a variable anxious-retarded phenotype [53]. As already mentioned above, the current situation regarding the efficient treatment of anxiety and depression is far from satisfactory despite the fact that there are multiple pharmacological agents on the market. The rate at which novel antidepressants are developed and introduced into clinical practice is very slow. This motivates research into different treatment modalities, of which V1B antagonists seem to be one promising option [54][55].
4. Melanocortins
Melanocortins are a group of peptide hormones derived from pro-opiomelanocortin (POMC). This group includes the adrenocorticotropic hormone (ACTH), alfa-, beta-, and gamma-melanocyte stimulating hormones (MSH), beta-endorphin, and corticotropin-like intermediate peptide (CLIP), the adrenocorticotropic hormone fragment [56]. Melanocortin receptors are associated with obesity, erectile dysfunction, cachexia, pain, depression, and anxiety [57]. Melanocortins work through the melanocortin receptors. The five melanocortin receptors are GPCRs identified as MC1 to five receptors. To date, only MC3R and MC4R with their ligand, the alfa-MSH (also gamma-MSH in the case of MC3R) were connected to anxiety and depression [58]. The main expression sites of melanocortin neurons are located in the brainstem and the hypothalamus, in the arcuate nucleus (ARC), closely communicating with AgRP- and Neuropeptide Y- (NPY) expressing neurons, thus indicating their participation in the system [59][60]. The alpha-MSH was reported to suppress the effects of NPY, thus reducing its antidepressant effect [61]. The administration of alpha-MSH antagonist with NPY shows a synergistic anxiolytic effect [62]. The inhibition of MC4 receptors in the dorsal raphe nucleus (DRN) using alpha-MSH induces anxiety and depression and reduces feeding in mice [63]. PVN, ARC, and DRN are predominant locations of the alpha-MSH effect [64]. The circle of regulation in the melanocortin system is closed by the receptor antagonist, the Agouti-related peptide (AgRP), with an orexigenic effect [65]. Chronic administration of a high-fat diet blunts AgRP response to anxiety and depression signals, as well as hunger by reducing GABA-ergic outputs from AgRP aimed at MC4R. However, GABA-ergic stimulation and suppression of the 5-HT3R within the MC4R neurons in the bed nucleus causes cessation of the effect of the high-fat diet-induced anxiety and depression [66]. The combined effect also reduces food intake and thus body weight. These findings indicate that initially AgRP regulates appetite, but later with a loss of effect, MC4Rs are a viable option for further research on anxiety and depression treatment [67][68]. Further associations are discussed in individual sections. A specific regulation protein, which affects the melanocortin system, is the melanocyte-stimulating hormone release-inhibiting factor-1 (MIF-1) with its analog Nemifitide. Both show low affinity to μ-opioid receptors and function as blockers of alpha-MSH release, thus reducing its inhibitory effect on NPY. Preclinical studies have shown effects in depressive disorder, but further research into this particular peptide is necessary to prove its efficacy.
5. Corticotropin-Releasing Hormone
Corticotropin-releasing hormone (CRH) is also known as the corticotropin-releasing factor (CRF); however, for precise nomenclature reasons, further on, the term CRH will be used. CRH is the central regulator of the hypothalamic–pituitary–adrenal (HPA) axis, which is the main organizer of the body’s response to stress. CRH consists of 41 amino acids and binds to CRHR1 and CRHR2, two GPCRs (not counting alpha and beta splicing variants), both in the central nervous system and the R2 also in peripheral tissues [69]. CHR receptors are expressed in the pituitary, amygdala, hippocampus, brain stem, and cingulate cortex (20). The CRH system consists of CRH itself, then Urocortin 1, 2, and 3, which all serve as ligands for the CRH receptors [70]. Effects of this system lie not only in the mediation of stress-related responses but also in the regulation of inflammation, with a close relationship with inflammation-related pain, as macrophages, monocytes, or mast cells do express both CRH receptors, thus serving as targets of this system [71]. CRH-induced stress-related responses through the HPA axis have a wide area of effect, recently measured in human hair follicles connected to hair loss interestingly antagonizable by caffeine [72]. It can be observed how deteriorative an effect the CRH-HPA system can have on the human body and how broad the effect is. It is not surprising that many human and animal studies report CRHs’ effect on the HPA axis regulation. Chronic stress activates the CRH-related signaling in the bed nucleus and induces maladaptive behavior in mice [73]. This goes in line with observed HPA axis dysfunction in patients with major depression or schizophrenia, where the continuous effect of CRH on the HPA axis causes disbalance (increased cortisol, increased ACTH, reduced feedback) and results in a pathology [74][75]. This disbalance is targeted by antidepressants, which after a period of time have the potential to revert changes in HPA axis hyperactivation. The CRH system is long associated with serotonergic mediation, emotional disbalance, behavioral changes, anxiety, and depression [76][77][78]. CRHR1 gene variants have been linked to a higher susceptibility to depression or panic disorder [79][80]. A sex-specific stress reaction in females has been observed in an animal model, with sex hormones affecting CRH regulation [81]. Transgenic mice with induced CRH expression show hyperactivation of the HPA axis and an increase in stress-mediated processes and behaviors, treatable with CRHR1 antagonists [82].
Further members of the CRH family are the urocortins. Urocortin 1, 2, and 3 are a group of three peptides (UCN1, UCN2, and UCN3). UCN1 is a 40-amino acid peptide, while UCN2 and 3 are paralogous 38-amino acid peptides that bind to CRHR2 [83]. UCN1 is produced by the hypothalamus, substantia nigra, and the pituitary gland and has a high affinity for both CRH receptors, suggesting its role in behavior and interestingly also regulation of food intake [84]. The intracerebrovascular injection of UCN1 has anxiogenic and “anti-social” effects antagonizable by CRHR1 antagonists [85][86]. However, the CRHR1 antagonist had no effects on social interaction on its own but antagonized the decreases in social interaction induced by stress or UCN1 [87]. Summarized by Tanaka et al., UCN2 and UCN3 show an association with depression-like behavior, where stimulation by UCN1 (CRHR1-2) is antagonized by UCN2 and UCN3 (CRHR2), thus resulting in no effect [88].
The latest members associated with the CRH family are four teneurins, or teneurin C-terminal associated peptides (TACPs), which share homology with the amino acid sequence of CRH and bind to latrophilins (a group of highly conserved GPCRs). Their effect is tissue-specific, and reports show an ability of TACP-1 to reduce anxiety, addiction, and depression in stress-induced behaviors [89].
On the other hand, studies and preclinical models also show mixed results [90]. In some cases, CRH or TACP receptor antagonists have different effects on anxiety, based on the animal test line, the baseline of depression, or perceived anxiety or the treatment regimen, but also depending on the test, as these therapeutics do not work under basal conditions. However, if depression- or anxiety-related symptoms or signaling are present, these therapeutics do have a significant effect [91]. In other words, a stressor (depression or anxiety) must be present for antidepressants and anxiolytics to work [92]. These conclusions are not surprising, as urocortins and their over-expression in CRH-deficient individuals are often induced as a compensatory mechanism in absence of CRH. The same result is for models with CRH over-expression antagonizable by CRHR1 antagonists, where some lines do exhibit higher anxiety and vulnerability to stress, but in other lines that do not exhibit these traits, a UCN1 downregulation was found [93][94].
6. Pituitary Adenylate Cyclase-Activating Peptide
Pituitary adenylate cyclase-activating peptide (PACAP) is a neuropeptide existing in two isoforms, either 27 or 38 amino acids long. PACAP is relatively well-conserved across many species [95]. Wide expression in central and peripheral tissues indicates a role in multiple physiological functions, such as the modulation of nociception, regulation of prolactin release, and food intake, along with stress, anxiety, and depression [96][97]. PACAP has the highest affinity for the ADCYAP1R1 receptor, known also as the PAC1 receptor [98]. Studies have associated this neuropeptide with stress-related mood disorders and adaptation [1][99]. PASAP is predominately expressed in the amygdala and the bed nucleus [100][101]. Dore et al. observed pro-depressive and anxiety-like effects on rats after intracerebroventricular PACAP administration [102]. Human studies show an association of PACAP/PAC1 receptor with behavioral and endocrine responses, also in neuropsychiatric disorders such as schizophrenia or post-traumatic stress disorder (PTSD) [103][104][105]. A recent animal model of PACAP-mutated mice under chronic variable mild stress exhibited viable results for future depression-targeted tests [106]. PACAP-KO mice exhibit decreased c-Fos expression, which shows a close connection not only with CRH and wider melanocortin signaling [107][108]. These results go in line with previous results on PACAP’s influence on CRH [109]. Additionally, a study on the consequences of chronic stress on PACAP expression in the bed nucleus goes in hand with CRH, indicating that synergic modulation exists in anxiety-like behavior [110]. The anxiety-associated effect of PACAP was further studied in mice, where the induction of PACAP-firing neurons aimed at the bed nucleus enhanced anxiety-like behavior [111].
7. Melanin-Concentrating Hormone
Melanin-concentrating hormone (MCH), or pro-melanin-concentrating hormone (PMCH), exists in a cyclic form and consists of 19 amino acids. MCH binds to two GPCRs, the MCH-1R and MCH-2R, of which only the 1R type is expressed in rodents, while both are present in humans [112]. Receptors are widely expressed in the brain, but MCH secretion, depending on species, is limited to sections of the hypothalamus and zona increta [113]. MCH is involved in the regulation of feeding and energy homeostasis, also stress-related adaptation, emotions, and cognitive functions, some of which are due to the MCH interaction with neurotransmitters of serotonergic and cholinergic systems [114][115]. MCH reverses activation of the HPA axis and also interacts with associated serotonergic and cholinergic pathways [116][117][118]. MCH-1R exhibits a potential for anxiety and depression treatment because even in animal models, MCH-1R knockouts exhibit an anxiety-resistant phenotype [119][120]. This potential has been studied by the administration of different MCH-1R antagonists that produced antidepressant results in rats [121][122]. MCH-1R receptor antagonists infused in the nucleus accumbens are producing faster anxiolytic and antidepressant effects in mice and rats compared to the selective serotonin reuptake inhibitors (SSRIs), while receptor agonists produce opposite effects [123]. This happens concurrently with induced sensitivity of dopamine D2 and D3 receptors [123]. The nucleus accumbens has a relation to the mediation of relative motivation for rewards. A recent animal study observed reduced stress-induced anxiety and depression after intranasal administration of MCH [124]. These results in animal studies are further proof of the important role of MCH in the etiology of anxiety and depression with connections to the HPA axis via CRH, where the MCH-1R antagonists act as inhibitors of this connection.
8. Beta-Endorphin
Beta-endorphin (β-endorphin) is a 31-amino acid endogenous opioid neuropeptide that binds to opioid receptors. The mature form is derived from precursor POMC. There have been conflicting findings in research regarding its potential role in depression, but recent studies show decreased β-endorphin activity during negative moods, and a decreased quantity of its μ receptors has been found in the brains of depressed suicide victims [125]. There is also a suggestion that its plasmatic levels might correlate with the response to depression treatment [126]. There has also been evidence that the opioid system is involved in the regulation of anxiety, and its activation has been demonstrated to have an anxiolytic effect in humans [127]. Savic et al. created a model on plasma beta-endorphin in which only anxiety and hyperarousal were directly associated with peripheral beta-endorphin fluctuations [128]. Patients with Meniere’s disease or dyscirculatory encephalopathy with the vestibular ataxic syndrome were examined for beta-endorphin levels before and after pharmacological and physical rehabilitative treatment where the levels correlated with the degree of anxiety and depression, but not the vestibular-related symptoms [129]. The intracerebroventricular administration of beta-endorphin antagonized suppressive effects of alfa-MSH on food intake and weight gain for a limited time and later lost effect, which indicates an intricate cross-regulation in the melanocortin system [130].
In a recent study on the effects of auricular point sticking therapy in patients undergoing partial lung resection, beta-endorphin concentration was used as a marker of peri- and post-operative pain, anxiety, and depression. Authors attributed the analgesic mechanism to the increase in plasma concentration of β-endorphin [131]. In a model on opiate-mediated analgesia in rats suffering from osteoarthritis (OA) -like symptoms combined with normal or anxiety-like behavior, baseline plasma levels of β-endorphin were significantly lower in the OA + anxiety group [132]. However, this effect was also observed after an intra-articular injection of saline in both groups, which is possible since, in a meta-analysis, the efficacy of saline injections in articular pain management of OA patients was found to be on par with other injectable options [133].
9. Neuropeptide Y
Neuropeptide Y (NPY), a 36-amino acid neuropeptide, has a wide distribution in the central nervous system (CNS) with connections to the melanocortin system. NPY belongs to the most conserved proteins in evolution. In the past, NPY levels were linked to affective disorders, and measured plasma levels were low in suicidal depressed patients [134][135]. The effect of NPY through its receptors has been reviewed by Morales-Medina et al., with the conclusion that animal models on Y1 and Y2 receptors provided robust data on their role in emotional responses and stress [136]. Holzer et al. reported the role of NPY, peptide YY (PYY), and pancreatic polypeptide (PP) on depression-related behavior through the gut–brain axis [137]. A recent study on a murine model demonstrated that depression increased IL6 levels and promoted myeloid cell infiltration by a sympathetic-NPY signal [138]. A different murine model reported depression-like behavior associated with the decreased expression of NPY in the hypothalamus and hippocampus, while also inducing changes in the microbiome and brain metabolome in mice on a high-fat diet [139]. NPY is partially connected to the HPG axis via kisspeptin neuron fibers on the ARC neurons that express POMC, the precursor of melanocortins, and indirectly inhibits the ARC neurons expressing NPY. A review by Dr. Domin suggested neuroprotective and antidepressant properties of ligands of NPY receptors Y2R and Y5R, currently backed by the murine model [140]. A correlation between rats with anxiety-like behavior on levels of NPY, calcitonin gene-related peptide, and neurokinin has been reported by Carboni et al. 2022. A most recent study studied transcriptional and translational levels of NPY receptors in the prefrontal cortex and hippocampus of normal brains (control subjects) and suicidal subjects (study group) [141]. In both studied parts of the brain, a significant decrease in NPY mRNA and also upregulation of Y1R and Y2R mRNA was observed in the study group, along with a significant decrease in expression of the NPY protein in the prefrontal cortex of the study group [142].
10. Neuropeptide S
Neuropeptide S (NPS), a 20-amino acid neuropeptide, is implicated in sleep, arousal, feeding behavior, anxiety, and stress adaptation [1][2]. In a study using Flinders Sensitive Line (FSL) versus Flinders Resistant Line (FRL) rats, the intracerebral application of NPS resulted in reduced depression and anxiety-related behaviors in FSL animals, suggesting its anxiolytic and antidepressant role [143]. In humans, a polymorphism of the NPS receptor in the amygdala was identified in males with panic disorder [144]. The mechanism of the anxiolytic effect of NPS was examined in a study, which investigated the interactions between NPS and other mediators, namely noradrenaline, serotonin, glutamate, GABA, dopamine, and acetylcholine, using mouse frontal cortex synaptosomes labeled with radioactive neurotransmitters. They found out that NPS binds to neurons in the frontal cortex, reducing evoked serotonin and norepinephrine release [145]. A later review described the interplay between NPS and oxytocinergic systems [146]. More recent evidence supports the anxiolytic role of NPS. Tillmann et al. used an adeno-associated viral vector to induce the overexpression of NPS in rat amygdala. The NPS overexpression had a massive anxiolytic effect in rats. However, the study did not confirm the antidepressant properties of NPS [147].
11. Neuropeptide FF
Neuropeptide FF (NPFF), an 8-amino acid neuropeptide, belongs to RF-amide-related peptides—the RFRPs, which play a role in physiological processes, such as the modulation of morphine-induced analgesia, blood pressure elevation, and increased pancreatic somatostatin secretion [148][149]. NPFF utilizes two receptors, NPFFR1 and NPFFR2, which also represent new therapeutic targets [150]. NPFF and different RFRPs do have similar effects: they do share the NPFFR1; however, further effects in mammals are not well researched yet [151]. A connection with the HPA and HPG axis in relation to NPFF receptors has been studied. NPFFR2 has been found to activate the HPA axis and induce anxiety in rats and mice [152]. NPFF has a role in the reproductive system, the HPG axis, the autonomic nervous system, and also in pathways affecting stress response, food intake, and energy balance [153][154]. A recent study reported stronger negative feedback on the HPA axis (trend toward resistance) after a single prolonged stress event in NPFFR2 knockout mice [155]. The prevention of lipopolysaccharide-induced depressive-like responses in mice was achieved in NPFFR2 knockout mice [156].
References
- Kormos, V.; Gaszner, B. Role of Neuropeptides in Anxiety, Stress, and Depression: From Animals to Humans. Neuropeptides 2013, 47, 401–419.
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the Role of Neuropeptides in Depression and Anxiety. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 114, 110478.
- Hoyer, D.; Bartfai, T. Neuropeptides and Neuropeptide Receptors: Drug Targets, and Peptide and Non-Peptide Ligands: A Tribute to Prof. Dieter Seebach. Chem. Biodivers. 2012, 9, 2367–2387.
- OMIM—Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD, USA). 25 May 2022. Available online: https://omim.org/ (accessed on 3 June 2022).
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59.
- Miller, W.L. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm. Res. Paediatr. 2018, 89, 212–223.
- Daskalakis, N.P.; Meijer, O.C.; de Kloet, E.R. Mineralocorticoid Receptor and Glucocorticoid Receptor Work Alone and Together in Cell-Type-Specific Manner: Implications for Resilience Prediction and Targeted Therapy. Neurobiol. Stress 2022, 18, 100455.
- Belvederi Murri, M.; Pariante, C.; Mondelli, V.; Masotti, M.; Atti, A.R.; Mellacqua, Z.; Antonioli, M.; Ghio, L.; Menchetti, M.; Zanetidou, S.; et al. HPA axis and Aging in Depression: Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2014, 41, 46–62.
- Belvederi Murri, M.; Prestia, D.; Mondelli, V.; Pariante, C.; Patti, S.; Olivieri, B.; Arzani, C.; Masotti, M.; Respino, M.; Antonioli, M.; et al. The HPA axis in Bipolar Disorder: Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2016, 63, 327–342.
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical Depression and Non-Atypical Depression: Is HPA axis Function a Biomarker? A Systematic Review. J. Affect. Disord. 2018, 233, 45–67.
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995.
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain Behav. Immun. 2017, 66, 9–17.
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489.
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The Microbiota and the Hypothalamus-Pituitary-Adrenocortical (HPA) Axis, Implications for Anxiety and Stress Disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82.
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA axis and Anxiety. In Anxiety Disorders; Kim, Y.-K., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1191, pp. 141–153. ISBN 978-981-329-704-3.
- Mohapatra, S.S.; Mukherjee, J.; Banerjee, D.; Das, P.K.; Ghosh, P.R.; Das, K. RFamide Peptides, the Novel Regulators of Mammalian HPG Axis: A Review. Vet. World 2021, 14, 1867–1873.
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-Related Peptide-3 Stimulates GH Secretion, Inhibits LH Secretion, and Has Variable Effects on Sex Behavior in the Adult Male Rat. Horm. Behav. 2007, 51, 171–180.
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress Increases Putative Gonadotropin Inhibitory Hormone and Decreases Luteinizing Hormone in Male Rats. Proc. Natl. Acad. Sci. USA 2009, 106, 11324–11329.
- Ubuka, T.; Mizuno, T.; Fukuda, Y.; Bentley, G.E.; Wingfield, J.C.; Tsutsui, K. RNA Interference of Gonadotropin-Inhibitory Hormone Gene Induces Aggressive and Sexual Behaviors in Birds. Gen. Comp. Endocrinol. 2013, 181, 179–186.
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Irahara, M. Gonadotropin-Inhibitory Hormone Plays Roles in Stress-Induced Reproductive Dysfunction. Front. Endocrinol. 2017, 8, 62.
- Suszka-Świtek, A.; Pałasz, A.; Filipczyk, Ł.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The Gn RH Analogues Affect Novel Neuropeptide SMIM 20/Phoenixin and GPR 173 Receptor Expressions in the Female Rat Hypothalamic–Pituitary–Gonadal ( HPG ) Axis. Clin. Exp. Pharm. Physiol. 2019, 46, 350–359.
- Gebert, D.; Auer, M.K.; Stieg, M.R.; Freitag, M.T.; Lahne, M.; Fuss, J.; Schilbach, K.; Schopohl, J.; Stalla, G.K.; Kopczak, A. De-Masking Oxytocin-Deficiency in Craniopharyngioma and Assessing Its Link with Affective Function. Psychoneuroendocrinology 2018, 88, 61–69.
- Myers, A.J.; Williams, L.; Gatt, J.M.; McAuley-Clark, E.Z.; Dobson-Stone, C.; Schofield, P.R.; Nemeroff, C.B. Variation in the Oxytocin Receptor Gene Is Associated with Increased Risk for Anxiety, Stress and Depression in Individuals with a History of Exposure to Early Life Stress. J. Psychiatr. Res. 2014, 59, 93–100.
- Love, T.M. The Impact of Oxytocin on Stress: The Role of Sex. Curr. Opin. Behav. Sci. 2018, 23, 136–142.
- MacDonald, K.; Feifel, D. Oxytocin׳s Role in Anxiety: A Critical Appraisal. Brain Res. 2014, 1580, 22–56.
- Boccia, M.L.; Petrusz, P.; Suzuki, K.; Marson, L.; Pedersen, C.A. Immunohistochemical Localization of Oxytocin Receptors in Human Brain. Neuroscience 2013, 253, 155–164.
- Grace, S.A.; Rossell, S.L.; Heinrichs, M.; Kordsachia, C.; Labuschagne, I. Oxytocin and Brain Activity in Humans: A Systematic Review and Coordinate-Based Meta-Analysis of Functional MRI Studies. Psychoneuroendocrinology 2018, 96, 6–24.
- Engel, S.; Laufer, S.; Knaevelsrud, C.; Schumacher, S. The Endogenous Oxytocin System in Depressive Disorders: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2019, 101, 138–149.
- Costa, B.; Pini, S.; Baldwin, D.S.; Silove, D.; Manicavasagar, V.; Abelli, M.; Coppedè, F.; Martini, C. Oxytocin Receptor and G-Protein Polymorphisms in Patients with Depression and Separation Anxiety. J. Affect. Disord. 2017, 218, 365–373.
- Parris, M.S.; Grunebaum, M.F.; Galfalvy, H.C.; Andronikashvili, A.; Burke, A.K.; Yin, H.; Min, E.; Huang, Y.; Mann, J.J. Attempted Suicide and Oxytocin-Related Gene Polymorphisms. J. Affect. Disord. 2018, 238, 62–68.
- Acevedo-Rodriguez, A.; Mani, S.K.; Handa, R.J. Oxytocin and Estrogen Receptor β in the Brain: An Overview. Front. Endocrinol. 2015, 6, 160.
- Mottolese, R.; Redouté, J.; Costes, N.; Le Bars, D.; Sirigu, A. Switching Brain Serotonin with Oxytocin. Proc. Natl. Acad. Sci. USA 2014, 111, 8637–8642.
- Broadbear, J.H.; Kabel, D.; Tracy, L.; Mak, P. Oxytocinergic Regulation of Endogenous as Well as Drug-Induced Mood. Pharmacol. Biochem. Behav. 2014, 119, 61–71.
- Smith, A.S.; Tabbaa, M.; Lei, K.; Eastham, P.; Butler, M.J.; Linton, L.; Altshuler, R.; Liu, Y.; Wang, Z. Local Oxytocin Tempers Anxiety by Activating GABAA Receptors in the Hypothalamic Paraventricular Nucleus. Psychoneuroendocrinology 2016, 63, 50–58.
- Li, K.; Nakajima, M.; Ibañez-Tallon, I.; Heintz, N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.
- Scantamburlo, G.; Hansenne, M.; Fuchs, S.; Pitchot, W.; Maréchal, P.; Pequeux, C.; Ansseau, M.; Legros, J.J. Plasma Oxytocin Levels and Anxiety in Patients with Major Depression. Psychoneuroendocrinology 2007, 32, 407–410.
- Jobst, A.; Sabaß, L.; Hall, D.; Brücklmeier, B.; Buchheim, A.; Hall, J.; Sarubin, N.; Zill, P.; Falkai, P.; Brakemeier, E.-L.; et al. Oxytocin Plasma Levels Predict the Outcome of Psychotherapy: A Pilot Study in Chronic Depression. J. Affect. Disord. 2018, 227, 206–213.
- Lancaster, K.; Goldbeck, L.; Pournajafi-Nazarloo, H.; Connelly, J.J.; Carter, C.S.; Morris, J.P. The Role of Endogenous Oxytocin in Anxiolysis: Structural and Functional Correlates. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 618–625.
- Tabak, B.A.; Rosenfield, D.; Sunahara, C.S.; Alvi, T.; Szeto, A.; Mendez, A.J. Social Anxiety Is Associated with Greater Peripheral Oxytocin Reactivity to Psychosocial Stress. Psychoneuroendocrinology 2022, 140, 105712.
- Bethlehem, R.A.I.; Baron-Cohen, S.; van Honk, J.; Auyeung, B.; Bos, P.A. The Oxytocin Paradox. Front. Behav. Neurosci. 2014, 8, 48.
- Gadassi Polack, R.; Joormann, J.; Orbach, M.; Silverman, W.K.; Lebowitz, E.R. Maternal Depression and Mother-Child Oxytocin Synchrony in Youth with Anxiety Disorders. Res. Child Adolesc. Psychopathol. 2021, 49, 381–392.
- Young, W.S., 3rd; Gainer, H. Transgenesis and the Study of Expression, Cellular Targeting and Function of Oxytocin, Vasopressin and Their Receptors. Neuroendocrinology 2003, 78, 185–203.
- Caldwell, H.K.; Lee, H.-J.; Macbeth, A.H.; Young, W.S. Vasopressin: Behavioral Roles of an “Original” Neuropeptide. Prog. Neurobiol. 2008, 84, 1–24.
- Zheng, H.; Lim, J.Y.; Kim, Y.; Jung, S.T.; Hwang, S.W. The Role of Oxytocin, Vasopressin, and Their Receptors at Nociceptors in Peripheral Pain Modulation. Front. Neuroendocrinol. 2021, 63, 100942.
- Neumann, I.D.; Landgraf, R. Balance of Brain Oxytocin and Vasopressin: Implications for Anxiety, Depression, and Social Behaviors. Trends Neurosci. 2012, 35, 649–659.
- Hodgson, R.A.; Mullins, D.; Lu, S.X.; Guzzi, M.; Zhang, X.; Bleickardt, C.J.; Scott, J.D.; Miller, M.W.; Stamford, A.W.; Parker, E.M.; et al. Characterization of a Novel Vasopressin V1b Receptor Antagonist, V1B-30N, in Animal Models of Anxiety-like and Depression-like Behavior. Eur. J. Pharmacol. 2014, 730, 157–163.
- Ranieri, M.; Di Mise, A.; Tamma, G.; Valenti, G. Vasopressin Type 2 Receptor Agonists and Antagonists. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; pp. 656–669. ISBN 978-0-12-801238-3.
- Ishizuka, Y.; Abe, H.; Tanoue, A.; Kannan, H.; Ishida, Y. Involvement of Vasopressin V1b Receptor in Anti-Anxiety Action of SSRI and SNRI in Mice. Neurosci. Res. 2010, 66, 233–237.
- Purba, J.S. Increased Number of Vasopressin- and Oxytocin-Expressing Neurons in the Paraventricular Nucleus of the Hypothalamus in Depression. Arch. Gen. Psychiatry 1996, 53, 137.
- Vollebregt, O.; Koyama, E.; Zai, C.C.; Shaikh, S.A.; Lisoway, A.J.; Kennedy, J.L.; Beitchman, J.H. Evidence for Association of Vasopressin Receptor 1A Promoter Region Repeat with Childhood Onset Aggression. J. Psychiatr. Res. 2021, 140, 522–528.
- Müller, M.B.; Landgraf, R.; Keck, M.E. Vasopressin, Major Depression, and Hypothalamic–Pituitary–Adrenocortical Desensitization. Biol. Psychiatry 2000, 48, 330–333.
- Mlynarik, M.; Zelena, D.; Bagdy, G.; Makara, G.B.; Jezova, D. Signs of Attenuated Depression-like Behavior in Vasopressin Deficient Brattleboro Rats. Horm. Behav. 2007, 51, 395–405.
- Goekoop, J.G.; de Winter, R.P.F.; de Rijk, R.; Zwinderman, K.H.; Frankhuijzen-Sierevogel, A.; Wiegant, V.M. Depression with Above-Normal Plasma Vasopressin: Validation by Relations with Family History of Depression and Mixed Anxiety and Retardation. Psychiatry Res. 2006, 141, 201–211.
- Louis, C.; Cohen, C.; Depoortère, R.; Griebel, G. Antidepressant-like Effects of the Corticotropin-Releasing Factor 1 Receptor Antagonist, SSR125543, and the Vasopressin 1b Receptor Antagonist, SSR149415, in a DRL-72 s Schedule in the Rat. Neuropsychopharmacol 2006, 31, 2180–2187.
- Hodgson, R.A.; Higgins, G.A.; Guthrie, D.H.; Lu, S.X.; Pond, A.J.; Mullins, D.E.; Guzzi, M.F.; Parker, E.M.; Varty, G.B. Comparison of the V1b Antagonist, SSR149415, and the CRF1 Antagonist, CP-154,526, in Rodent Models of Anxiety and Depression. Pharmacol. Biochem. Behav. 2007, 86, 431–440.
- Rocha, A.; Godino-Gimeno, A.; Cerdá-Reverter, J.M. Evolution of Proopiomelanocortin. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2019; Volume 111, pp. 1–16. ISBN 978-0-12-818858-3.
- Fosgerau, K.; Raun, K.; Nilsson, C.; Dahl, K.; Wulff, B.S. Novel α-MSH Analog Causes Weight Loss in Obese Rats and Minipigs and Improves Insulin Sensitivity. J. Endocrinol. 2014, 220, 97–107.
- Copperi, F.; Kim, J.D.; Diano, S. Melanocortin Signaling Connecting Systemic Metabolism With Mood Disorders. Biol. Psychiatry 2022, 91, 879–887.
- Mountjoy, K.G. Distribution and Function of Melanocortin Receptors within the Brain. In Melanocortins: Multiple Actions and Therapeutic Potential; Catania, A., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 681, pp. 29–48. ISBN 978-1-4419-6353-6.
- Nyamugenda, E.; Griffin, H.; Russell, S.; Cooney, K.A.; Kowalczyk, N.S.; Islam, I.; Phelan, K.D.; Baldini, G. Selective Survival of Sim1/MC4R Neurons in Diet-Induced Obesity. iScience 2020, 23, 101114.
- Goyal, S.; Kokare, D.; Chopde, C.; Subhedar, N. Alpha-Melanocyte Stimulating Hormone Antagonizes Antidepressant-like Effect of Neuropeptide Y in Porsolt’s Test in Rats. Pharmacol. Biochem. Behav. 2006, 85, 369–377.
- Kokare, D.M.; Dandekar, M.P.; Chopde, C.T.; Subhedar, N. Interaction between Neuropeptide Y and Alpha-Melanocyte Stimulating Hormone in Amygdala Regulates Anxiety in Rats. Brain Res. 2005, 1043, 107–114.
- Bruschetta, G.; Jin, S.; Liu, Z.-W.; Kim, J.D.; Diano, S. MC4R Signaling in Dorsal Raphe Nucleus Controls Feeding, Anxiety, and Depression. Cell Rep. 2020, 33, 108267.
- Kokare, D.M.; Dandekar, M.P.; Singru, P.S.; Gupta, G.L.; Subhedar, N.K. Involvement of α-MSH in the Social Isolation Induced Anxiety- and Depression-like Behaviors in Rat. Neuropharmacology 2010, 58, 1009–1018.
- Fu, L.-Y.; van den Pol, A.N. Agouti-Related Peptide and MC3/4 Receptor Agonists Both Inhibit Excitatory Hypothalamic Ventromedial Nucleus Neurons. J. Neurosci. 2008, 28, 5433–5449.
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal Control of Obesity and Anxiety–Depressive Disorder via a GABA and Serotonin Neural Circuit. Mol. Psychiatry 2021, 26, 2837–2853.
- Chaki, S.; Okubo, T. Melanocortin-4 Receptor Antagonists for the Treatment of Depression and Anxiety Disorders. Curr. Top. Med. Chem. 2007, 7, 1145–1151.
- Sternson, S.M.; Atasoy, D. Agouti-Related Protein Neuron Circuits That Regulate Appetite. Neuroendocrinology 2014, 100, 95–102.
- Dermitzaki, E.; Venihaki, M.; Tsatsanis, C.; Gravanis, A.; Avgoustinaki, P.D.; Liapakis, G.; Margioris, A.N. The Multi-Faceted Profile of Corticotropin-Releasing Factor (CRF) Family of Neuropeptides and of Their Receptors on the Paracrine/Local Regulation of the Inflammatory Response. Curr. Mol. Pharmacol. 2018, 11, 39–50.
- Vuppaladhadiam, L.; Ehsan, C.; Akkati, M.; Bhargava, A. Corticotropin-Releasing Factor Family: A Stress Hormone-Receptor System’s Emerging Role in Mediating Sex-Specific Signaling. Cells 2020, 9, 839.
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345.
- Fischer, T.W.; Bergmann, A.; Kruse, N.; Kleszczynski, K.; Skobowiat, C.; Slominski, A.T.; Paus, R. New Effects of Caffeine on Corticotropin-releasing Hormone (CRH)-induced Stress along the Intrafollicular Classical Hypothalamic–Pituitary–Adrenal (HPA) Axis (CRH-R1/2, IP3 -R, ACTH, MC-R2) and the Neurogenic Non-HPA axis (Substance P, P75 NTR and TrkA) in Ex Vivo Human Male Androgenetic Scalp Hair Follicles. Br. J. Dermatol. 2021, 184, 96–110.
- Hu, P.; Liu, J.; Maita, I.; Kwok, C.; Gu, E.; Gergues, M.M.; Kelada, F.; Phan, M.; Zhou, J.-N.; Swaab, D.F.; et al. Chronic Stress Induces Maladaptive Behaviors by Activating Corticotropin-Releasing Hormone Signaling in the Mouse Oval Bed Nucleus of the Stria Terminalis. J. Neurosci. 2020, 40, 2519–2537.
- Sze, Y.; Brunton, P.J. Sex, Stress and Steroids. Eur. J. Neurosci. 2020, 52, 2487–2515.
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298.
- Forster, G.L.; Pringle, R.B.; Mouw, N.J.; Vuong, S.M.; Watt, M.J.; Burke, A.R.; Lowry, C.A.; Summers, C.H.; Renner, K.J. Corticotropin-Releasing Factor in the Dorsal Raphe Nucleus Increases Medial Prefrontal Cortical Serotonin via Type 2 Receptors and Median Raphe Nucleus Activity. Eur. J. Neurosci. 2008, 28, 299–310.
- Lukkes, J.L.; Forster, G.L.; Renner, K.J.; Summers, C.H. Corticotropin-Releasing Factor 1 and 2 Receptors in the Dorsal Raphé Differentially Affect Serotonin Release in the Nucleus Accumbens. Eur. J. Pharmacol. 2008, 578, 185–193.
- Keen-Rhinehart, E.; Michopoulos, V.; Toufexis, D.J.; Martin, E.I.; Nair, H.; Ressler, K.J.; Davis, M.; Owens, M.J.; Nemeroff, C.B.; Wilson, M.E. Continuous Expression of Corticotropin-Releasing Factor in the Central Nucleus of the Amygdala Emulates the Dysregulation of the Stress and Reproductive Axes. Mol. Psychiatry 2009, 14, 37–50.
- Liu, Z.; Zhu, F.; Wang, G.; Xiao, Z.; Wang, H.; Tang, J.; Wang, X.; Qiu, D.; Liu, W.; Cao, Z.; et al. Association of Corticotropin-Releasing Hormone Receptor1 Gene SNP and Haplotype with Major Depression. Neurosci. Lett. 2006, 404, 358–362.
- Keck, M.E.; Kern, N.; Erhardt, A.; Unschuld, P.G.; Ising, M.; Salyakina, D.; Müller, M.B.; Knorr, C.C.; Lieb, R.; Hohoff, C.; et al. Combined Effects of Exonic Polymorphisms in CRHR1 and AVPR1B Genes in a Case/Control Study for Panic Disorder. Am. J. Med. Genet. 2008, 147B, 1196–1204.
- Rosinger, Z.J.; Jacobskind, J.S.; Bulanchuk, N.; Malone, M.; Fico, D.; Justice, N.J.; Zuloaga, D.G. Characterization and Gonadal Hormone Regulation of a Sexually Dimorphic Corticotropin-Releasing Factor Receptor 1 Cell Group. J. Comp. Neurol. 2019, 527, 1056–1069.
- Lu, X.; Ross, B.; Sanchez-Alavez, M.; Zorrilla, E.P.; Bartfai, T. Phenotypic Analysis of GalR2 Knockout Mice in Anxiety- and Depression-Related Behavioral Tests. Neuropeptides 2008, 42, 387–397.
- Vaughan, J.; Donaldson, C.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a Mammalian Neuropeptide Related to Fish Urotensin I and to Corticotropin-Releasing Factor. Nature 1995, 378, 287–292.
- Pan, W.; Kastin, A. Urocortin and the Brain. Prog. Neurobiol. 2008, 84, 148–156.
- Gehlert, D.R.; Shekhar, A.; Morin, S.M.; Hipskind, P.A.; Zink, C.; Gackenheimer, S.L.; Shaw, J.; Fitz, S.D.; Sajdyk, T.J. Stress and Central Urocortin Increase Anxiety-like Behavior in the Social Interaction Test via the CRF1 Receptor. Eur. J. Pharmacol. 2005, 509, 145–153.
- Spiga, F.; Lightman, S.L.; Shekhar, A.; Lowry, C.A. Injections of Urocortin 1 into the Basolateral Amygdala Induce Anxiety-like Behavior and c-Fos Expression in Brainstem Serotonergic Neurons. Neuroscience 2006, 138, 1265–1276.
- de Groote, L.; Penalva, R.G.; Flachskamm, C.; Reul, J.M.H.M.; Linthorst, A.C.E. Differential Monoaminergic, Neuroendocrine and Behavioural Responses after Central Administration of Corticotropin-Releasing Factor Receptor Type 1 and Type 2 Agonists. J. Neurochem. 2005, 94, 45–56.
- Tanaka, M.; Telegdy, G. Antidepressant-like Effects of the CRF Family Peptides, Urocortin 1, Urocortin 2 and Urocortin 3 in a Modified Forced Swimming Test in Mice. Brain Res. Bull. 2008, 75, 509–512.
- Woelfle, R.; D’Aquila, A.L.; Pavlović, T.; Husić, M.; Lovejoy, D.A. Ancient Interaction between the Teneurin C-Terminal Associated Peptides (TCAP) and Latrophilin Ligand-Receptor Coupling: A Role in Behavior. Front. Neurosci. 2015, 9, 146.
- Holsboer, F.; Ising, M. Central CRH System in Depression and Anxiety—Evidence from Clinical Studies with CRH1 Receptor Antagonists. Eur. J. Pharmacol. 2008, 583, 350–357.
- Rotzinger, S.; Lovejoy, D.A.; Tan, L.A. Behavioral Effects of Neuropeptides in Rodent Models of Depression and Anxiety. Peptides 2010, 31, 736–756.
- Overstreet, D.H.; Knapp, D.J.; Breese, G.R. Can CRF1 Receptor Antagonists Become Antidepressant and/or Anxiolytic Agents? Drug Dev. Res. 2005, 65, 191–204.
- Weninger, S.C.; Peters, L.L.; Majzoub, J.A. Urocortin Expression in the Edinger-Westphal Nucleus Is Up-Regulated by Stress and Corticotropin-Releasing Hormone Deficiency1. Endocrinology 2000, 141, 256–263.
- Kozicz, T.; Korosi, A.; Korsman, C.; Tilburg-Ouwens, D.; Groenink, L.; Veening, J.; van Der Gugten, J.; Roubos, E.; Olivier, B. Urocortin Expression in the Edinger-Westphal Nucleus Is down-Regulated in Transgenic Mice over-Expressing Neuronal Corticotropin-Releasing Factor. Neuroscience 2004, 123, 589–594.
- Gargiulo, A.T.; Curtis, G.R.; Barson, J.R. Pleiotropic Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP): Novel Insights into the Role of PACAP in Eating and Drug Intake. Brain Res. 2020, 1729, 146626.
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharm. Rev. 2009, 61, 283–357.
- Stojakovic, A.; Ahmad, S.M.; Malhotra, S.; Afzal, Z.; Ahmed, M.; Lutfy, K. The Role of Pituitary Adenylyl Cyclase-Activating Polypeptide in the Motivational Effects of Addictive Drugs. Neuropharmacology 2020, 171, 108109.
- Liao, C.; de Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417.
- Hashimoto, R.; Hashimoto, H.; Shintani, N.; Ohi, K.; Hori, H.; Saitoh, O.; Kosuga, A.; Tatsumi, M.; Iwata, N.; Ozaki, N.; et al. Possible Association between the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Gene and Major Depressive Disorder. Neurosci. Lett. 2010, 468, 300–302.
- Hashimoto, H.; Shintani, N.; Tanaka, K.; Mori, W.; Hirose, M.; Matsuda, T.; Sakaue, M.; Miyazaki, J.; Niwa, H.; Tashiro, F.; et al. Altered Psychomotor Behaviors in Mice Lacking Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP). Proc. Natl. Acad. Sci. USA 2001, 98, 13355–13360.
- Hannibal, J. Pituitary Adenylate Cyclase-Activating Peptide in the Rat Central Nervous System: An Immunohistochemical and in Situ Hybridization Study. J. Comp. Neurol. 2002, 453, 389–417.
- Dore, R.; Iemolo, A.; Smith, K.L.; Wang, X.; Cottone, P.; Sabino, V. CRF Mediates the Anxiogenic and Anti-Rewarding, But Not the Anorectic Effects of PACAP. Neuropsychopharmacol 2013, 38, 2160–2169.
- Lutfy, K.; Shankar, G. Emerging Evidence for the Role of Pituitary Adenylate Cyclase-Activating Peptide in Neuropsychiatric Disorders. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 167, pp. 143–157. ISBN 978-0-12-818855-2.
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-Traumatic Stress Disorder Is Associated with PACAP and the PAC1 Receptor. Nature 2011, 470, 492–497.
- Almli, L.M.; Mercer, K.B.; Kerley, K.; Feng, H.; Bradley, B.; Conneely, K.N.; Ressler, K.J. ADCYAP1R1 Genotype Associates with Post-traumatic Stress Symptoms in Highly Traumatized African-American Females. Am. J. Med. Genet. 2013, 162, 262–272.
- Farkas, J.; Kovács, L.Á.; Gáspár, L.; Nafz, A.; Gaszner, T.; Ujvári, B.; Kormos, V.; Csernus, V.; Hashimoto, H.; Reglődi, D.; et al. Construct and Face Validity of a New Model for the Three-Hit Theory of Depression Using PACAP Mutant Mice on CD1 Background. Neuroscience 2017, 354, 11–29.
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The Behavioral Phenotype of Pituitary Adenylate-Cyclase Activating Polypeptide-Deficient Mice in Anxiety and Depression Tests Is Accompanied by Blunted c-Fos Expression in the Bed Nucleus of the Stria Terminalis, Central Projecting Edinger–Westphal Nucleus, Ventral Lateral Septum, and Dorsal Raphe Nucleus. Neuroscience 2012, 202, 283–299.
- Iemolo, A.; Seiglie, M.; Blasio, A.; Cottone, P.; Sabino, V. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in the Central Nucleus of the Amygdala Induces Anxiety via Melanocortin Receptors. Psychopharmacology 2016, 233, 3269–3277.
- Agarwal, A.; Halvorson, L.M.; Legradi, G. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Mimics Neuroendocrine and Behavioral Manifestations of Stress: Evidence for PKA-Mediated Expression of the Corticotropin-Releasing Hormone (CRH) Gene. Mol. Brain Res. 2005, 138, 45–57.
- Hammack, S.E.; Roman, C.W.; Lezak, K.R.; Kocho-Shellenberg, M.; Grimmig, B.; Falls, W.A.; Braas, K.; May, V. Roles for Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Expression and Signaling in the Bed Nucleus of the Stria Terminalis (BNST) in Mediating the Behavioral Consequences of Chronic Stress. J. Mol. Neurosci. 2010, 42, 327–340.
- Boucher, M.N.; Aktar, M.; Braas, K.M.; May, V.; Hammack, S.E. Activation of Lateral Parabrachial Nucleus (LPBn) PACAP-Expressing Projection Neurons to the Bed Nucleus of the Stria Terminalis (BNST) Enhances Anxiety-like Behavior. J. Mol. Neurosci. 2022, 72, 451–458.
- Chung, S.; Parks, G.S.; Lee, C.; Civelli, O. Recent Updates on the Melanin-Concentrating Hormone (MCH) and Its Receptor System: Lessons from MCH1R Antagonists. J. Mol. Neurosci. 2011, 43, 115–121.
- Bencze, J.; Pocsai, K.; Murnyák, B.; Gergely, P.A.; Juhász, B.; Szilvássy, Z.; Hortobágyi, T. The Melanin-Concentrating Hormone System in Human, Rodent and Avian Brain. Open Med. 2018, 13, 264–269.
- Alldredge, B. Pathogenic Involvement of Neuropeptides in Anxiety and Depression. Neuropeptides 2010, 44, 215–224.
- Al-Massadi, O.; Dieguez, C.; Schneeberger, M.; López, M.; Schwaninger, M.; Prevot, V.; Nogueiras, R. Multifaceted Actions of Melanin-Concentrating Hormone on Mammalian Energy Homeostasis. Nat. Rev. Endocrinol. 2021, 17, 745–755.
- Millan, M.J.; Gobert, A.; Panayi, F.; Rivet, J.-M.; Dekeyne, A.; Brocco, M.; Ortuno, J.-C.; Di Cara, B. The Melanin-Concentrating Hormone1 Receptor Antagonists, SNAP-7941 and GW3430, Enhance Social Recognition and Dialysate Levels of Acetylcholine in the Frontal Cortex of Rats. Int. J. Neuropsychopharm. 2008, 11, 1105.
- Smith, D.G.; Hegde, L.G.; Wolinsky, T.D.; Miller, S.; Papp, M.; Ping, X.; Edwards, T.; Gerald, C.P.; Craig, D.A. The Effects of Stressful Stimuli and Hypothalamic–Pituitary–Adrenal Axis Activation Are Reversed by the Melanin-Concentrating Hormone 1 Receptor Antagonist SNAP 94847 in Rodents. Behav. Brain Res. 2009, 197, 284–291.
- Presse, F.; Conductier, G.; Rovere, C.; Nahon, J.-L. The Melanin-Concentrating Hormone Receptors: Neuronal and Non-Neuronal Functions. Int. J. Obes. Suppl. 2014, 4, S31–S36.
- Shimazaki, T.; Yoshimizu, T.; Chaki, S. Melanin-Concentrating Hormone MCH1 Receptor Antagonists: A Potential New Approach to the Treatment of Depression and Anxiety Disorders. CNS Drugs 2006, 20, 801–811.
- García-Fuster, M.J.; Parks, G.S.; Clinton, S.M.; Watson, S.J.; Akil, H.; Civelli, O. The Melanin-Concentrating Hormone (MCH) System in an Animal Model of Depression-like Behavior. Eur. Neuropsychopharmacol. 2012, 22, 607–613.
- Georgescu, D. The Hypothalamic Neuropeptide Melanin-Concentrating Hormone Acts in the Nucleus Accumbens to Modulate Feeding Behavior and Forced-Swim Performance. J. Neurosci. 2005, 25, 2933–2940.
- Chaki, S.; Yamaguchi, J.; Yamada, H.; Thomsen, W.; Tran, T.-A.; Semple, G.; Sekiguchi, Y. ATC0175: An Orally Active Melanin-Concentrating Hormone Receptor 1 Antagonist for the Potential Treatment of Depression and Anxiety. CNS Drug Rev. 2006, 11, 341–352.
- Marsteller, D.A.; Gerald, C.P.G.; Kong, R.; Cajina, M.; Craig, D.A.; Swanson, C.J. The MCH1 Receptor Antagonist SNAP 94847 Induces Sensitivity to Dopamine D2/D3 Receptor Agonists in Rats and Mice. Eur. J. Pharmacol. 2009, 602, 66–72.
- Oh, J.-Y.; Liu, Q.F.; Hua, C.; Jeong, H.J.; Jang, J.-H.; Jeon, S.; Park, H.-J. Intranasal Administration of Melanin-Concentrating Hormone Reduces Stress-Induced Anxiety- and Depressive-like Behaviors in Rodents. Exp. Neurobiol. 2020, 29, 453–469.
- Hegadoren, K.M.; O’Donnell, T.; Lanius, R.; Coupland, N.J.; Lacaze-Masmonteil, N. The Role of β-Endorphin in the Pathophysiology of Major Depression. Neuropeptides 2009, 43, 341–353.
- Kubryak, O.; Emelyanova, I. 269—Increase of Beta-Endorphin Plasma Level as a Potential Marker of Positive Response to Treatment of Depression. Eur. Psychiatry 2013, 28, 1.
- Colasanti, A.; Rabiner, E.; Lingford-Hughes, A.; Nutt, D. Opioids and Anxiety. J. Psychopharmacol. 2011, 25, 1415–1433.
- Savic, D.; Knezevic, G.; Matic, G.; Damjanovic, S.; Spiric, Z. Posttraumatic and Depressive Symptoms in β-Endorphin Dynamics. J. Affect. Disord. 2015, 181, 61–66.
- Kunel’skaya, N.L.; Guseva, A.L.; Chistov, S.D. The Level of β-Endorphin, Chronic Stress, and Depression Associated with Vestibular Pathology. Vestn. Otorinolaringol. 2015, 80, 12.
- Dutia, R.; Meece, K.; Dighe, S.; Kim, A.J.; Wardlaw, S.L. β-Endorphin Antagonizes the Effects of α-MSH on Food Intake and Body Weight. Endocrinology 2012, 153, 4246–4255.
- Li, Y.; Du, J.-L.; Hao, P.-L.; Zhang, M.-X.; Jiang, Y.-B.; Shu, M.; Guan, L. Effect of auricular point sticking therapy on perioperative pain in patients with partial lung resection. Zhongguo Zhen Jiu 2021, 41, 603–607.
- Lillywhite, A.; Woodhams, S.G.; Gonçalves, S.V.; Watson, D.J.G.; Li, L.; Burston, J.J.; Gowler, P.R.W.; Canals, M.; Walsh, D.A.; Hathway, G.J.; et al. Anxiety Enhances Pain in a Model of Osteoarthritis and Is Associated with Altered Endogenous Opioid Function and Reduced Opioid Analgesia. Pain Rep. 2021, 6, e956.
- Gazendam, A.; Ekhtiari, S.; Bozzo, A.; Phillips, M.; Bhandari, M. Intra-Articular Saline Injection Is as Effective as Corticosteroids, Platelet-Rich Plasma and Hyaluronic Acid for Hip Osteoarthritis Pain: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2021, 55, 256–261.
- Redrobe, J.P.; Dumont, Y.; Quirion, R. Neuropeptide Y (NPY) and Depression: From Animal Studies to the Human Condition. Life Sci. 2002, 71, 2921–2937.
- Widerlöv, E.; Lindström, L.H.; Wahlestedt, C.; Ekman, R. Neuropeptide Y and Peptide YY as Possible Cerebrospinal Fluid Markers for Major Depression and Schizophrenia, Respectively. J. Psychiatr. Res. 1988, 22, 69–79.
- Morales-Medina, J.C.; Dumont, Y.; Quirion, R. A Possible Role of Neuropeptide Y in Depression and Stress. Brain Res. 2010, 1314, 194–205.
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, Peptide YY and Pancreatic Polypeptide in the Gut–Brain Axis. Neuropeptides 2012, 46, 261–274.
- Cheng, Y.; Tang, X.-Y.; Li, Y.-X.; Zhao, D.-D.; Cao, Q.-H.; Wu, H.-X.; Yang, H.-B.; Hao, K.; Yang, Y. Depression-Induced Neuropeptide Y Secretion Promotes Prostate Cancer Growth by Recruiting Myeloid Cells. Clin. Cancer Res 2019, 25, 2621–2632.
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Fröhlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-Fat Diet Induces Depression-like Behaviour in Mice Associated with Changes in Microbiome, Neuropeptide Y, and Brain Metabolome. Nutr. Neurosci. 2019, 22, 877–893.
- Domin, H. Neuropeptide Y Y2 and Y5 Receptors as Potential Targets for Neuroprotective and Antidepressant Therapies: Evidence from Preclinical Studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110349.
- Carboni, L.; El Khoury, A.; Beiderbeck, D.I.; Neumann, I.D.; Mathé, A.A. Neuropeptide Y, Calcitonin Gene-Related Peptide, and Neurokinin A in Brain Regions of HAB Rats Correlate with Anxiety-like Behaviours. Eur. Neuropsychopharmacol. 2022, 57, 1–14.
- Sharma, A.; Ren, X.; Zhang, H.; Pandey, G.N. Effect of Depression and Suicidal Behavior on Neuropeptide Y (NPY) and Its Receptors in the Adult Human Brain: A Postmortem Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 112, 110428.
- Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S Alters Anxiety, but Not Depression-like Behaviour in Flinders Sensitive Line Rats: A Genetic Animal Model of Depression. Int. J. Neuropsychopharm. 2012, 15, 375–387.
- Okamura, N.; Hashimoto, K.; Iyo, M.; Shimizu, E.; Dempfle, A.; Friedel, S.; Reinscheid, R.K. Gender-Specific Association of a Functional Coding Polymorphism in the Neuropeptide S Receptor Gene with Panic Disorder but Not with Schizophrenia or Attention-Deficit/Hyperactivity Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1444–1448.
- Raiteri, L.; Luccini, E.; Romei, C.; Salvadori, S.; Calò, G. Neuropeptide S Selectively Inhibits the Release of 5-HT and Noradrenaline from Mouse Frontal Cortex Nerve Endings: Neuropeptide S and Neurotransmitter Release. Br. J. Pharmacol. 2009, 157, 474–481.
- Gupta, P.R.; Prabhavalkar, K. Combination Therapy with Neuropeptides for the Treatment of Anxiety Disorder. Neuropeptides 2021, 86, 102127.
- Tillmann, S.; Skibdal, H.E.; Christiansen, S.H.; Gøtzsche, C.R.; Hassan, M.; Mathé, A.A.; Wegener, G.; Woldbye, D.P.D. Sustained Overexpression of Neuropeptide S in the Amygdala Reduces Anxiety-like Behavior in Rats. Behav. Brain Res. 2019, 367, 28–34.
- Gouardères, C.; Sutak, M.; Zajac, J.-M.; Jhamandas, K. Antinociceptive Effects of Intrathecally Administered F8Famide and FMRFamide in the Rat. Eur. J. Pharmacol. 1993, 237, 73–81.
- Fehmann, H.C.; McGregor, G.; Weber, V.; Eissele, R.; Göke, R.; Göke, B.; Arnold, R. The Effects of Two FMRFamide Related Peptides (A-18-F-Amide and F-8-F-Amide; ‘Morphine Modulating Peptides’) on the Endocrine and Exocrine Rat Pancreas. Neuropeptides 1990, 17, 87–92.
- Nguyen, T.; Marusich, J.; Li, J.-X.; Zhang, Y. Neuropeptide FF and Its Receptors: Therapeutic Applications and Ligand Development. J. Med. Chem. 2020, 63, 12387–12402.
- Kim, J.S. What’s in a Name? Roles of RFamide-Related Peptides Beyond Gonadotrophin Inhibition. J Neuroendocr. 2016, 28.
- Lin, Y.-T.; Yu, Y.-L.; Hong, W.-C.; Yeh, T.-S.; Chen, T.-C.; Chen, J.-C. NPFFR2 Activates the HPA axis and Induces Anxiogenic Effects in Rodents. Int. J. Mol. Sci. 2017, 18, 1810.
- Kovács, A.; László, K.; Gálosi, R.; Tóth, K.; Ollmann, T.; Péczely, L.; Lénárd, L. Microinjection of RFRP-1 in the Central Nucleus of Amygdala Decreases Food Intake in the Rat. Brain Res. Bull. 2012, 88, 589–595.
- Lin, Y.-T.; Chen, J.-C. Neuropeptide FF Modulates Neuroendocrine and Energy Homeostasis through Hypothalamic Signaling. Chin. J. Physiol. 2019, 62, 47.
- Lin, Y.-T.; Huang, Y.-L.; Tsai, S.-C.; Chen, J.-C. Ablation of NPFFR2 in Mice Reduces Response to Single Prolonged Stress Model. Cells 2020, 9, 2479.
- Yu, Z.; Lin, Y.-T.; Chen, J.-C. Knockout of NPFFR2 Prevents LPS-Induced Depressive-like Responses in Mice. Int. J. Mol. Sci. 2021, 22, 7611.
More
Information
Subjects:
Neurosciences; Psychology, Applied; Behavioral Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
13 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




