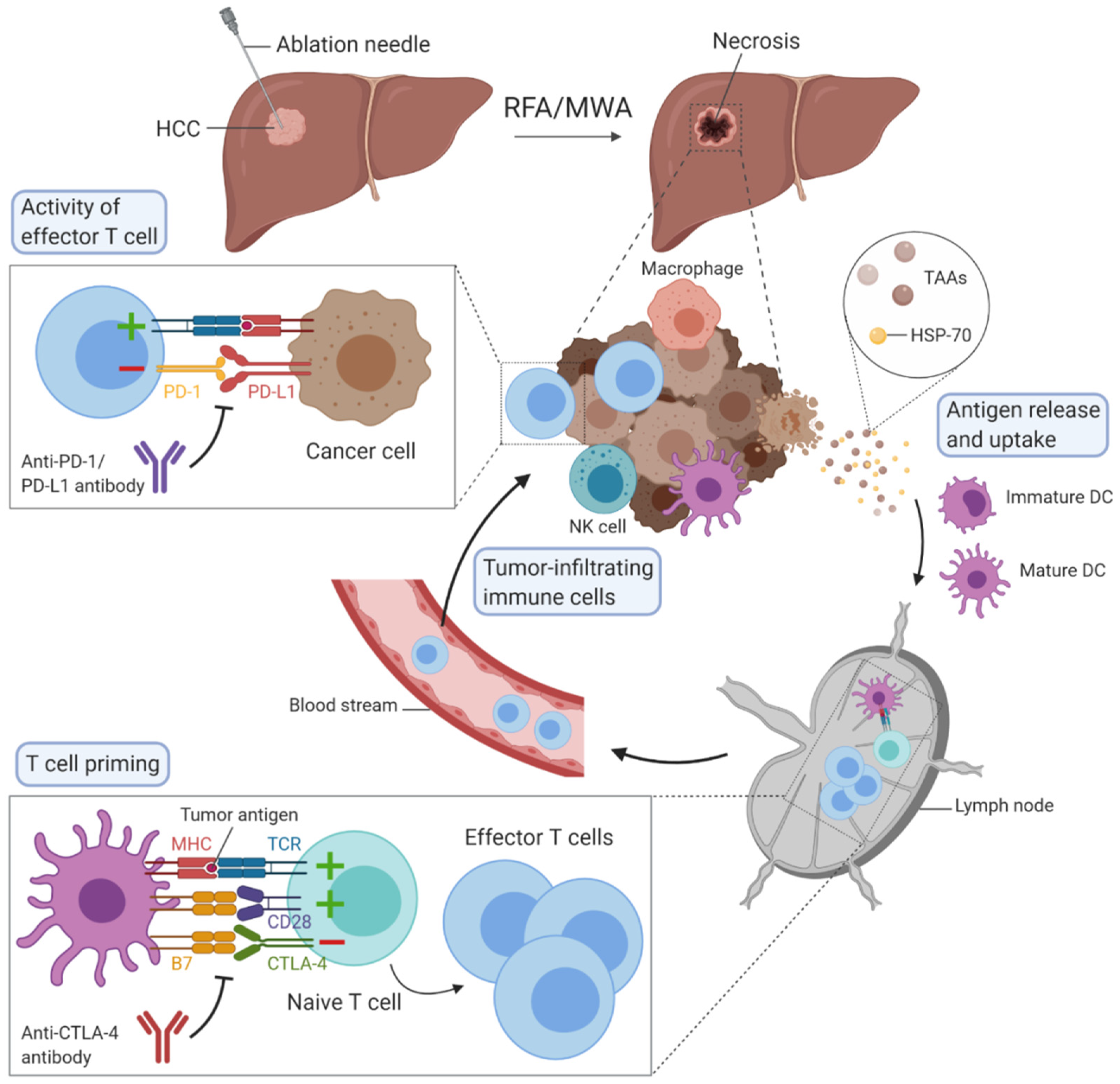
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related deaths worldwide and its incidence is rising. Percutaneous locoregional therapies, such as radiofrequency ablation and microwave ablation, are widely used as curative treatment options for patients with small HCC, but their effectiveness remains restricted because of the associated high rate of recurrence, occurring in about 70% of patients at five years. These thermal ablation techniques have the particularity to induce immunomodulation by destroying tumours, although this is not sufficient to raise an effective antitumour immune response. Ablative therapies combined with immunotherapies could act synergistically to enhance antitumour immunity.
1. Thermal Ablation Techniques
RFA and MWA are both heat-based percutaneous ablation techniques used to treat small liver tumours
[1]. RFA is the most validated technique and the most commonly employed in early stage disease for tumours smaller than 3 cm in diameter. Radiofrequency waves are supplied by an electrode in a needle inserted through the skin at the tumour site under imaging guidance
[2]. An electrical circuit is created and completed through grounding pads attached to the thighs or back of the patient. A continuous alternative current generates heat that increases the temperature in the tissue (between 60 and 100 °C), leading to tumour cell death by coagulation necrosis around the electrode
[3]. The larger proportion of the final ablation zone is attributed to thermal conduction into more peripheral areas around the electrode. Tissue boiling and charring act as electrical insulators and limit the effect of RFA through increased impedance; hence, the important tissue properties for RFA are electrical and thermal conductivities. Since the first experimental hepatic RFA performed in 1990
[4], there has been extensive work done on RFA of liver tumours.
More recently, MWA has gained attention. It delivers a microwave oscillating electric field through a needle that greatly increases the temperature (more than 100 °C) in the targeted tissue, inducing coagulative necrosis that results in tumour cell death
[3]. This method is faster than RFA and seems to be more suited to treating larger tumours as it has the ability to achieve better heating of greater tumour volumes, although no significant difference in the efficacy of these methods was reported
[5]. MWA was first introduced in 1994
[6] and since that time—as a result of several significant improvements in the clinical application and advancements in the technology—has been increasingly used.
Cryoablation is another thermal percutaneous ablation technique that uses freezing for tumour cell destruction. Cryoablation can be considered an old technique; the first use of cold to destroy tumour tissue is credited to James Arnott (1797–1883), an English physician, who successfully used cold temperatures created by salt and ice solutions. Today, liquid gas—such as argon or nitrogen—is delivered to the tumour tissue under imaging guidance through a cryoprobe to decrease the temperature by the Thomson effect. In fact, these gases cool as they expand, generating local tissue freezing and vascular injury
[1]. Several cycles of freezing followed by thawing are usually performed and the effect of cryoablation depends on the variables such as the number of cycles, rate of cooling, duration of the minimum temperature, the rate of thawing etc. Cryoablation results in necrotic cell death in a small radius around the probe. However, in the peripheral cryoablation zones, hypothermia is not directly lethal for cells but induces cell injury followed by delayed apoptosis
[7].
2. Immunomodulation Mediated by Ablation Techniques
Percutaneous thermal ablation techniques induce immunomodulation in patients with HCC. Indeed, the heat delivered to cancer tissues provokes cell death by a process called necrosis in cases of RFA and MWA or by necrosis and apoptosis in the case of cryoablation.
Cell necrosis results in an uncontrolled release of cellular components into the extracellular space which initiates an inflammatory response. Initially, innate immune cells rapidly react and infiltrate into the tissue site, followed by activation of adaptive immune cells
[8][9]. This process also triggers an antitumour response, since these tumour-specific antigens become accessible to whole immune system components (
Figure 1). The phenomenon, whereby a locally applied therapy triggers a distant antitumour response, is well known in radiation therapy as the abscopal effect
[10]. For conventional surgical resections, there is no such phenomenon as there is direct tumour removal instead of local destruction
[9].
Figure 1. Overview of the possible mechanisms of immunomodulation induced by radiofrequency ablation (RFA) or microwave ablation (MWA) in hepatocellular carcinoma. The figure was created with BioRender.
Cell apoptosis, which occurs during cryoablation, is described as an active, programmed process of autonomous cellular death that induces immunosuppression rather than antitumour response stimulation.
3. Immunotherapy in HCC
Immune checkpoint inhibitors constitute the most promising treatment for HCC in the future, especially PD-1 and PD-L1 inhibitors
[11][12]. The goal of this immune checkpoint is to prevent the overstimulation of immune response. PD-1 receptor is expressed on many immune cells such as T cells and especially CD8+ T cells, while its ligand can be found mainly on cancer cells’ and antigen-presenting cells’ surfaces. When PD-1 is engaged with its ligand, effector T cell-mediated immune responses are impaired. Antibodies blocking PD-1 have been developed including nivolumab and pembrolizumab, while durvalumab, avelumab and atezolizumab target PD-L1. Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) is another immune checkpoint expressed on regulatory T cells and activated T cells. This receptor inhibits T cell activation by recognising the ligand B7 on DCs with a higher affinity than the co-stimulatory molecule receptor CD28. Tremelimumab and ipilimumab are antibodies against CTLA-4. T cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene 3 (LAG-3), T cell immunoreceptor with Ig and ITIM domains (TIGIT) or V-domain Ig Suppressor of T cell Activation (VISTA) are other immune checkpoints, with ongoing clinical trials to demonstrate their clinical efficacy and outcomes in patients with HCC
[12][13]. Co-expression of multiple immune checkpoints has been associated with a severely exhausted T cell state, typical for CD8+ T cells in the tumour microenvironment. Blocking of one immune checkpoint pathway—for example, PD-1/PD-L1—often results in compensatory upregulation of additional immune checkpoint molecules, which limits the efficacy of monotherapy approaches
[14][15]. In order to enhance antitumour efficacy and clinical success, future therapeutic strategies require combinations between immune checkpoint inhibitors or between checkpoint inhibitors and other therapeutic strategies. The best example today is the combination of PD-L1 and vascular endothelial growth factor (VEGF) antibodies, particularly the combination of atezolizumab and bevacizumab, which represents a significant breakthrough in the first-line treatment of unresectable HCC
[12][16][17].
4. Combination of Ablation Techniques with Immunotherapy in HCC
All the observations mentioned above show that thermal ablation techniques can induce immunomodulation, as summarised in
Table 1. However, this effect is insufficient to prevent tumour recurrence in patients. These studies point out the advantages of combining RFA or MWA with immunotherapy to boost the antitumour immune response and thereby prevent recurrence following ablation and improve beneficial outcomes
[18]. The combination of thermal ablation therapies with cellular immunotherapy has raised interest due to its potential to improve the strength of tumour-specific immunity. Cui et al. used the combination of RFA and cellular immunotherapy based on immune cells, comprising cytotoxic T cells, NKT, NK and γδT cells that were harvested in the form of peripheral blood mononuclear cells (PBMCs) from patients with HCC before RFA, expanded and then, injected back intravenously after RFA. Authors demonstrated that the risk of tumour recurrence was significantly reduced compared to patients treated with RFA alone
[19]. Recently, the therapeutic efficacy of irreversible electroporation in combination with the intratumoural injection of the immunogenic adjuvant was successfully tested in an animal model of HCC
[20].
Table 1. Summary of principal human studies focused on RFA-, MWA- or cryoablation-induced immunomodulation in hepatocellular carcinoma.
The combination of MWA and cellular immunotherapy was assessed in a phase I clinical trial, with immune cells collected from the peripheral blood of HCC patients. Immature DCs and effector cells were injected into the marginal area of ablated tumours under contrast-enhanced sonographic guidance. The first observations were encouraging, as a depletion of regulatory T cells occurred and was associated with an increase in effector CD8+ CD28- T cells one month after the RFA treatment, even though this difference was no longer significant after six months
[28].
The combination of MWA and cellular immunotherapy was assessed in a phase I clinical trial, with immune cells collected from the peripheral blood of HCC patients. Immature DCs and effector cells were injected into the marginal area of ablated tumours under contrast-enhanced sonographic guidance. The first observations were encouraging, as a depletion of regulatory T cells occurred and was associated with an increase in effector CD8+ CD28- T cells one month after the RFA treatment, even though this difference was no longer significant after six months
[28].
Similarly, adoptive transfer of co-cultured DCs and effector cells was tested in combination with cryoablation in patients with metastatic HCC. Patients who received cryoablation with cellular immunotherapy exhibited improved survival outcomes in comparison to patients who received only single treatment
[29].
A more recent promising approach for the treatment of HCC was the combination of heat-based ablation therapies with immune checkpoint inhibitors. The use of a CTLA-4 inhibitor, tremelimumab, with RFA was proved as safe and without dose-limiting toxicity. Tumour biopsies revealed that PD-1 expression was increased on the T cell surfaces and a higher number of tumour infiltrating T cells was observed following the combination treatment, especially CD8+ T cells
[30]. On the contrary, incomplete percutaneous ablation may worsen the prognosis and induce immunotherapy resistance, as reported in a mouse model of metastatic colorectal cancer
[31].
Nonetheless, many questions remain unanswered as to which ablation technique is the most efficient in inducing immunomodulation, what is the best time to start immunotherapy and which type of immunotherapy is the most promising for this strategy. In addition, local ablation can be used as a treatment during the waiting time prior to liver transplantation. In such cases, the combination with immunotherapy may not be recommended due to fear of possible future liver rejection. Moreover, it should be noted that some patients may experience serious, immunotherapy-related adverse events and a careful selection of patients for combination of ablation techniques with immunotherapy will be therefore required.
Today, several ongoing clinical studies are evaluating the combination of ablation techniques and immunotherapy in HCC patients, or the use of, as summarised in Table 2. The efficacy and safety results are eagerly awaited.
Table 2. Summary of principal clinical studies evaluating efficacy of RFA or MWA in combination with immunotherapy.
| Clinical Trials (Identifier) |
Phase |
Intervention/Treatment |
Number of Participants |
Estimated Study Completion Date |
| LKSM001 (NCT03674073) |
Phase 1 |
Personalized neoantigen-based DC vaccine in combination with MWA |
24 |
December 2020 |
| RI11330 (NCT03864211) |
Phase 1/2 |
Thermal ablation, RFA or MWA, followed by Toripalimab |
120 |
March 2021 |
| ZS-IR-2019B (NCT04220944) |
Phase 1 |
MWA in combination with simultaneous TACE plus Sintilimab |
45 |
September 2021 |
| 160135 (NCT02821754) |
Phase 2 |
Combination of tremelimumab and durvalumab with ablative therapies, TACE, RFA or cryoablation |
90 |
December 2021 |
| HCC 004 (NCT02678013) |
Phase 3 |
RFA combined with highly-purified CTLs |
210 |
January 2022 |
| IMMULAB (NCT03753659) |
Phase 2 |
Pembrolizumab in combination with local ablation via RFA or MWA |
30 |
September 2022 |
| EMERALD-2 (NCT03847428) |
Phase 3 |
Durvalumab in combination with bevacizumab or durvalumab alone in patients with hepatocellular carcinoma who are at high risk of recurrence after surgical resection or ablation |
888 |
June 2023 |
| S2019-128-02 (NCT04204577) |
Phase 2 |
Thermal ablation combined with Apatinib and Carilimub |
90 |
November 2023 |
| MK-3475-937/KEYNOTE-937 (NCT03867084) |
Phase 3 |
Pembrolizumab in comparison with placebo in HCC patients with complete radiological response after surgical resection or ablation |
950 |
June 2025 |
| CheckMate 9DX (NCT03383458) |
Phase 3 |
Nivolumab in comparison with placebo in HCC patients at high risk of recurrence after surgical resection or ablation |
530 |
June 2025 |
| 1102320191018 (NCT04150744) |
Phase 2 |
RFA combined with Carrizumab and Apatinib |
120 |
December 2026 |
| IMbrave050 (NCT04102098) |
Phase 3 |
Atezolizumab plus bevacizumab in comparison with active surveillance in HCC patients at high risk of recurrence after surgical resection or ablation |
662 |
July 2027 |