Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jingmei Li | -- | 2954 | 2022-09-08 05:42:35 | | | |
| 2 | Vivi Li | -33 word(s) | 2921 | 2022-09-09 05:00:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lim, Y.X.; Lim, Z.L.; Ho, P.J.; Li, J. Breast Cancer in Asia. Encyclopedia. Available online: https://encyclopedia.pub/entry/27005 (accessed on 07 February 2026).
Lim YX, Lim ZL, Ho PJ, Li J. Breast Cancer in Asia. Encyclopedia. Available at: https://encyclopedia.pub/entry/27005. Accessed February 07, 2026.
Lim, Yu Xian, Zi Lin Lim, Peh Joo Ho, Jingmei Li. "Breast Cancer in Asia" Encyclopedia, https://encyclopedia.pub/entry/27005 (accessed February 07, 2026).
Lim, Y.X., Lim, Z.L., Ho, P.J., & Li, J. (2022, September 08). Breast Cancer in Asia. In Encyclopedia. https://encyclopedia.pub/entry/27005
Lim, Yu Xian, et al. "Breast Cancer in Asia." Encyclopedia. Web. 08 September, 2022.
Copy Citation
Nearly all breast cancer patients survive for more than five years when the tumor is found early and in the localized stage. Regular clinical breast examinations, mammograms, and monthly self-exams of the breasts all contribute to early detection. However, late-stage breast cancers are common in many Asian countries. Low-income countries suffer from a lack of resources for breast cancer screening. Close to half (45.4%) of the 2.3 million breast cancers (BC) diagnosed in 2020 were from Asia.
Asian breast cancers
mammography screening
risk-based screening
mammography screening recommendations
risk stratification
1. Introduction
1.1. Breast Cancer Is a Significant Public Health Problem in Asia
In 2020, 2.3 million new breast cancer cases were diagnosed worldwide, overtaking lung cancer as the most common cancer [1]. Breast cancer accounts for 24.5% of all female cancers [1]. Close to half of the breast cancer patients (45.4%) were diagnosed in Asia [1]. Hubert H. Humphrey, an American politician and pharmacist who served as the United States’ 38th vice president, once commented that, “Asia is rich in people, rich in culture, and rich in resources. It is also rich in trouble”. When it comes to the public health problem of breast cancer, he may not be wrong.
1.2. Debate on Whether Breast Cancer Is a Different Disease in Asia Due to Earlier Onset of Age
Breast cancer strikes Asian women earlier than it does Western women [2][3]. In Asian countries, the peak age is between 40 and 50 years, while in Western countries, it is between 60 and 70 years [2][3]. This observation has sparked a debate on whether breast cancer is the same disease in Asian and Western countries [2].
It should be noted that confounding by calendar-period and/or birth cohort effects may be an issue in cross-sectional analyses [4]. The younger mean age at diagnosis may be due to the younger population [5]. Using an age-period-cohort approach, Mousavi-Jarrrahi et al. examined the data from 29 European cancer registries and nine Asian registries for the period between 1953 and 2002 [6]. Their results showed that a strong cohort effect was the main reason for the observed difference in age of onset of breast cancer [6]. Interestingly, Sung et al. used similar age-period-cohort models to analyze cancer registry data from China, Hong Kong, South Korea, Taiwan, Singapore, and the United States, and concluded that the extrapolated estimates of onset ages for the most recent cohorts in certain Asian countries were actually later than in the United States [4]. Indeed, the age at breast cancer presentation has risen over time in Asia, likely because of the later generations being exposed to more risk factors, the introduction of breast cancer screening in women over 50 years, and a longer lifespan [7]. Ultimately, breast cancer is likely the same disease, regardless of geographical location.
2. Trends of Breast Cancer in Asia
The risk of developing breast cancer increases with age [8]. The age-standardized incidence rate (ASIR) of breast cancer refers to the rate at which new breast cancers are diagnosed over a specified period, accounting for the population age structure. The breast cancer ASIR in 2020, expressed per 100,000 females, is lowest in Asia (36.8), compared to Africa (40.7), Latin America and the Caribbean (51.9), Europe (74.3), Oceania (87.8), and Northern America (89.4) [9].
The age-standardized mortality rate (ASMR) of breast cancer is measured as the number of deaths resulting from the disease over a specified period, accounting for the population age structure. The ASMR in 2020, expressed per 100,000 females, for Asia (11.6) is also the lowest in the world, compared to Oceania (13.2), Latin America and the Caribbean (13.5), Europe (14.8), Northern America (16.9), and Africa (19.4) [9].
2.1. Inequities in Breast Cancer Outcomes
The mortality-to-incidence ratio (M/I), defined as the number of deaths that occurs compared to the number of breast cancers diagnosed each year, is generally used as a high-level comparative measure to identify inequities in cancer outcomes. Although Asia has the lowest ASMR and ASIR, the M/I in Asia (0.32) is higher than the world’s average (0.28), and the second-highest in the world by region [9]. In contrast, M/I in Oceania (0.15), Northern America (0.19), Europe (0.20), and Latin America and the Caribbean (0.26) are lower, despite higher ASIRs—a smaller proportion of women die from the disease in these areas [9].
Within Asia, there is a large variation in M/I [9][10][11] (Figure 1). In the East Asia and Pacific, Europe and Central Asia, and South Asia regions, high-income countries generally have higher breast cancer incidence and lower mortality rates (Figure 1). Examples include Singapore, Japan, South Korea, Brunei, and Israel. The corresponding M/I in the East Asia and Pacific region (0.26) is the lowest; it is also the only sub-region with a M/I lower than the world’s average (0.28) [9]. In contrast, M/I is the highest in South Asia at 0.52 [9]. This indicates that the burden of the disease is twice as high in South Asia, compared to the East Asia and Pacific sub-region. M/I in Europe and Central Asia and the Middle East and North Africa are similar, at around 0.34 [9].
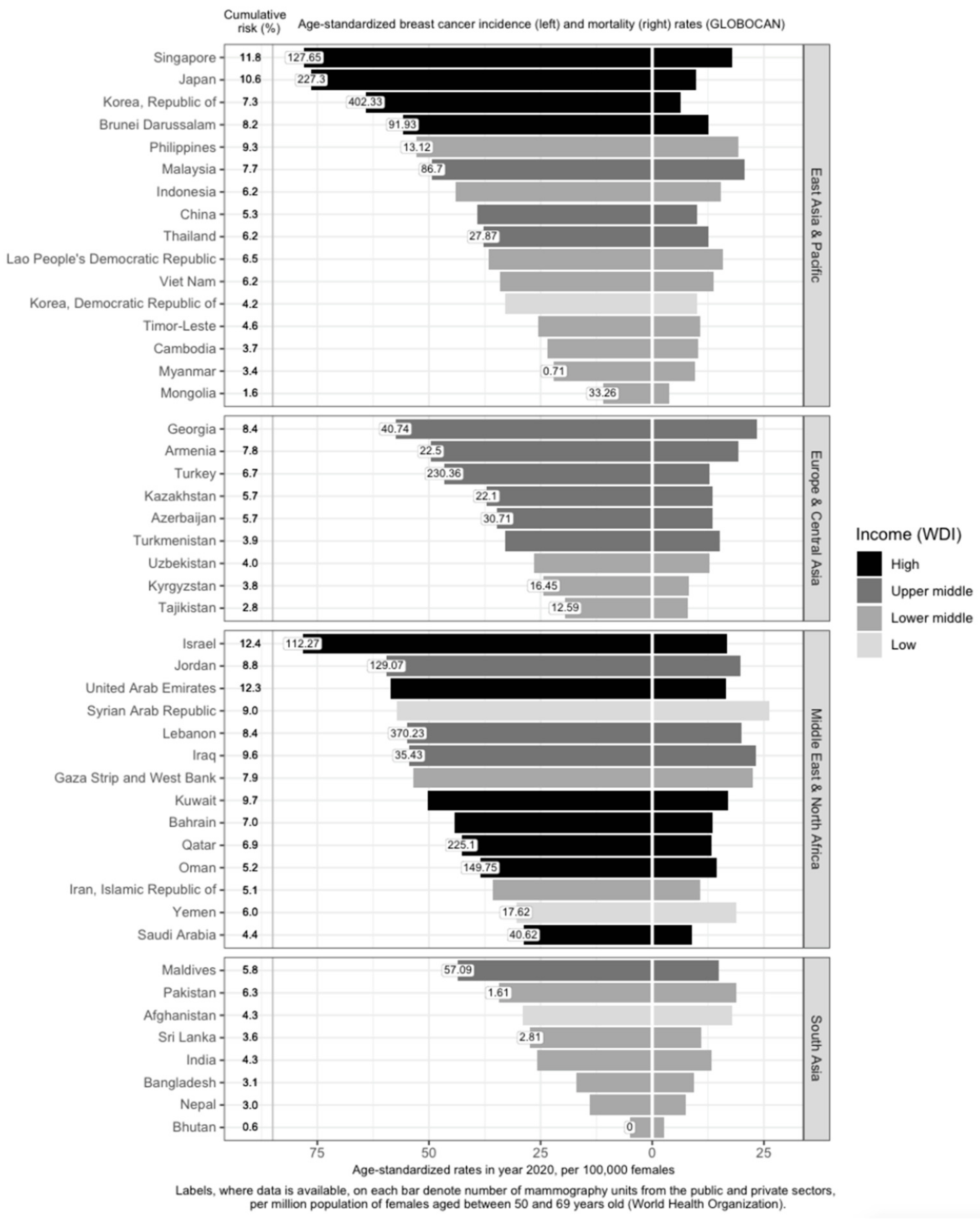
Figure 1. Variation of breast cancer burden and availability of breast cancer screening resources (mammography units) in Asia by region, country, and income level. Age-standardized incidence rate (ASIR) of breast cancer, age-standardized mortality rate (ASMR) of breast cancer, income group, cumulative risk up to 74 years (%), and number of mammography units per 1 million females aged 50 to 69 years in Asia. GLOBOCAN and income statistics from the year 2020. Information on mammography units per million female residents retrieved from World Health Organization (2022). Missing labels denote mammography resource information not available for the respective country. WDI: World Development Index.
2.2. Affluence and Breast Cancer Incidence
Income is directly associated with ASIR and inversely associated with ASMR [12][13][14][15] (Figure 1). Affluent women are more likely to have delayed births, breastfeed less, and use hormone supplements, all of which are risk factors for breast cancer [16]. In addition, they are more capable of affording mammograms, which detect many malignancies that would otherwise remain undetected till a later stage [16]. High-income countries are more likely to offer population-based mammography screening programs [17][18][19][20][21][22][23][24][25][26][27][28][29][30] and have more resources in terms of qualified physicians and mammogram units per capita (Figure 1), which contributes to higher breast cancer incidence through increased screening. However, high-income countries such as Kuwait, Bahrain, Qatar, Oman, and Saudi Arabia have much lower incidence rates, as compared to low- and low-middle-income countries (LMICs) such as Jordan, Syrian Arab Republic, Lebanon, Iraq, and the Gaza Strip and West Bank. This may be due to the higher fertility rates reducing the breast cancer risk in these higher-income countries [31]. Nonetheless, it should be noted that, after correcting for social-economic status, differences in breast cancer risk and outcomes across countries are greatly reduced, indicating that affluence is the main factor driving such differences [32][33].
3. Importance of Breast Cancer Screening
3.1. Delayed Diagnosis Is the Deadliest Threat to Survival
Recently, Kerlikowske and team reported that the most accurate way to define advanced cancer associated with breast cancer death was the American Joint Committee on Cancer (AJCC) prognostic pathologic stage IIA or higher [34]. According to breast cancer statistics published by Cancer Research UK, the majority of women with Stage I breast cancer (~98%) will live five years or longer after diagnosis; nearly nine in ten Stage II breast cancer patients will survive five years or more [35]. The five-year survival rate drops to 70% for Stage III breast cancers. Tumors that have metastasized to distant parts of the body (Stage IV) are associated with poor survival rates (25%). Early detection by means of routine mammography screening finds smaller and less advanced breast cancers that are associated with lower treatment costs and a higher survival rate [36]. Previous studies have shown similar breast cancer prognosis between populations, after accounting for stage [37].
Breast cancer mortality rates in LMICs are higher than in their high-income counterparts (Figure 1). Timely and accurate diagnoses, as well as the quality of treatment and care, are critical factors that drive breast cancer survival outcomes [38]. In terms of timeliness, the stage at presentation of breast cancer varies widely throughout Asia. The median proportions of localized (Stage I and II) breast cancers detected in Asian countries, in order of income categories, are 33.6%, 43.0%, 50.0%, and 63.4% [39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81]. The corresponding numbers for Stage I breast cancer are 7.2%, 10.7%, 25.6%, and 35.0% (Figure 2). Notably, more than seven in ten breast cancers diagnosed in high-income countries such as Qatar, Singapore, and Japan are Stage II and below. Over half of the breast cancers diagnosed in Singapore are Stage I.
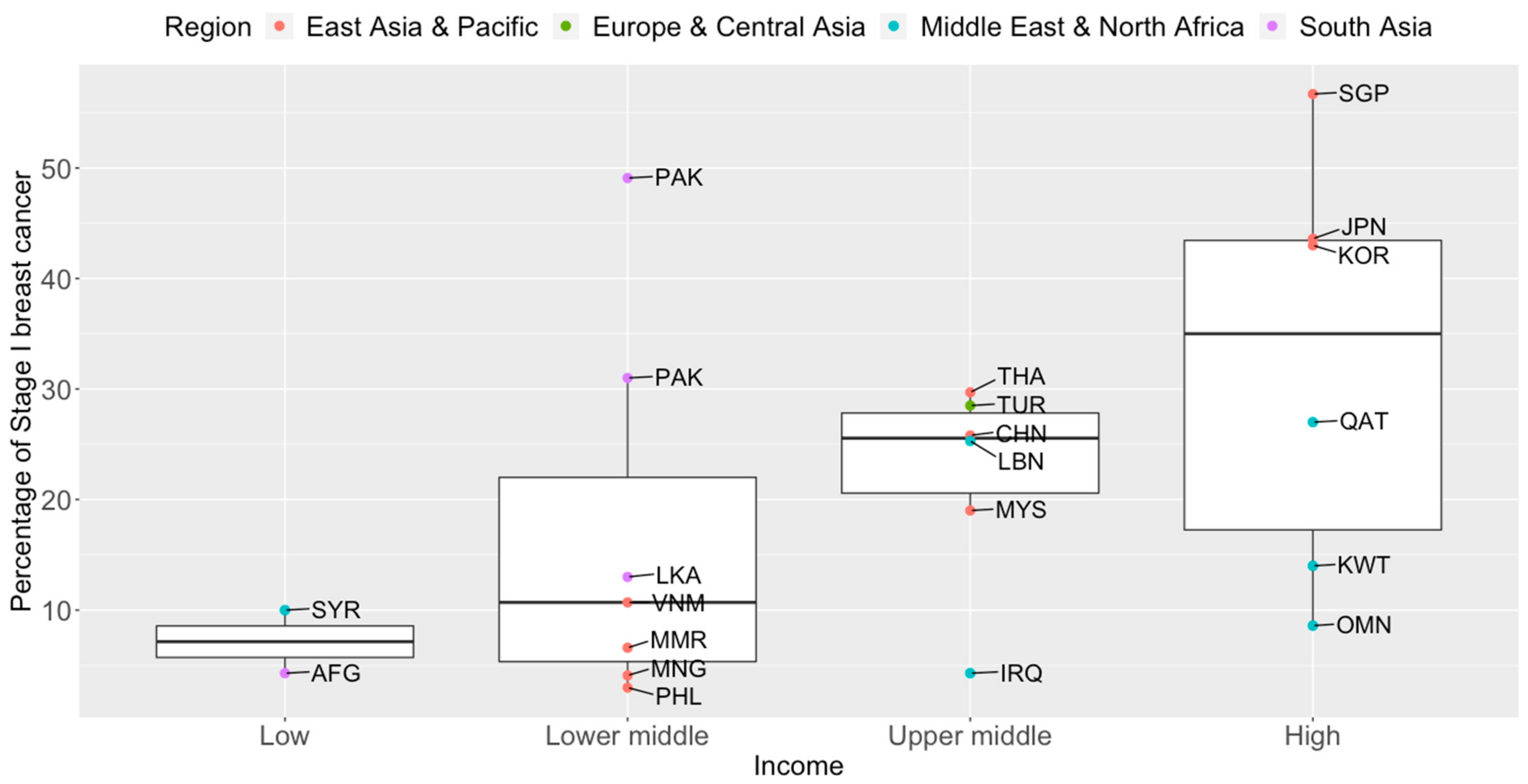
Figure 2. Box plots of early-stage breast cancers diagnosed (Stage I only) by income groups and regions in Asia. Source of income level data: World Development Index, 2020. AFG: Afghanistan, CHN: China, IRQ: Iraq, JPN: Japan, KOR: Korea, Republic of, KWT: Kuwait, LBN: Lebanon, LKA: Sri Lanka, MYS: Malaysia, MNG: Mongolia, MMR: Myanmar, OMN: Oman, PAK: Pakistan, PHL: Philippines, QAT: Qatar, SGP: Singapore, SYR: Syrian Arab Republic, THA: Thailand, TUR: Turkey, VNM: Vietnam.
The high proportion of late-stage breast cancers at diagnosis may pose a bigger healthcare burden on low-income countries, as the cost of breast cancer treatment increases with more advanced cancers [82]. At the individual level, more than 75% of patients die or face financial ruin within a year in southeast Asia [83].
3.2. Early Detection as a Prerequisite to Life after Breast Cancer
Between the 1930s and 1970s, breast cancer mortality rates remained stable [84]. Breast cancer survival improved in the 1980s in countries after the introduction of early detection programs [85]. Common breast screening methods include breast self-examination, clinical breast examination, MRI, ultrasound, and mammography. However, the gold standard for breast screening is mammography, which is a low-dose X-ray of the breast. It is the only approach proven to effectively reduce breast cancer deaths by early detection in a population-based screening setting [86]. A combined analysis of eight prospective randomized clinical trials showed that screening mammography produced a mortality benefit of ~22% for women aged 50 to 69 years old in populations invited to screening [87].
3.3. Nipping Breast Cancer in the Bud
Serial mammography screening in asymptomatic women can detect breast abnormalities early before any symptoms or signs are present [88]. Evidence from European populations shows that the number of lives saved by mammography screening is substantial [89]. When a participation rate of 70 to 75% within the target population receives mammography, a significant reduction in breast cancer mortality at the population level can be expected after 7–10 years [89]. In a more recent study, it is estimated that absolute benefits of 8.8 and 5.7 breast cancer deaths were avoided per 1000 women screened for 20 years, beginning at age 50, in Sweden and England respectively [90]. At the 2018 Kyoto Breast Cancer Consensus Conference, a poll showed that ~87% of the participants agreed that screening was an effective way to reduce breast cancer mortality, and 78% were supportive of establishing systematic mammography screening programs in all developed countries [91].
Mammography screening is often an opportunistic event in Asia, while several European countries have reported mammography participation rates of over 75% [92]. Only 13 of the 47 Asian countries have organized population-based mammography screening programs (Figure 3) [17][18][19][20][21][22][23][24][25][26][27][28][29][30]. Among these countries, only Israel comes close to achieving the ideal mammography attendance rate of 70% [20]. Despite the presence of highly subsidized, nationwide mammography screening programs established in the early 2000s in high-income Asian countries such as Korea, Japan, Taiwan, and Singapore, the uptake of screening mammography remains low. The participation rate in Korea was the highest among the countries, with organized mammography screening at 59.7% in 2015 [93]. In 2016, only 44.9% of the target women in Japan had undergone mammography screening within the past 2 years [28]. In Taiwan, the biennial participation rate was slightly below 40% in 2014 [94]. In a similar time period (2015–2016), less than 40% of the target population in Singapore attended timely mammography screening [95].
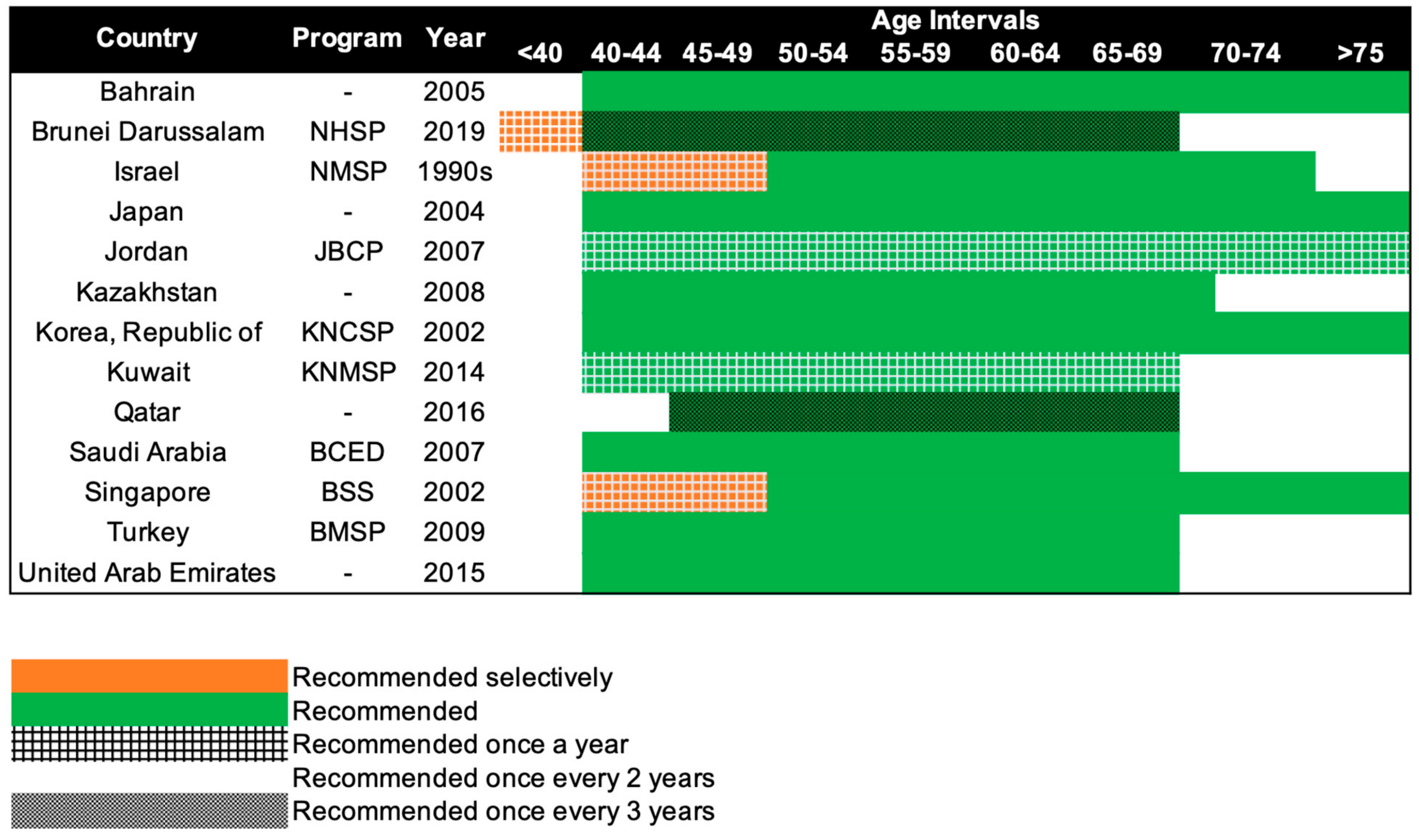
Figure 3. Recommendations of national breast cancer screening programs in Asia. NHSP: National Health Screening Program, NMSP: National Mammography Screening Program, JBCP: Jordan Breast Cancer Program, KNCSP: Korean National Cancer Screening Program, KNMSP: Kuwait National Mammography Screening Program, BCED: Breast Cancer Early Detection, BSS: BreastScreen Singapore, BMSP: Bahcesehir Mammography Screening Project.
3.4. Mammography Screening Guidelines in Asia
Beginning in the 1990s, 13 countries in Asia have progressively implemented population-based mammography screening, starting as early as the 1990s in Israel and only in 2019 in Brunei (Figure 3). Overall, the recommendations for mammography screening are relatively similar among the 13 countries. The most common screening recommendation is biennial screening beginning from 40 years of age. Seven of the 13 countries, namely, Kazakhstan, Turkey, Bahrain, Saudi Arabia, United Arab Emirates, Japan, and South Korea (Republic of Korea), recommend this as part of their national screening program [17][18][19][24][25][26][28][29]. Singapore and Israel have similar guidelines, but the first 10 years of screening are selectively offered annually to women, only upon request or referral [20][30]. Kuwait and Jordan provide their women with the highest frequency of screening, with annual screening from the age of 40 years [21][22]. The screening interval is the longest for Brunei and Qatar, with screening recommended only every 3 years, from the age of 40 and 45 respectively [23][27]. Despite Brunei having the longest screening interval, it does recommend annual screening for women with high genetic risk (i.e., BRCA1/2 mutation carriers) starting from the age of 25 [27].
3.5 Tailoring Screening for Asian Populations
The current standard of care for breast cancer screening provides a uniform strategy for women in the target population based only on their age, while the best recommendations for specific subgroups of high-risk women may vary [96][97][98][99]. Around half of the Asian women are diagnosed with breast cancer before they reach the typical mammography screening age of 50, implying that age limits may need to be adjusted [100]. While the evidence for mammography as a screening tool for women aged 50 and above is based on high-quality meta-analyses and systematic reviews of randomized controlled trials, the evidence for younger women is not as convincing [101]. Mammography is associated with poor diagnostic performance in younger women [88]. Furthermore, Asian women tend to have small breasts with high mammographic density, which might make early and small breast tumors difficult to detect [102]. The lower incidence of breast cancer among Asian women compared to women of European ancestry also implies that the positive predictive value of screening mammography will be lower [103].
It has been proposed that to improve the risk-benefit ratio of mammography screening, the age-based strategy should be replaced with a stratified approach (risk-based) [104][105]. A stratified approach would be to invite women to screen based on their individual risk of developing breast cancer and to give tailored recommendations [104][105].
Several efforts worldwide are underway to refine and tailor breast cancer screening based on individual risk [106][107]. A press release by the Government of the Hong Kong Special Administrative Region announced a stratified breast cancer screening pilot program in late 2021 [108]. Women aged 44 to 69 who have certain combinations of individual risk factors that place them at elevated risk of breast cancer are recommended to attend mammography screening every two years, according to the latest Cancer Expert Working Group on Cancer Prevention and Screening recommendations [109]. The breast cancer risk assessment tools developed by the University of Hong Kong can be found at the Cancer Online Resource Hub: www.cancer.gov.hk/en/bctool (accessed on 1 July 2022) [110][111].
In Taiwan, general population screening was deemed not cost-effective and unnecessary, due to the low incidence rate of breast cancer [112][113]. Hence, a stratified approach was taken in the Keelung Community-based Integrated Screening (KCIS) to prioritize women who may benefit from mammography screening [112]. Risk factors used in the stratification included family history of breast cancer or risk scores computed from self-reported menstrual and reproductive characteristics [112]. Women identified to be in the high-risk group were recommended to attend a biennial mammography screening [112]. Women not identified to be at high risk were recommended to undergo annual physical examinations [112]. In the same study, comprising 1,429,890 asymptomatic women enrolled in three screening programs (clinical breast examination, universal mammography screening, and risk-based mammography screening), universal biennial mammography, compared to clinical breast examination, was associated with a 41% mortality reduction and a 30% reduction of breast cancers that are Stage II and above [112]. In contrast, risk-based mammography screening was not associated with a statistically significant mortality reduction.
BREAst screening Tailored for HEr (BREATHE) is a pilot stratified mammography screening study in Singapore [114]. The program integrates both non-genetic and genetic breast cancer risk prediction tools to personalize screening recommendations. Predictions are based on the following: (1) Gail model (non-genetic), (2) mammographic density and recall, (3) BOADICEA predictions (breast cancer predisposition genes), and (4) breast cancer polygenic risk score (PRS) [114]. The BREATHE’s risk classification decision tree is adapted from the established WISDOM Personalized Breast Cancer Screening Trial [106]. WISDOM uses a five-year absolute risk threshold of 6% (risk of an average BRCA carrier) for stratification based on genetic risk factors [106]. However, confirmatory clinical genetic testing was not performed in BREATHE. Based only on predicted genetic risks, BREATHE is testing lower five-year absolute risk thresholds for disease stratification (~3%).
4. Conclusion
Breast cancer is a growing public health problem in most parts of Asia. Despite the establishment of screening guidelines globally, Asia has been slow to adopt breast cancer screening. High-income countries are not benefiting fully from national breast screening programs due to an underutilization of the preventive healthcare services available. On the other hand, LMICs are unable to adopt screening programs implemented in high-income countries, due to resource constraints. The full potential of mammography screening cannot be achieved, as there is still room for improvements in the procedure (e.g., reducing overdiagnosis and increasing screening sensitivity for dense breasts). These gaps may be filled by incorporating stratified screening, with the use of both genetic and non-genetic risk factors. However, while studies are underway to evaluate the use of these risk factors to refine individual breast cancer risk in healthy populations, questions regarding appropriate risk thresholds to define above-average risk, type of personalized screening recommendations offered, and implementation challenges, among others, remain to be answered before the verdict is out on the utility of risk-based screening. Ultimately, it is important to note that mammography screening is an imperfect test that is associated with limitations and biases, and these may undermine real survival benefits. It is important to weigh the hazards of screening against the risks of not screening.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249.
- Leong, S.P.L.; Shen, Z.-Z.; Liu, T.-J.; Agarwal, G.; Tajima, T.; Paik, N.-S.; Sandelin, K.; Derossis, A.; Cody, H.; Foulkes, W.D. Is breast cancer the same disease in Asian and western countries? World J. Surg. 2010, 34, 2308–2324.
- Green, M.; Raina, V. Epidemiology, screening and diagnosis of breast cancer in the Asia-Pacific region: Current perspectives and important considerations. Asia-Pac. J. Clin. Oncol. 2008, 4, S5–S13.
- Sung, H.; Rosenberg, P.S.; Chen, W.-Q.; Hartman, M.; Lim, W.-y.; Chia, K.S.; Wai-Kong Mang, O.; Chiang, C.-J.; Kang, D.; Ngan, R.K.-C.; et al. Female breast cancer incidence among Asian and western populations: More similar than expected. J. Natl. Cancer Inst. 2015, 107, djv107.
- Yip, C.-H. Breast cancer in Asia. Methods Mol. Biol. 2009, 471, 51–64.
- Mousavi-Jarrrahi, H.S.; Kasaeian, A.; Mansori, K.; Ranjbaran, M.; Khodadost, M.; Mosavi-Jarrahi, A. Addressing the younger age at onset in breast cancer patients in Asia: An age-period-cohort analysis of fifty years of quality data from the International Agency for Research on Cancer. ISRN Oncol. 2013, 2013, 429862.
- Fan, L.; Goss, P.E.; Strasser-Weippl, K. Current status and future projections of breast cancer in Asia. Breast Care 2015, 10, 372–378.
- Ozsoy, A.; Barca, N.; Dolek, B.A.; Aktas, H.; Elverici, E.; Araz, L.; Ozkaraoglu, O. The relationship between breast cancer and risk factors: A single-center study. Eur. J. Breast Health 2017, 13, 145–149.
- World Health Organisation (WHO). Estimated Age-Standardized Incidence Rates (World) in 2020, Breast, Females, All Ages, Asia. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 3 February 2022).
- The World Bank. World Health Organization’s Global Health Workforce Statistics, OECD. (Physicians (per 1000 People). Available online: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS?locations=8S-Z4-Z7-ZQ (accessed on 3 February 2022).
- World Health Organisation (WHO). Estimated Cumulative Risk of Incidence in 2020, Breast, Females, All Ages, Asia. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 3 February 2022).
- Ng, J.C.; Teo, C.H.; Abdullah, N.; Tan, W.P.; Tan, H.M. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer 2015, 15, 613.
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 2018, 36, 25–33.
- Sparano, A.J.; Brawley, O.W. Deconstructing racial and ethnic disparities in breast cancer. JAMA Oncol. 2021, 7, 355.
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—A systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813.
- Lehrer, S.; Green, S.; Rosenzweig, K.E. Affluence and breast cancer. Breast J. 2016, 22, 564–567.
- Salikhanov, I.; Crape, B.; Howie, P. Cost-effectiveness of mammography screening program in a resource-limited post-Soviet country of Kazakhstan. Asian Pac. J. Cancer Prev. 2019, 20, 3153–3160.
- Ozmen, V.; Gurdal, S.O.; Cabioglu, N.; Ozcinar, B.; Ozaydin, A.N.; Kayhan, A.; Aribal, E.; Sahin, C.; Saip, P.; Alagoz, O. Cost-effectiveness of breast cancer screening in Turkey, a developing country: Results from Bahçeşehir mammography screening project. Eur. J. Breast Health. 2017, 13, 117–122.
- Bahrain Cancer Society. Early Detection of Breast Disease. Available online: https://www.bahraincancer.com/cancer-prevention-screening/early-detection-of-of-breast-disease/ (accessed on 1 May 2022).
- Israel Cancer Association (ICA). Breast Cancer. Available online: https://en.cancer.org.il/template_e/default.aspx?PageId=7749 (accessed on 1 May 2022).
- Al-Mousa, D.S.; Alakhras, M.; Hossain, S.Z.; Al-Sa’di, A.G.; Al Hasan, M.; Al-Hayek, Y.; Brennan, P.C. Knowledge, attitude and practice around breast cancer and mammography screening among Jordanian women. Breast Cancer Targets Ther. 2020, 12, 231–242.
- Mango, V.L.; Al-Khawari, H.; Dershaw, D.D.; Ashkanani, M.H.; Pennisi, B.; Turner, P.; Thornton, C.; Morris, E.A. Initiating a national mammographic screening program: The Kuwait experience training with a US cancer center. J. Am. Coll. Radiol. 2019, 16, 202–207.
- National Cancer Program of the Ministry of Public Health. (Early Detection of Cancer). Available online: https://www.moph.gov.qa/english/derpartments/healthaffairs/healthpromotion/nationalcancerprogram/cancerscreening/Pages/default.aspx (accessed on 1 May 2022).
- Ministry of Health (MOH). Breast Cancer Early Detection. 2020. Available online: https://www.moh.gov.sa/en/Ministry/Projects/breast-cancer/Pages/default.aspx (accessed on 1 May 2022).
- Al-Shamsi, H.O.; Alrawi, S. Breast cancer screening in the United Arab Emirates: Is it time to call for a screening at an earlier age? J. Cancer Prev. Curr. Res. 2018, 9, 00334.
- United Arab Emirates Ministry of Health and Prevention. The National Guidelines For Breast Cancer Screening and Diagnosis. 2014. Available online: https://www.isahd.ae/content/docs/Guidelines%20For%20Breast%20Cancer%20Screening_Booklet.pdf (accessed on 1 May 2022).
- Noncommunicable Diseases Prevention Unit, Ministry of Health. National Health Screening Guideline on Noncommunicable Diseases (NCDs). 2019. Available online: https://www.moh.gov.bn/Shared%20Documents/MOH_National%20Health%20Screening%20Guideline%20on%20NCDs_23%20Jul%202020.pdf (accessed on 1 May 2022).
- Satoh, M.; Sato, N. Relationship of attitudes toward uncertainty and preventive health behaviors with breast cancer screening participation. BMC Women Health 2021, 21, 171.
- Choi, E.; Lee, Y.Y.; Suh, M.; Lee, E.Y.; Mai, T.T.X.; Ki, M.; Oh, J.-K.; Cho, H.; Park, B.; Jun, J.K.; et al. Socioeconomic inequalities in cervical and breast cancer screening among women in Korea, 2005–2015. Yonsei Med. J. 2018, 59, 1026.
- Loy, E.Y.; Molinar, D.; Chow, K.Y.; Fock, C. National breast cancer screening programme, Singapore: Evaluation of participation and performance indicators. J. Med. Screen. 2015, 22, 194–200.
- Zahedi, R.; Molavi Vardanjani, H.; Baneshi, M.R.; Haghdoost, A.A.; Malekpour Afshar, R.; Ershad Sarabi, R.; Tavakoli, F.; Zolala, F. Incidence trend of breast Cancer in women of eastern Mediterranean region countries from 1998 to 2019: A systematic review and meta-analysis. BMC Women Health 2020, 20, 53.
- Shariff-Marco, S.; Yang, J.; John, E.M.; Kurian, A.W.; Cheng, I.; Leung, R.; Koo, J.; Monroe, K.R.; Henderson, B.E.; Bernstein, L.; et al. Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer. J. Community Health 2015, 40, 1287–1299.
- Linnenbringer, E.; AGeronimus, T.; Davis, K.L.; Bound, J.; Ellis, L.; Gomez, S.L. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among black and white women. Breast Cancer Res. Treat. 2020, 180, 437–447.
- Kerlikowske, K.; Bissell, M.C.S.; Sprague, B.L.; Buist, D.S.M.; Henderson, L.M.; Lee, J.M.; Miglioretti, D.L. Advanced breast cancer definitions by staging system examined in the breast cancer surveillance consortium. J. Natl. Cancer Inst. 2021, 113, 909–916.
- Cancer Research UK. Survival. 2020. Available online: https://www.cancerresearchuk.org/about-cancer/breast-cancer/survival (accessed on 23 August 2022).
- Wong, J.Z.Y.; Chai, J.H.; Yeoh, Y.S.; Mohamed Riza, N.K.; Liu, J.; Teo, Y.-Y.; Wee, H.L.; Hartman, M. Cost effectiveness analysis of a polygenic risk tailored breast cancer screening programme in Singapore. BMC Health Serv. Res. 2021, 21, 379.
- Tan, B.K.T.; Lim, G.H.; Czene, K.; Hall, P.; Chia, K.S. Do Asian breast cancer patients have poorer survival than their western counterparts? A comparison between Singapore and Stockholm. Breast Cancer Res. 2009, 11, R4.
- Martei, Y.M.; Pace, L.E.; Brock, J.E.; Shulman, L.N. Breast cancer in low and middle-income countries. Clin. Lab. Med. 2018, 38, 161–173.
- Niazi, A.-u.-R.; Jami, A.A.; Shams, A.Z.; Mahmoodi, A.S.; Krapfl, E.; Falk, S.; Buia, A.; Hanisch, E. Establishing a breast cancer center in Herat, Afghanistan: An implementation study. Glob. Health J. 2021, 5, 204–208.
- Ahmad Jawad, F. Factors contributing to delayed diagnosis and treatment of breast cancer and its outcome in Jamhoriat Hospital Kabul, Afghanistan. In Proceedings of the Breast Cancer 2021 & Pediatrics 2021, Webinar, 4 October 2021.
- Bedirian, K.; Aghabekyan, T.; Mesrobian, A.; Shekherdimian, S.; Zohrabyan, D.; Safaryan, L.; Sargsyan, L.; Avagyan, A.; Harutyunyan, L.; Voskanyan, A.; et al. Overview of cancer control in Armenia and policy implications. Front. Oncol. 2022, 11, 782581.
- Ryzhov, A.; Corbex, M.; Piñeros, M.; Barchuk, A.; Andreasyan, D.; Djanklich, S.; Ghervas, V.; Gretsova, O.; Kaidarova, D.; Kazanjan, K.; et al. Comparison of breast cancer and cervical cancer stage distributions in ten newly independent states of the former Soviet Union: A population-based study. Lancet Oncol. 2021, 22, 361–369.
- Alam, N.E.; Islam, M.S.; Ullah, H.; Molla, M.T.; Shifat, S.K.; Akter, S.; Aktar, S.; Khatun, M.M.; Ali, M.R.; Sen, T.C.; et al. Evaluation of knowledge, awareness and attitudes towards breast cancer risk factors and early detection among females in Bangladesh: A hospital based cross-sectional study. PLoS ONE 2021, 16, e0257271.
- Ley, P.; Hong, C.; Varughese, J.; Camp, L.; Bouy, S.; Maling, E. Challenges in the management of breast cancer in a low resource setting in South East Asia. Asian Pac. J. Cancer Prev. 2016, 17, 3459–3463.
- Zeng, H.; Ran, X.; An, L.; Zheng, R.; Zhang, S.; Ji, J.S.; Zhang, Y.; Chen, W.; Wei, W.; He, J. Disparities in stage at diagnosis for five common cancers in China: A multicentre, hospital-based, observational study. Lancet Public Health 2021, 6, e887.
- Panato, C.; Abusamaan, K.; Bidoli, E.; Hamdi-Cherif, M.; Pierannunzio, D.; Ferretti, S.; Daher, M.; Elissawi, F.; Serraino, D. Survival after the diagnosis of breast or colorectal cancer in the GAZA Strip from 2005 to 2014. BMC Cancer 2018, 18, 632.
- Sathwara, J.A.; Balasubramaniam, G.; Bobdey, S.C.; Jain, A.; Saoba, S. Sociodemographic factors and late-stage diagnosis of breast cancer in India: A hospital-based study. Indian J. Med. Paediatr. Oncol. 2017, 38, 277–281.
- Anwar, S.L.; Raharjo, C.A.; Herviastuti, R.; Dwianingsih, E.K.; Setyoheriyanto, D.; Avanti, W.S.; Choridah, L.; Harahap, W.A.; Darwito; Aryandono, T.; et al. Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: A hospital-based study. BMC Women’s Health 2019, 19, 28.
- Montazeri, A.; Ebrahimi, M.; Mehrdad, N.; Ansari, M.; Sajadian, A. Delayed presentation in breast cancer: A study in Iranian women. BMC Women Health 2003, 3, 4.
- Foroozani, E.; Ghiasvand, R.; Mohammadianpanah, M.; Afrashteh, S.; Bastam, D.; Kashefi, F.; Shakarami, S.; Dianatinasab, M. Determinants of delay in diagnosis and end stage at presentation among breast cancer patients in Iran: A multi-center study. Sci. Rep. 2020, 10, 21477.
- Mutar, M.T.; Goyani, M.S.; Had, A.M.; Mahmood, A.S. Pattern of presentation of patients with breast cancer in Iraq in 2018: A cross-sectional study. J. Glob. Oncol. 2019, 5, 00041.
- Keinan-Boker, L.; Baron-Epel, O.; Fishler, Y.; Liphshitz, I.; Barchana, M.; Dichtiar, R.; Goodman, M. Breast cancer trends in Israeli Jewish and Arab women, 1996–2007. Eur. J. Cancer Prev. 2013, 22, 112–120.
- Kubo, M.; Kumamaru, H.; Isozumi, U.; Miyashita, M.; Nagahashi, M.; Kadoya, T.; Kojima, Y.; Aogi, K.; Hayashi, N.; Tamura, K.; et al. Annual report of the Japanese breast cancer society registry for 2016. Breast Cancer 2020, 27, 511–518.
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K.; et al. Breast cancer statistics in Korea in 2017: Data from a breast cancer registry. J. Breast Cancer 2020, 23, 115.
- Fayaz, M.S.; El-Sherify, M.S.; El-Basmy, A.; Zlouf, S.A.; Nazmy, N.; George, T.; Samir, S.; Attia, G.; Eissa, H. Clinicopathological features and prognosis of triple negative breast cancer in Kuwait: A comparative/perspective analysis. Rep. Pract. Oncol. Radiother. 2014, 19, 173–181.
- Luangxay, T.; Virachith, S.; Hando, K.; Vilayvong, S.; Xaysomphet, P.; Arounlangsy, P.; Phongsavan, K.; Mieno, M.N.; Honma, N.; Kitagawa, M.; et al. Subtypes of breast cancer in Lao, P.D.R.: A study in a limited-resource setting. Asian Pac. J. Cancer Prev. 2019, 20, 589–594.
- El Saghir, N.S.; Daouk, S.; Saroufim, R.; Moukalled, N.; Ghosn, N.; Assi, H.; Tfaily, A.; Mukherji, D.; Charafeddine, M.; Al-Darazi, M.; et al. Rise of metastatic breast cancer incidence in Lebanon: Effect of refugees and displaced people from Syria, and patients from war-torn Iraq. Breast 2017, 36, S74.
- Norsa’adah, B.; Rampal, K.G.; Rahmah, M.A.; Naing, N.N.; Biswal, B.M. Diagnosis delay of breast cancer and its associated factors in Malaysian women. BMC Cancer 2011, 11, 141.
- Angarmurun, D.; Batzorig, B.; Undram, L.; Gantuya, D.; Chimedsuren, O.; Avirmed, D. Breast cancer survival in Mongolian women. OALib 2014, 1, 1100396.
- San, T.H.; Fujisawa, M.; Fushimi, S.; Soe, L.; Min, N.W.; Yoshimura, T.; Ohara, T.; Yee, M.M.; Oda, S.; Matsukawa, A. Molecular subtypes of breast cancers from Myanmar women: A study of 91 cases at two pathology centers. Asian Pac. J. Cancer Prev. 2017, 18, 1617–1621.
- Pun, C.B.; Shrestha, S.; Bhatta, R.R.; Pandey, G.; Uprety, S.; Bastakoti, S.; Dhungana, I.; Jha, N. A retrospective analysis of breast cancer at BPKMCH, Nepal. Nepal. J. Cancer 2020, 4, 98–101.
- Jerudong Park Medical Centre. JPMC Held Health Talk for PEKERTI on Breast Cancer Awareness. 2020. Available online: https://www.jpmcbrunei.com/jpmc-held-health-talk-for-pekerti-on-breast-cancer-awareness/ (accessed on 10 July 2022).
- Suhair Khalifa, A.S.; Akbar, J.A. Breast cancer risk factors and stage at presentation. Bahrain Med. Bull. 2006, 28, 111–115.
- Medical Aid for Palestinians. Breast Cancer in Occupied Palestine. Available online: https://www.map.org.uk/downloads/map-breast-cancer-fact-sheet.pdf (accessed on 10 July 2022).
- Jordan Breast Cancer Program Breast Cancer Screening and Diagnosis Guidelines. 2011. Available online: https://www.iccp-portal.org/system/files/plans/jor_D1_guidlines%2021.4.2011%20breast%20cancer.pdf/ (accessed on 10 July 2022).
- Al-Moundhri, M.; Al-Bahrani, B.; Pervez, I.; Ganguly, S.S.; Nirmala, V.; Al-Madhani, A.; Al-Mawaly, K.; Grant, C. The outcome of treatment of breast cancer in a developing country—Oman. Breast 2004, 13, 139–145.
- Aziz, Z.; Iqbal, J.; Akram, M. Effect of social class disparities on disease stage, quality of treatment and survival outcomes in breast cancer patients from developing countries. Breast J. 2008, 14, 372–375.
- De Leon Matsuda, M.L.; Liede, A.; Kwan, E.; Mapua, C.A.; Cutiongco, E.M.C.; Tan, A.; Borg, Å.; Narod, S.A. BRCA1 and BRCA2 mutations among breast cancer patients from the Philippines. Int. J. Cancer 2002, 98, 596–603.
- Bujassoum, S.M. Epidemiology of breast cancer in Qatar 1999–2000. QATAR Med. J. 2005, 14, 34–36.
- Ezzat, A.; Raja, M.; Rostom, A.; Zwaan, F.; Akhtar, M.; Bazarbashi, S.; Ingemansson, S.; Al-Abdulkareem, A. An overview of breast cancer. Ann. Saudi Med. 1997, 17, 10–15.
- Health Promotion Board. Singapore Cancer Registry Annual Report 2019. 2022. Available online: https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/scr-2019_annual-report_final.pdf?sfvrsn=fa847590_0 (accessed on 10 July 2022).
- Wijeratne, D.T.; Gunasekera, S.; Booth, C.M.; Promod, H.; Jalink, M.; Jayarajah, U.; Seneviratne, S. Demographic, tumour, and treatment characteristics of female patients with breast cancer in Sri Lanka; results from a hospital-based cancer registry. BMC Cancer 2021, 21, 1175.
- Ghazal, F.; Mutasem, M.; Feras Al, J.; Nidal, K.; Ehab, A.; Maher, S.; Maha, M.; Saad Aldeen, J.; Eyad, A.; Ahmad, F. Rapid Assessment of Cancer Management Care in Syria. 2016. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/Final%20report-%20cancer%20study.pdf (accessed on 1 May 2022).
- Kotepui, M.; Chupeerach, C. Age distribution of breast cancer from a Thailand population-based cancer registry. Asian Pac. J. Cancer Prev. 2013, 14, 3815–3817.
- Filomeno, M. TL Needs Mammography Unit for Early Detection of Breast Cancer. Tatoli. 2021. Available online: http://www.tatoli.tl/en/2021/09/28/tl-needs-mammography-unit-for-early-detection-of-breast-cancer/ (accessed on 22 August 2022).
- Ozmen, V.; Ozmen, T.; Dogru, V. Breast cancer in Turkey; an analysis of 20,000 patients with breast cancer. Eur. J. Breast Health 2019, 15, 141–146.
- Akkazieva, B.; Tello, J.; Smith, B.; Jakab, M.; Krasovsky, K.; Sautenkova, N.; Yuldasheva, L.; Shoismatuloeva, M. Better non-Communicable Disease Outcomes: Challenges and Opportunities for Health Systems. Tajikistan Country Assessment. World Health Organization. Regional Office for Europe. 2015. Available online: https://apps.who.int/iris/handle/10665/153907 (accessed on 1 May 2022).
- Elobaid, Y.; Aamir, M.; Grivna, M.; Suliman, A.; Attoub, S.; Mousa, H.; Ahmed, L.A.; Oulhaj, A. Breast cancer survival and its prognostic factors in the United Arab Emirates: A retrospective study. PLoS ONE 2021, 16, e0251118.
- Trieu, P.D.Y.; Mello-Thoms, C.; Brennan, P.C. Female breast cancer in Vietnam: A comparison across Asian specific regions. Cancer Biol. Med. 2015, 12, 238–245.
- Harhra, N.A.; Basaleem, H.O. Trends of breast cancer and its management in the last twenty years in aden and adjacent governorates, Yemen. Asian Pac. J. Cancer Prev. 2012, 13, 4347–4351.
- Ernawati; Oktaviana, D.; Mantasia; Yusuf, R.A.; Sumarmi. The effect of health education based on the health belief model about pap smear test on women in rural district Indonesia. Med. Leg. Update 2021, 21, 7–12.
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am. Health Drug Benefits 2016, 9, 23–32.
- Kimman, M.; Peters, S.; Jan, S.; Bhoo-Pathy, N.; Yip, C.H.; Joore, M.; Woodward, M. The Economic Impact of Breast Cancer in the South-East Asian Region, in Breast Cancer: Global Quality Care; Joore, M., Pouwels, X., Ramaekers, B., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 298–306.
- World Health Organisation (WHO). Breast Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 26 March 2021).
- Glass, A.G.; Lacey, J.V.; Carreon, J.D.; Hoover, R.N. Breast cancer incidence, 1980–2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J. Natl. Cancer Inst. 2007, 99, 1152–1161.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-Cancer Screening—Viewpoint of the IARC working group. N. Engl. J. Med. 2015, 372, 2353–2358.
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. preventive services task force recommendation. Ann. Intern. Med. 2016, 164, 244.
- Hollingsworth, A.B. Redefining the sensitivity of screening mammography: A review. Am. J. Surg. 2019, 218, 411–418.
- Day, N.E.; Williams, D.R.; Khaw, K.T. Breast cancer screening programmes: The development of a monitoring and evaluation system. Br. J. Cancer 1989, 59, 954–958.
- Duffy, S.W.; Tabar, L.; Olsen, A.H.; Vitak, B.; Allgood, P.C.; Chen, T.H.; Yen, A.M.; Smith, R.A. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the breast screening programme in England. J. Med. Screen. 2010, 17, 25–30.
- Jatoi, I.; Benson, J.R.; Toi, M. Breast cancer over-diagnosis: An adverse consequence of mammography screening—highlights of the 2018 Kyoto Breast Cancer Consensus Conference. Future Oncol. 2019, 15, 1193–1196.
- Eurostat. Healthcare Activities Statistics—Preventive Services. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_activities_statistics_-_preventive_services#Breast_cancer_screening (accessed on 22 June 2022).
- Choi, E.; Jun, J.K.; Suh, M.; Jung, K.-W.; Park, B.; Lee, K.; Jung, S.-Y.; Lee, E.S.; Choi, K.S. Effectiveness of the Korean national cancer screening program in reducing breast cancer mortality. Breast Cancer 2021, 7, 83.
- Yang, C.-C. Breast cancer trend in Taiwan. Women’s Health 2017, 6, 00153.
- Ministry of Health and Health Promotion Board, Singapore. National Population Health Survey 2020. 2020. Available online: https://www.moh.gov.sg/docs/librariesprovider5/default-document-library/nphs-2020-survey-report.pdf (accessed on 1 July 2022).
- Rebolj, M.; Assi, V.; Brentnall, A.; Parmar, D.; Duffy, S.W. Addition of ultrasound to mammography in the case of dense breast tissue: Systematic review and meta-analysis. Br. J. Cancer 2018, 118, 1559–1570.
- Gareth, E.D.; Nisha, K.; Yit, L.; Soujanye, G.; Emma, H.; Massat, N.J.; Maxwell, A.J.; Sarah, I.; Rosalind, E.; Leach, M.O.; et al. MRI breast screening in high-risk women: Cancer detection and survival analysis. Breast Cancer Res. Treat. 2014, 145, 663–672.
- Brake, M. A doctor’s kid. JAMA 2012, 307, 465.
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S.; et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. JAMA 2019, 322, 652.
- Bhoo-Pathy, N.; Yip, C.H.; Taib, N.A.; Hartman, M.; Saxena, N.; Iau, P.; Bulgiba, A.M.; Lee, S.C.; Lim, S.E.; Wong, J.E.L.; et al. Breast cancer in a multi-ethnic Asian setting: Results from the Singapore–Malaysia hospital-based breast cancer registry. Breast 2011, 20, S75–S80.
- U.S. Preventive Services Task Force. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2009, 151, 716.
- Rajaram, N.; Mariapun, S.; Eriksson, M.; Tapia, J.; Kwan, P.Y.; Ho, W.K.; Harun, F.; Rahmat, K.; Czene, K.; Taib, N.A.M.; et al. Differences in mammographic density between Asian and Caucasian populations: A comparative analysis. Breast Cancer Res. Treat. 2017, 161, 353–362.
- Bhoo-Pathy, N.; Yip, C.-H.; Hartman, M.; Uiterwaal, C.S.P.M.; Devi, B.C.R.; Peeters, P.H.M.; Taib, N.A.; van Gils, C.H.; Verkooijen, H.M. Breast cancer research in Asia: Adopt or adapt western knowledge? Eur. J. Cancer 2013, 49, 703–709.
- Pashayan, N.; Morris, S.; Gilbert, F.J.; Pharoah, P.D.P. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer. JAMA Oncol. 2018, 4, 1504.
- Morris, E.; Feig, S.A.; Drexler, M.; Lehman, C. Implications of overdiagnosis: Impact on screening mammography practices. Popul. Health Manag. 2015, 18, S1.
- Esserman, L.J. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. Npj Breast Cancer 2017, 3, 34.
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Stover Fiscalini, A.; Ziv, E.; van’t Veer, L.J.; Esserman, L.J.; Tice, J.A. Breast cancer screening in the precision medicine era: Risk-based screening in a population-based trial. J. Natl. Cancer Inst. 2017, 109, djw290.
- Gabriel, M.; Leung, M. Hong Kong Breast Cancer Study. Available online: https://ClinicalTrials.gov/show/NCT02889458 (accessed on 1 July 2022).
- Tsang, T.H.F.; Wong, K.H.; Allen, K.; Chan, K.K.L.; Chan, M.C.M.; Chao, D.V.K.; Cheung, A.N.; Fan, C.Y.M.; Hui, E.P.; Ip, D.K.M.; et al. Update on the recommendations on breast cancer screening by the cancer expert working group on cancer prevention and screening. Hong Kong Med. J. 2022, 28, 161–168.
- Wang, F.; Dai, J.; Li, M.; Chan, W.-C.; Kwok, C.C.-H.; Leung, S.-L.; Wu, C.; Li, W.; Yu, W.-C.; Tsang, K.-H.; et al. Risk assessment model for invasive breast cancer in Hong Kong women. Medicine 2016, 95, e4515.
- Hong Kong Breast Cancer Study. Breast Cancer Risk Assessment Tool. Available online: www.cancer.gov.hk/en/bctool (accessed on 22 June 2022).
- Yen, A.M.-F.; Tsau, H.-S.; Fann, J.C.-Y.; Chen, S.L.-S.; Chiu, S.Y.-H.; Lee, Y.-C.; Pan, S.-L.; Chiu, H.-M.; Kuo, W.-H.; Chang, K.-J.; et al. Population-based breast cancer screening with risk-based and universal mammography screening compared with clinical breast examination. JAMA Oncol. 2016, 2, 915.
- Chen, T.H.-H.; Chiu, Y.-H.; Luh, D.-L.; Yen, M.-F.; Wu, H.-M.; Chen, L.-S.; Tung, T.-H.; Huang, C.-C.; Chan, C.-C.; Shiu, M.-N.; et al. Community-based multiple screening model. Cancer 2004, 100, 1734–1743.
- Liu, J.; Ho, P.J.; Tan, T.H.L.; Yeoh, Y.S.; Chew, Y.J.; Mohamed Riza, N.K.; Khng, A.J.; Goh, S.-A.; Wang, Y.; Oh, H.B.; et al. BREAst screening Tailored for HEr (BREATHE)—A study protocol on personalised risk-based breast cancer screening programme. PLoS ONE 2022, 17, e0265965.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
864
Revisions:
2 times
(View History)
Update Date:
09 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




