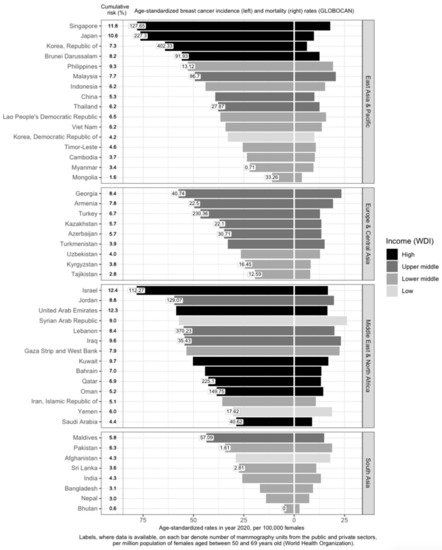
Income is directly associated with ASIR and inversely associated with ASMR [
15,
16,
17,
18] (
Figure 1). Affluent women are more likely to have delayed births, breastfeed less, and use hormone supplements, all of which are risk factors for breast cancer [
19]. In addition, they are more capable of affording mammograms, which detect many malignancies that would otherwise remain undetected till a later stage [
19]. High-income countries are more likely to offer population-based mammography screening programs [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33] and have more resources in terms of qualified physicians and mammogram units per capita (
Figure 1), which contributes to higher breast cancer incidence through increased screening. However, high-income countries such as Kuwait, Bahrain, Qatar, Oman, and Saudi Arabia have much lower incidence rates, as compared to low- and low-middle-income countries (LMICs) such as Jordan, Syrian Arab Republic, Lebanon, Iraq, and the Gaza Strip and West Bank. This may be due to the higher fertility rates reducing the breast cancer risk in these higher-income countries [
34]. Nonetheless, it should be noted that, after correcting for social-economic status, differences in breast cancer risk and outcomes across countries are greatly reduced, indicating that affluence is the main factor driving such differences [
35,
36].
3. Importance of Breast Cancer Screening
3.1. Delayed Diagnosis Is the Deadliest Threat to Survival
Recently, Kerlikowske and team reported that the most accurate way to define advanced cancer associated with breast cancer death was the American Joint Committee on Cancer (AJCC) prognostic pathologic stage IIA or higher [
37]. According to breast cancer statistics published by Cancer Research UK, the majority of women with Stage I breast cancer (~98%) will live five years or longer after diagnosis; nearly nine in ten Stage II breast cancer patients will survive five years or more [
38]. The five-year survival rate drops to 70% for Stage III breast cancers. Tumors that have metastasized to distant parts of the body (Stage IV) are associated with poor survival rates (25%). Early detection by means of routine mammography screening finds smaller and less advanced breast cancers that are associated with lower treatment costs and a higher survival rate [
39]. Previous studies have shown similar breast cancer prognosis between populations, after accounting for stage [
40].
Breast cancer mortality rates in LMICs are higher than in their high-income counterparts (
Figure 1). Timely and accurate diagnoses, as well as the quality of treatment and care, are critical factors that drive breast cancer survival outcomes [
41]. In terms of timeliness, the stage at presentation of breast cancer varies widely throughout Asia. The median proportions of localized (Stage I and II) breast cancers detected in Asian countries, in order of income categories, are 33.6%, 43.0%, 50.0%, and 63.4% [
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84]. The corresponding numbers for Stage I breast cancer are 7.2%, 10.7%, 25.6%, and 35.0% (
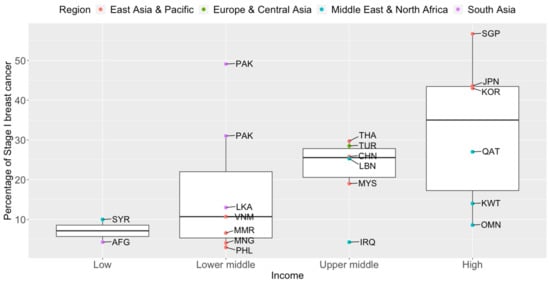
Figure 2). Notably, more than seven in ten breast cancers diagnosed in high-income countries such as Qatar, Singapore, and Japan are Stage II and below. Over half of the breast cancers diagnosed in Singapore are Stage I.
Figure 2. Box plots of early-stage breast cancers diagnosed (Stage I only) by income groups and regions in Asia. Source of income level data: World Development Index, 2020. AFG: Afghanistan, CHN: China, IRQ: Iraq, JPN: Japan, KOR: Korea, Republic of, KWT: Kuwait, LBN: Lebanon, LKA: Sri Lanka, MYS: Malaysia, MNG: Mongolia, MMR: Myanmar, OMN: Oman, PAK: Pakistan, PHL: Philippines, QAT: Qatar, SGP: Singapore, SYR: Syrian Arab Republic, THA: Thailand, TUR: Turkey, VNM: Vietnam.
The high proportion of late-stage breast cancers at diagnosis may pose a bigger healthcare burden on low-income countries, as the cost of breast cancer treatment increases with more advanced cancers [
85]. At the individual level, more than 75% of patients die or face financial ruin within a year in southeast Asia [
86].
3.2. Early Detection as a Prerequisite to Life after Breast Cancer
Between the 1930s and 1970s, breast cancer mortality rates remained stable [
87]. Breast cancer survival improved in the 1980s in countries after the introduction of early detection programs [
88]. Common breast screening methods include breast self-examination, clinical breast examination, MRI, ultrasound, and mammography. However, the gold standard for breast screening is mammography, which is a low-dose X-ray of the breast. It is the only approach proven to effectively reduce breast cancer deaths by early detection in a population-based screening setting [
89]. A combined analysis of eight prospective randomized clinical trials showed that screening mammography produced a mortality benefit of ~22% for women aged 50 to 69 years old in populations invited to screening [
90].
3.3. Nipping Breast Cancer in the Bud
Serial mammography screening in asymptomatic women can detect breast abnormalities early before any symptoms or signs are present [
91]. Evidence from European populations shows that the number of lives saved by mammography screening is substantial [
92]. When a participation rate of 70 to 75% within the target population receives mammography, a significant reduction in breast cancer mortality at the population level can be expected after 7–10 years [
92]. In a more recent study, it is estimated that absolute benefits of 8.8 and 5.7 breast cancer deaths were avoided per 1000 women screened for 20 years, beginning at age 50, in Sweden and England respectively [
93]. At the 2018 Kyoto Breast Cancer Consensus Conference, a poll showed that ~87% of the participants agreed that screening was an effective way to reduce breast cancer mortality, and 78% were supportive of establishing systematic mammography screening programs in all developed countries [
94].
Mammography screening is often an opportunistic event in Asia, while several European countries have reported mammography participation rates of over 75% [
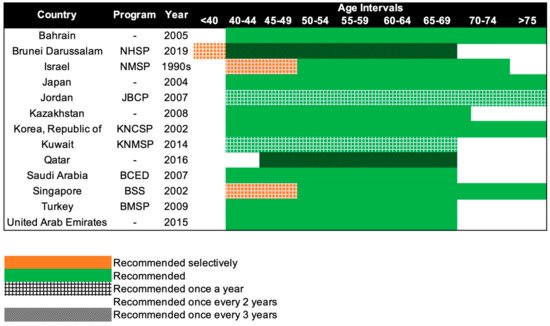
95]. Only 13 of the 47 Asian countries have organized population-based mammography screening programs (
Figure 3) [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33]. Among these countries, only Israel comes close to achieving the ideal mammography attendance rate of 70% [
23]. Despite the presence of highly subsidized, nationwide mammography screening programs established in the early 2000s in high-income Asian countries such as Korea, Japan, Taiwan, and Singapore, the uptake of screening mammography remains low. The participation rate in Korea was the highest among the countries, with organized mammography screening at 59.7% in 2015 [
96]. In 2016, only 44.9% of the target women in Japan had undergone mammography screening within the past 2 years [
31]. In Taiwan, the biennial participation rate was slightly below 40% in 2014 [
97]. In a similar time period (2015–2016), less than 40% of the target population in Singapore attended timely mammography screening [
98].
Figure 3. Recommendations of national breast cancer screening programs in Asia. NHSP: National Health Screening Program, NMSP: National Mammography Screening Program, JBCP: Jordan Breast Cancer Program, KNCSP: Korean National Cancer Screening Program, KNMSP: Kuwait National Mammography Screening Program, BCED: Breast Cancer Early Detection, BSS: BreastScreen Singapore, BMSP: Bahcesehir Mammography Screening Project.
3.4. Mammography Screening Guidelines in Asia
Beginning in the 1990s, 13 countries in Asia have progressively implemented population-based mammography screening, starting as early as the 1990s in Israel and only in 2019 in Brunei (
Figure 3). Overall, the recommendations for mammography screening are relatively similar among the 13 countries. The most common screening recommendation is biennial screening beginning from 40 years of age. Seven of the 13 countries, namely, Kazakhstan, Turkey, Bahrain, Saudi Arabia, United Arab Emirates, Japan, and South Korea (Republic of Korea), recommend this as part of their national screening program [
20,
21,
22,
27,
28,
29,
31,
32]. Singapore and Israel have similar guidelines, but the first 10 years of screening are selectively offered annually to women, only upon request or referral [
23,
33]. Kuwait and Jordan provide their women with the highest frequency of screening, with annual screening from the age of 40 years [
24,
25]. The screening interval is the longest for Brunei and Qatar, with screening recommended only every 3 years, from the age of 40 and 45 respectively [
26,
30]. Despite Brunei having the longest screening interval, it does recommend annual screening for women with high genetic risk (i.e.,
BRCA1/2 mutation carriers) starting from the age of 25 [
30].
3.5 Tailoring Screening for Asian Populations
The current standard of care for breast cancer screening provides a uniform strategy for women in the target population based only on their age, while the best recommendations for specific subgroups of high-risk women may vary [173,174,175,176]. Around half of the Asian women are diagnosed with breast cancer before they reach the typical mammography screening age of 50, implying that age limits may need to be adjusted [177]. While the evidence for mammography as a screening tool for women aged 50 and above is based on high-quality meta-analyses and systematic reviews of randomized controlled trials, the evidence for younger women is not as convincing [178]. Mammography is associated with poor diagnostic performance in younger women [91]. Furthermore, Asian women tend to have small breasts with high mammographic density, which might make early and small breast tumors difficult to detect [121]. The lower incidence of breast cancer among Asian women compared to women of European ancestry also implies that the positive predictive value of screening mammography will be lower [179].
It has been proposed that to improve the risk-benefit ratio of mammography screening, the age-based strategy should be replaced with a stratified approach (risk-based) [180,181]. A stratified approach would be to invite women to screen based on their individual risk of developing breast cancer and to give tailored recommendations [180,181].
Several efforts worldwide are underway to refine and tailor breast cancer screening based on individual risk [
188,
189]. A press release by the Government of the Hong Kong Special Administrative Region announced a stratified breast cancer screening pilot program in late 2021 [
190]. Women aged 44 to 69 who have certain combinations of individual risk factors that place them at elevated risk of breast cancer are recommended to attend mammography screening every two years, according to the latest Cancer Expert Working Group on Cancer Prevention and Screening recommendations [
191]. The breast cancer risk assessment tools developed by the University of Hong Kong can be found at the Cancer Online Resource Hub:
www.cancer.gov.hk/en/bctool (accessed on 1 July 2022) [
192,
193].
In Taiwan, general population screening was deemed not cost-effective and unnecessary, due to the low incidence rate of breast cancer [
148,
194]. Hence, a stratified approach was taken in the Keelung Community-based Integrated Screening (KCIS) to prioritize women who may benefit from mammography screening [
148]. Risk factors used in the stratification included family history of breast cancer or risk scores computed from self-reported menstrual and reproductive characteristics [
148]. Women identified to be in the high-risk group were recommended to attend a biennial mammography screening [
148]. Women not identified to be at high risk were recommended to undergo annual physical examinations [
148]. In the same study, comprising 1,429,890 asymptomatic women enrolled in three screening programs (clinical breast examination, universal mammography screening, and risk-based mammography screening), universal biennial mammography, compared to clinical breast examination, was associated with a 41% mortality reduction and a 30% reduction of breast cancers that are Stage II and above [
148]. In contrast, risk-based mammography screening was not associated with a statistically significant mortality reduction.
BREAst screening Tailored for HEr (BREATHE) is a pilot stratified mammography screening study in Singapore [
195]. The program integrates both non-genetic and genetic breast cancer risk prediction tools to personalize screening recommendations. Predictions are based on the following: (1) Gail model (non-genetic), (2) mammographic density and recall, (3) BOADICEA predictions (breast cancer predisposition genes), and (4) breast cancer polygenic risk score (PRS) [
195]. The BREATHE’s risk classification decision tree is adapted from the established WISDOM Personalized Breast Cancer Screening Trial [
188]. WISDOM uses a five-year absolute risk threshold of 6% (risk of an average BRCA carrier) for stratification based on genetic risk factors [
188]. However, confirmatory clinical genetic testing was not performed in BREATHE. Based only on predicted genetic risks, BREATHE is testing lower five-year absolute risk thresholds for disease stratification (~3%).
4. Conclusion
Breast cancer is a growing public health problem in most parts of Asia. Despite the establishment of screening guidelines globally, Asia has been slow to adopt breast cancer screening. High-income countries are not benefiting fully from national breast screening programs due to an underutilization of the preventive healthcare services available. On the other hand, LMICs are unable to adopt screening programs implemented in high-income countries, due to resource constraints. The full potential of mammography screening cannot be achieved, as there is still room for improvements in the procedure (e.g., reducing overdiagnosis and increasing screening sensitivity for dense breasts). These gaps may be filled by incorporating stratified screening, with the use of both genetic and non-genetic risk factors. However, while studies are underway to evaluate the use of these risk factors to refine individual breast cancer risk in healthy populations, questions regarding appropriate risk thresholds to define above-average risk, type of personalized screening recommendations offered, and implementation challenges, among others, remain to be answered before the verdict is out on the utility of risk-based screening. Ultimately, it is important to note that mammography screening is an imperfect test that is associated with limitations and biases, and these may undermine real survival benefits. It is important to weigh the hazards of screening against the risks of not screening.