Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related deaths worldwide and its incidence is rising. Percutaneous locoregional therapies, such as radiofrequency ablation and microwave ablation, are widely used as curative treatment options for patients with small HCC, but their effectiveness remains restricted because of the associated high rate of recurrence, occurring in about 70% of patients at five years. These thermal ablation techniques have the particularity to induce immunomodulation by destroying tumours, although this is not sufficient to raise an effective antitumour immune response. Ablative therapies combined with immunotherapies could act synergistically to enhance antitumour immunity.
- liver
- HCC
- ablation
- RFA
- MWA
- immunomodulation
1. Thermal Ablation Techniques
2. Immunomodulation Mediated by Ablation Techniques
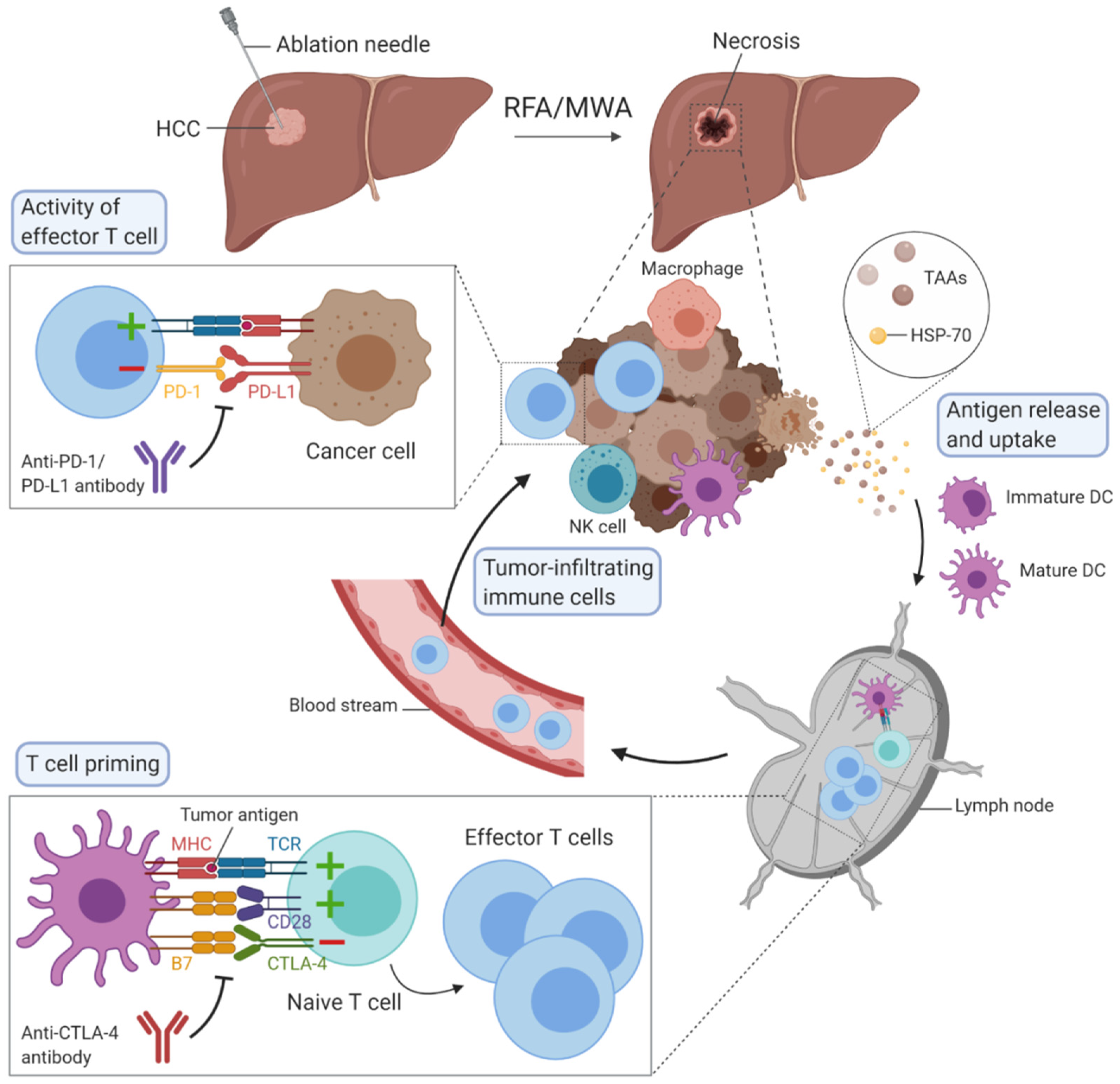
3. Immunotherapy in HCC
4. Combination of Ablation Techniques with Immunotherapy in HCC
| First Author Name et al. | Year | Ablation Technique | Number of Subjects (n) | Immunomodulation Observed |
|---|---|---|---|---|
| Haen et al. [21] | 2011 | RFA | 4 | Increase in serum levels of HSP-70 |
| Nobuoka et al. [22] | 2012 | RFA | 9 | Increase in circulating glypican-3-specific cytotoxic T cells |
| Mizukoshi et al. [23] | 2013 | RFA | 69 | Increase in the number of circulating TAA-specific T cells, mainly the central memory phenotype (CD45RA-/CCR7+) |
| Huang et al. [9] | 2019 | RFA | 6 | Decrease in circulating regulatory T cells; Increase in circulating CD8+ T cells and CD4+/CD45RO+ memory T cells; Decrease in TGF-β, IL-10; Increase in IFN-γ |
| Rochigneux et al. [24] | 2019 | RFA | 80 | Modifications of NKp30+ NK cells and plasmacytoid DC |
| Dong et al. [25] | 2003 | MWA | 82 | Increase in tumour-infiltrating NK cells, macrophages and T cells |
| Zhang et al. [26] | 2017 | MWA | 45 | Increase in circulating CD3+ cells and CD4+ cells; Increase in IL-12; Decrease in IL-4 and IL-10 |
| Zhou et al. [27] | 2010 | Cryoablation | 111 | Association of circulating regulatory T cells with tumour regression or progression Decrease in CD8+, CD4+, and FoxP3+ cells around the cryoablation zones. |
| Clinical Trials (Identifier) | Phase | Intervention/Treatment | Number of Participants | Estimated Study Completion Date |
|---|---|---|---|---|
| LKSM001 (NCT03674073) | Phase 1 | Personalized neoantigen-based DC vaccine in combination with MWA | 24 | December 2020 |
| RI11330 (NCT03864211) | Phase 1/2 | Thermal ablation, RFA or MWA, followed by Toripalimab | 120 | March 2021 |
| ZS-IR-2019B (NCT04220944) | Phase 1 | MWA in combination with simultaneous TACE plus Sintilimab | 45 | September 2021 |
| 160135 (NCT02821754) | Phase 2 | Combination of tremelimumab and durvalumab with ablative therapies, TACE, RFA or cryoablation | 90 | December 2021 |
| HCC 004 (NCT02678013) | Phase 3 | RFA combined with highly-purified CTLs | 210 | January 2022 |
| IMMULAB (NCT03753659) | Phase 2 | Pembrolizumab in combination with local ablation via RFA or MWA | 30 | September 2022 |
| EMERALD-2 (NCT03847428) | Phase 3 | Durvalumab in combination with bevacizumab or durvalumab alone in patients with hepatocellular carcinoma who are at high risk of recurrence after surgical resection or ablation | 888 | June 2023 |
| S2019-128-02 (NCT04204577) | Phase 2 | Thermal ablation combined with Apatinib and Carilimub | 90 | November 2023 |
| MK-3475-937/KEYNOTE-937 (NCT03867084) | Phase 3 | Pembrolizumab in comparison with placebo in HCC patients with complete radiological response after surgical resection or ablation | 950 | June 2025 |
| CheckMate 9DX (NCT03383458) | Phase 3 | Nivolumab in comparison with placebo in HCC patients at high risk of recurrence after surgical resection or ablation | 530 | June 2025 |
| 1102320191018 (NCT04150744) | Phase 2 | RFA combined with Carrizumab and Apatinib | 120 | December 2026 |
| IMbrave050 (NCT04102098) | Phase 3 | Atezolizumab plus bevacizumab in comparison with active surveillance in HCC patients at high risk of recurrence after surgical resection or ablation | 662 | July 2027 |
This entry is adapted from the peer-reviewed paper 10.3390/ijms21124398
References
- Nault, J.C.; Sutter, O.; Nahon, P.; Ganne-Carrié, N.; Séror, O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J. Hepatol. 2018, 68, 783–797.
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1054–1063.
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208.
- McGahan, J.P.; Browning, P.D.; Brock, J.M.; Tesluk, H. Hepatic ablation using radiofrequency electrocautery. Investig. Radiol. 1990, 25, 267–270.
- Glassberg, M.B.; Ghosh, S.; Clymer, J.W.; Qadeer, R.A.; Ferko, N.C.; Sadeghirad, B.; Wright, G.W.; Amaral, J.F. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. OncoTargets Ther. 2019, 12, 6407–6438.
- Seki, T.; Wakabayashi, M.; Nakagawa, T.; Itho, T.; Shiro, T.; Kunieda, K.; Sato, M.; Uchiyama, S.; Inoue, K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994, 74, 817–825.
- Mehta, A.; Oklu, R.; Sheth, R.A. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol. Res. Pract. 2016, 2016.
- Li, L.; Wang, W.; Pan, H.; Ma, G.; Shi, X.; Xie, H.; Liu, X.; Ding, Q.; Zhou, W.; Wang, S. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J. Transl. Med. 2017, 15, 23.
- Huang, K.W.; Jayant, K.; Lee, P.H.; Yang, P.C.; Hsiao, C.Y.; Habib, N.; Sodergren, M.H. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 385.
- Ng, J.; Dai, T. Radiation therapy and the abscopal effect: A concept comes of age. Ann. Transl. Med. 2016, 4, 118.
- Macek Jilkova, Z.; Aspord, C.; Decaens, T. Predictive Factors for Response to PD-1/PD-L1 Checkpoint Inhibition in the Field of Hepatocellular Carcinoma: Current Status and Challenges. Cancers 2019, 11, 1554.
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R.; et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 2020, 39, 3620–3637.
- Vivas, I.; Iribarren, K.; Lozano, T.; Cano, D.; Lasarte-Cia, A.; Chocarro, S.; Gorraiz, M.; Sarobe, P.; Hervás-Stubbs, S.; Bilbao, J.I.; et al. Therapeutic Effect of Irreversible Electroporation in Combination with Poly-ICLC Adjuvant in Preclinical Models of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. JVIR 2019, 30, 1098–1105.
- Johnston, M.P.; Khakoo, S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. WJG 2019, 25, 2977–2989.
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086.
- Macek Jilkova, Z.; Aspord, C.; Kurma, K.; Granon, A.; Sengel, C.; Sturm, N.; Marche, P.N.; Decaens, T. Immunologic Features of Patients With Advanced Hepatocellular Carcinoma Before and During Sorafenib or Anti-programmed Death-1/Programmed Death-L1 Treatment. Clin. Transl. Gastroenterol. 2019.
- Kudo, M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers 2020, 12, 1089.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Greten, T.F.; Mauda-Havakuk, M.; Heinrich, B.; Korangy, F.; Wood, B.J. Combined locoregional-immunotherapy for liver cancer. J. Hepatol. 2019, 70, 999–1007.
- Cui, J.; Wang, N.; Zhao, H.; Jin, H.; Wang, G.; Niu, C.; Terunuma, H.; He, H.; Li, W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int. J. Cancer 2014, 134, 342–351.
- Haen, S.P.; Gouttefangeas, C.; Schmidt, D.; Boss, A.; Clasen, S.; von Herbay, A.; Kosan, B.; Aebert, H.; Pereira, P.L.; Rammensee, H.G. Elevated serum levels of heat shock protein 70 can be detected after radiofrequency ablation. Cell Stress Chaperones 2011, 16, 495–504.
- Nobuoka, D.; Motomura, Y.; Shirakawa, H.; Yoshikawa, T.; Kuronuma, T.; Takahashi, M.; Nakachi, K.; Ishii, H.; Furuse, J.; Gotohda, N.; et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2012, 40, 63–70.
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457.
- Rochigneux, P.; Nault, J.C.; Mallet, F.; Chretien, A.S.; Barget, N.; Garcia, A.J.; Del Pozo, L.; Bourcier, V.; Blaise, L.; Grando-Lemaire, V.; et al. Dynamic of systemic immunity and its impact on tumor recurrence after radiofrequency ablation of hepatocellular carcinoma. Oncoimmunology 2019, 8.
- Dong, B.W.; Zhang, J.; Liang, P.; Yu, X.L.; Su, L.; Yu, D.J.; Ji, X.L.; Yu, G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int. J. Hyperth. Off. J. Eur. Soc. Hyperth. Oncol. North 2003, 19, 119–133.
- Zhang, H.; Hou, X.; Cai, H.; Zhuang, X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim. Invasive Ther. Allied Technol. MITAT Off. J. Soc. Minim. Invasive Ther. 2017, 26, 207–211.
- Zhou, L.; Fu, J.L.; Lu, Y.Y.; Fu, B.Y.; Wang, C.P.; An, L.J.; Wang, X.Z.; Zeng, Z.; Zhou, C.B.; Yang, Y.P.; et al. cells are associated with post-cryoablation prognosis in patients with hepatitis B virus-related hepatocellular carcinoma. J. Gastroenterol. 2010, 45, 968–978.
- Zhou, P.; Liang, P.; Dong, B.; Yu, X.; Han, Z.; Xu, Y. Phase clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol. Ther. 2011, 11, 450–456.
- Niu, L.Z.; Li, J.L.; Zeng, J.Y.; Mu, F.; Liao, M.T.; Yao, F.; Li, L.; Liu, C.Y.; Chen, J.B.; Zuo, J.S.; et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J. Gastroenterol. WJG 2013, 19, 3473–3480.
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551.
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 2019, 10, 1–13.
