Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaoding Lou | -- | 1779 | 2022-09-06 07:38:42 | | | |
| 2 | Camila Xu | -2 word(s) | 1777 | 2022-09-06 08:57:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, B.; Yuan, H.; Zhang, W.; Hu, J.; Lou, X.; Xia, F. Cell Membrane-Targeted Bioprobes for the Imaging of Organelles. Encyclopedia. Available online: https://encyclopedia.pub/entry/26904 (accessed on 07 February 2026).
Chen B, Yuan H, Zhang W, Hu J, Lou X, Xia F. Cell Membrane-Targeted Bioprobes for the Imaging of Organelles. Encyclopedia. Available at: https://encyclopedia.pub/entry/26904. Accessed February 07, 2026.
Chen, Bochao, Haotong Yuan, Wei Zhang, Jingjing Hu, Xiaoding Lou, Fan Xia. "Cell Membrane-Targeted Bioprobes for the Imaging of Organelles" Encyclopedia, https://encyclopedia.pub/entry/26904 (accessed February 07, 2026).
Chen, B., Yuan, H., Zhang, W., Hu, J., Lou, X., & Xia, F. (2022, September 06). Cell Membrane-Targeted Bioprobes for the Imaging of Organelles. In Encyclopedia. https://encyclopedia.pub/entry/26904
Chen, Bochao, et al. "Cell Membrane-Targeted Bioprobes for the Imaging of Organelles." Encyclopedia. Web. 06 September, 2022.
Copy Citation
Organelles are important subsystems of cells. The damage and inactivation of organelles are closely related to the occurrence of diseases. Organelles’ functional activity can be observed by fluorescence molecular tools. Nowadays, a series of aggregation-induced emission (AIE) bioprobes with organelles-targeting ability have emerged, showing great potential in visualizing the interactions between probes and different organelles.
organelles
peptide
fluorescence imaging
aggregation-induced emission
1. Introduction
One of the reasons why eukaryotes are more complicated than prokaryotes is that eukaryotes contain many independent inner membrane systems, which are called organelles or subcellular compartments [1][2]. Each organelle has unique structural characteristics and plays a different role in various physiological processes. They maintain cell biochemical reactions efficiently, diversely and stably. The organelles of eukaryotes contain a cell membrane, mitochondria, a nucleus, lysosomes, an endoplasmic reticulum, a Golgi apparatus, lipid droplets, ribosomes, etc. [3]. These organelles can not only work independently but also cooperate with each other. On one hand, working independently could guarantee orderly physiological activities. For instance, mitochondria are mostly responsible for producing energy, controlling the apoptosis progress and regulating intracellular calcium and reactive oxygen species (ROS) [4][5][6]. Lysosomes contain many hydrolases that can hydrolyze multiple waste or abnormal proteins [7][8]. On the other hand, complicated physiological activities require the cooperation of multiple organelles, such as the synthesis of proteins. At first, proteins need to be transcribed in the nucleus and translated in ribosomes. Subsequently, they are modified and packaged by the Golgi apparatus to obtain the active proteins with intended functions. In this case, dysfunction of organelles was able to cause various diseases such as cancer, metabolic disorders, cardiovascular and neurodegenerative diseases and so on [9][10][11][12][13][14]. For example, when mitochondria are damaged, cytochrome c and mitochondria outer membrane proteins are released into the cytoplasm and interact with apoptotic protease activator 1, which leads to the activation and recruitment of the caspase family, ultimately resulting in the apoptosis of cells [15][16][17]. Thus, it is very important to image and detect these organelles. It helps to study the different physiological and biochemical processes of cells, understand the operation mechanism of the life system and establish the foundation for biomedical research.
Fluorescence imaging technology is widely used in various fields due to its non-invasiveness, high sensitivity and high temporal spatial resolution [18][19][20]. Aggregation-induced emission (AIE) is a phenomenon by which luminogen does not emit fluorescence in a dilute solution but emits strong fluorescence in a high concentration or solid state [21][22][23][24]. AIE luminogens (AIEgens) generally have a molecular rotor structure. In the free state, the molecular rotor can rotate freely and activate non-radiative transitions to consume excitation energy. In the aggregated state, molecular rotor movement is restricted, and excitation energy is mainly released through radiative transitions, which results in the bright and stable fluorescence emission [25][26][27]. In recent years, due to their outstanding properties, such as a high quantum yield, a resistance to photobleaching, a large Stokes shift and photosensitivity, AIEgens have been widely used in biomarker detection and imaging [28][29], drug delivery [30][31], surgery navigation [32][33], anti-bacteria processes [34], phototherapy [35][36][37][38][39][40] and a series of biomedical application areas.
AIEgens can be conjugated with biological macromolecules (peptides, polysaccharides, nucleic acids, proteins, etc.) through covalent bonds [22][41][42]. Among them, peptides are widely developed in the biomedical field. When peptides bind to AIEgens, the addition of peptides restricts the rotation of AIEgens, which leads to AIEgens emitting fluorescence [27]. AIEgens have the advantages of photobleaching resistance, photosensitivity and a high quantum yield. At the same time, AIEgens are generally lipid-soluble and more hydrophobic and enter the cell difficultly. The introduction of peptides can improve the hydrophilicity of AIEgens and make them have good biocompatibility, which is conducive to the application of bioprobes in the biomedical field. In addition, the introduction of peptides can also give AIEgens various functions, such as the specific recognition function, toxic function, targeting function, etc. Simultaneously, the immunogenicity of bioprobes can be reduced. Therefore, the design of biomacromolecule-functionalized AIEgens can be used as a practical tool to obtain complex information at the biological level, analyze biomacromolecule interactions and understand disease mechanisms. At the same time, peptides are unstable and easily degraded by bioactive substances. Researchers hope that obstacles can be overcome in future research [43][44][45][46].
There are various strategies for designing peptide-targeting organelles. Among them, the strategy for the design of cell membrane-targeted peptides is the electrostatic interaction of arginine rich in positive charge with phosphate groups on the cell membrane. In addition, the cell membrane can be targeted by peptides that can bind to cell membrane receptors. The nuclear localization peptide (NLS) peptides could bind to the nucleoprotein. The peptides targeting the mitochondria are usually liposoluble cationic peptides because mitochondria have negative membrane potential and a hydrophobic environment. The lysosomal-targeted peptides are generally reactive under acidic conditions and can bind to lysosomal proteins. ER-targeted peptides could bind to the KDEL receptor (Figure 1). According to different organelles, this research reviews the recent progress of AIEgen-peptide bioprobes in organelles imaging. The structural characteristics and application strategies of AIEgen-peptide bioprobes in different organelles are summarized.
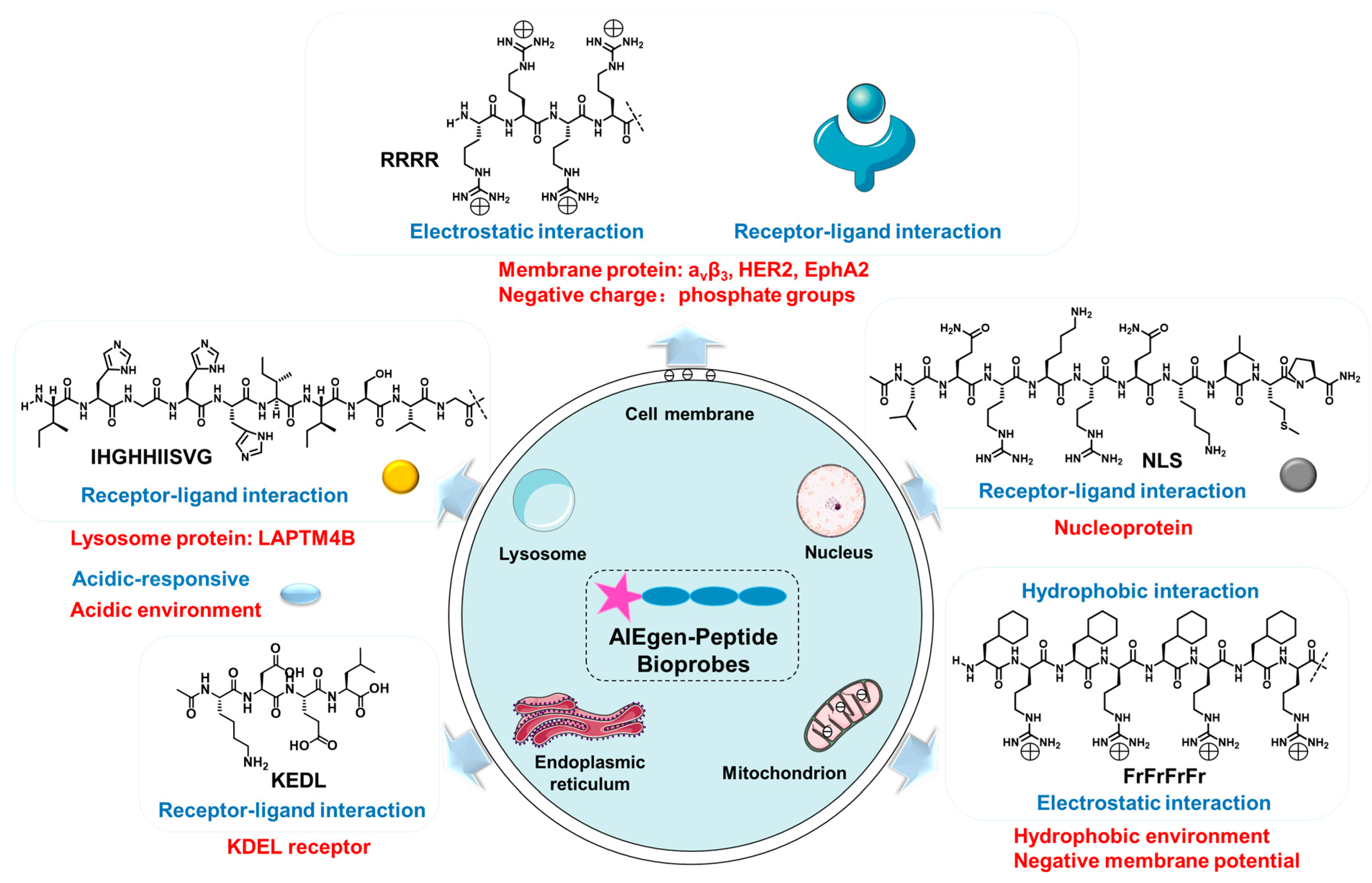
Figure 1. The mechanism of bioprobes-targeting organelles.
2. Cell Membrane-Targeted Bioprobes
The cell membrane is composed of an amphiphilic phospholipid bilayer with a large negative potential. It plays an important role in maintaining homeostasis, controlling substance transport and regulating signal transduction [16][17][47]. At present, cell membrane-targeting fluorescent probes usually consist of a fluorophore and an anchoring element [15]. The cell membrane-anchoring element is generally the substance including: penetrating peptides [48], alkyl chains [49], cholesterol [50], protein ligands [51], antibodies, aptamers [52], etc. Protein ligands can be utilized to target cell membrane receptors; for example, a peptide sequence RGD often acts as a targeted peptide of the integrin receptor in the cell membrane. As shown in Figure 2 compound (1), Liu et al. reported a new bioprobe TPS-2cRGD by integrating an AIEgen (TPS) with cyclic arginine-glycine-aspartic tripeptide (cRGD), a targeting ligand to the cell membrane integrin αvβ3 receptor [53]. The excitation wavelength of the bioprobe is 356 nm, and the maximum emission wavelength is 480 nm. The bioprobe overlapped well with commercial cell membrane dyes and was used to track αvβ3-positive cancer cells. The negative membrane potential and lipid solubility of the phospholipid bilayer lead to the enrichment of lipophilic and cations molecules in the cell membrane. Because of the abundant positive charge, cell-penetrating peptide RRRR was usually used to improve the targeting ability of the cell membrane. At the same time, palmitic acid was usually used to target the cell membrane due to its lipid solubility. Liang et al. reported an AIEgen-peptide bioprobe TR4 [54]. As shown in Figure 2 compound (2), the bioprobe consisted of three elements: palmitic acid, cell-penetrating peptide (RRRR) and tetraphenylethylene (TPE). The excitation wavelength of the bioprobe is 330 nm, and the maximum emission wavelength is 466 nm. When MCF-7 cells were incubated with TR4, the cell membrane was labeled. TR4 showed good photostability and biocompatibility and low toxicity. It opened the door of the TR4 in cell membrane imaging. To further improve cell membrane-targeting ability, researchers' group combined RRRR, RGD and palmitic acid to AIEgen to track the imaging of the cell membrane [55]. As shown in Figure 2 compound (3), RTP (λex: 330 nm, λem: 500 nm) consisted of three elements: a RGD-targeted peptide, a palmitic acid-modified hydrophilic peptide (Pal-RRRR) and AIEgen (T-MY). The palmitic acid had a cell membrane-targeting function and was inserted into the cell membrane through hydrophobic interactions. A RGD-targeted peptide was bound to integrin receptors on the cell membrane. A hydrophilic peptide (RRRR) was bound to the cell membrane through electrostatic interactions. Under the comprehensive combination of RGD and Pal-RRRR, RTP successfully achieved the imaging of the cell membrane precisely and robustly. Simultaneously, RTP showed durable stability and a strong resistance to photobleaching.
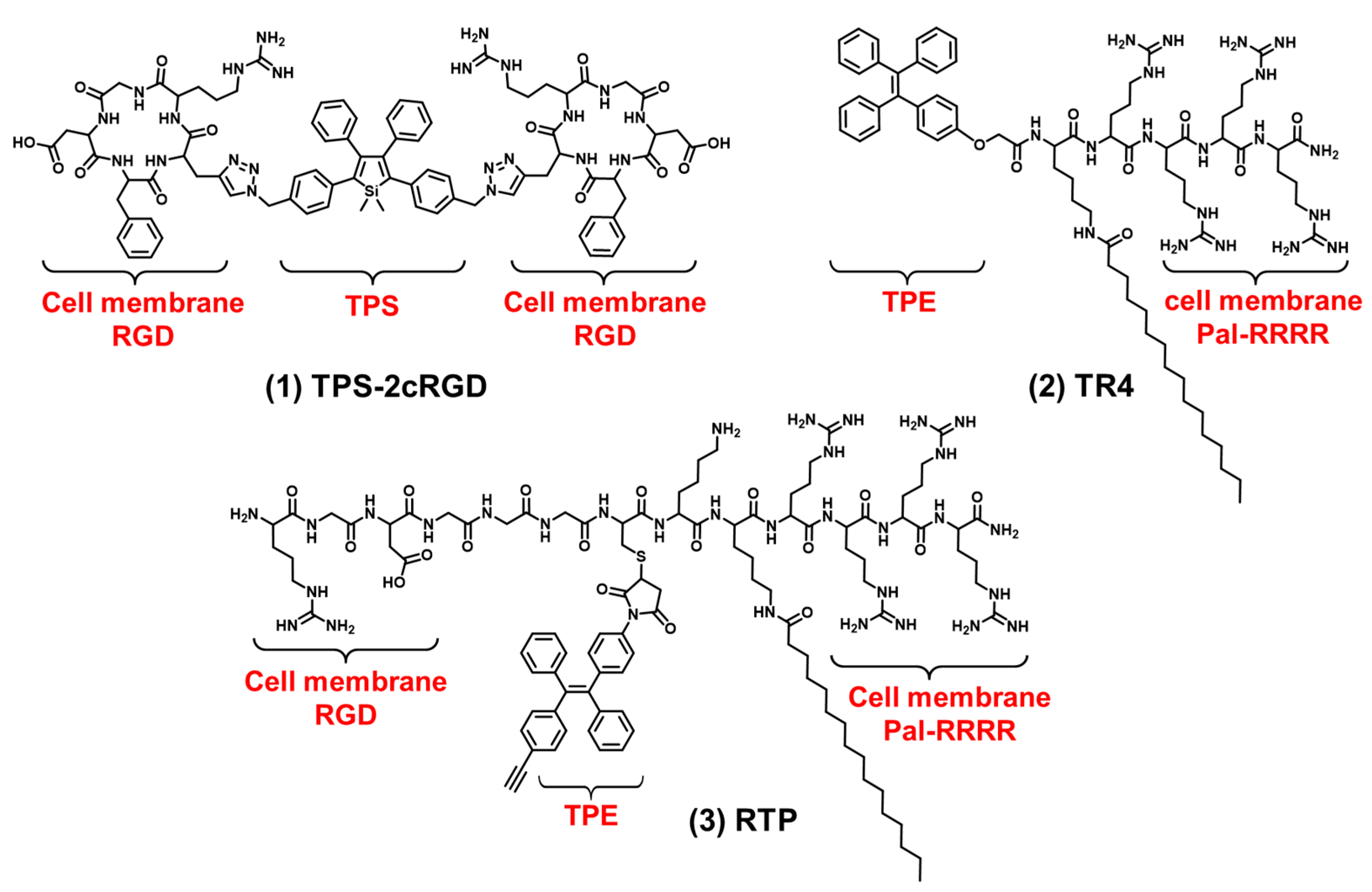
Figure 2. The chemical structures of cell membrane-targeting bioprobes.
In addition to the integrin receptors overexpressed in most cancer cells, some cancer cells also have specific receptors. For example, human epidermal growth factor receptor 2 (HER2) is overexpressed in breast cancers. HER2 is a tyrosine receptor kinase that can induce dimer formation with itself and then lead to downstream signal activation. Wang et al. designed a AIEgen-peptide bioprobe (TPM) that targeted the HER2 receptor on the cell membrane [56]. As shown in Figure 3 compound (4), the bis-pyrene (BP) element with the AIE property for fluorescence reporting, the peptide (YCDGFYACYMDV) bound to the HER2 receptor on the surface of cell membrane and the peptide (KLVFF) was used as a hydrophobic element that could be assembled into nanofibers. When the peptide bound to HER2 on the cancer cells’ surface, TPM (λex: 380 nm, λem: 520 nm), was converted into nanofibers and attached to the cell membrane strongly, this restricted the rotation of AIEgen and the imaged cell membrane. In addition, TPM also destroyed the formation of HER2 dimers, thereby blocking the downstream signaling pathway and leading to tumor cell apoptosis. This is a bioprobe targeting cell surface receptors in order to target the cell membrane which has favorable potential for future clinical applications. In addition, Eph receptor A2 (EphA2) is an adrenaline tyrosine receptor kinase overexpressed in tumor-specific membranes. EphA2 plays an important role in promoting cancer malignancy. Therefore, the specific imaging of EphA2 is of great significance for the diagnosis of tumors. Ding et al. constructed a self-assembling bioprobe (DBT-2FFGYSA) that selectively targeted the EphA2 protein on the cell membrane [57]. As shown in Figure 3 compound (5), it consisted of three elements: the middle element was AIEgen (DBT), the two aromatic phenylalanine (FF) were the self-assembled element and the peptide sequence YSAYPDSVPMMS (YSA) could specifically bind to EphA2. The excitation wavelength of the bioprobe is 490 nm, and the maximum emission wavelength is 642 nm. The bioprobe selectively targeted EphA2 receptors and caused AIEgen to aggregate in the cell membrane. In addition, this bioprobe could effectively transform cold tumors into hot tumors to stimulate immune response and inhibit tumor growth. In addition to the strategy of the bioprobe bound to the cell membrane receptors, 16-carbon alkyl chains can adhere firmly to the cell membrane. Zhang et al. reported a membrane-targeted AIEgen-peptide bioprobe (CTGP) imaging the cell membrane [58]. As shown in Figure 3 compound (6), it was composed of the AIEgen element (TPE), cathepsin B (CB) enzyme-responded peptide element (GFLG) and 16-carbon alkyl chain element (C16), which targeted cell membrane. The excitation wavelength of the bioprobe is 370 nm, and the maximum emission wavelength is 470 nm. The bioprobe was cleaved by CB when the bioprobe was present in tumor cells with CB overexpression. CTGP was transformed from spherical nanoparticles into nanofibers. TPE was encapsulated by nanofibers on the cell membrane, so CTGP can image the cell membrane obviously. In addition, this encapsulation characteristic could prevent the DOX efflux in tumor cells and prevent the drug resistance of tumor cells from inhibiting the drug efflux. This encapsulation of the cell membrane opened a new avenue for tumor imaging and drug resistance research.
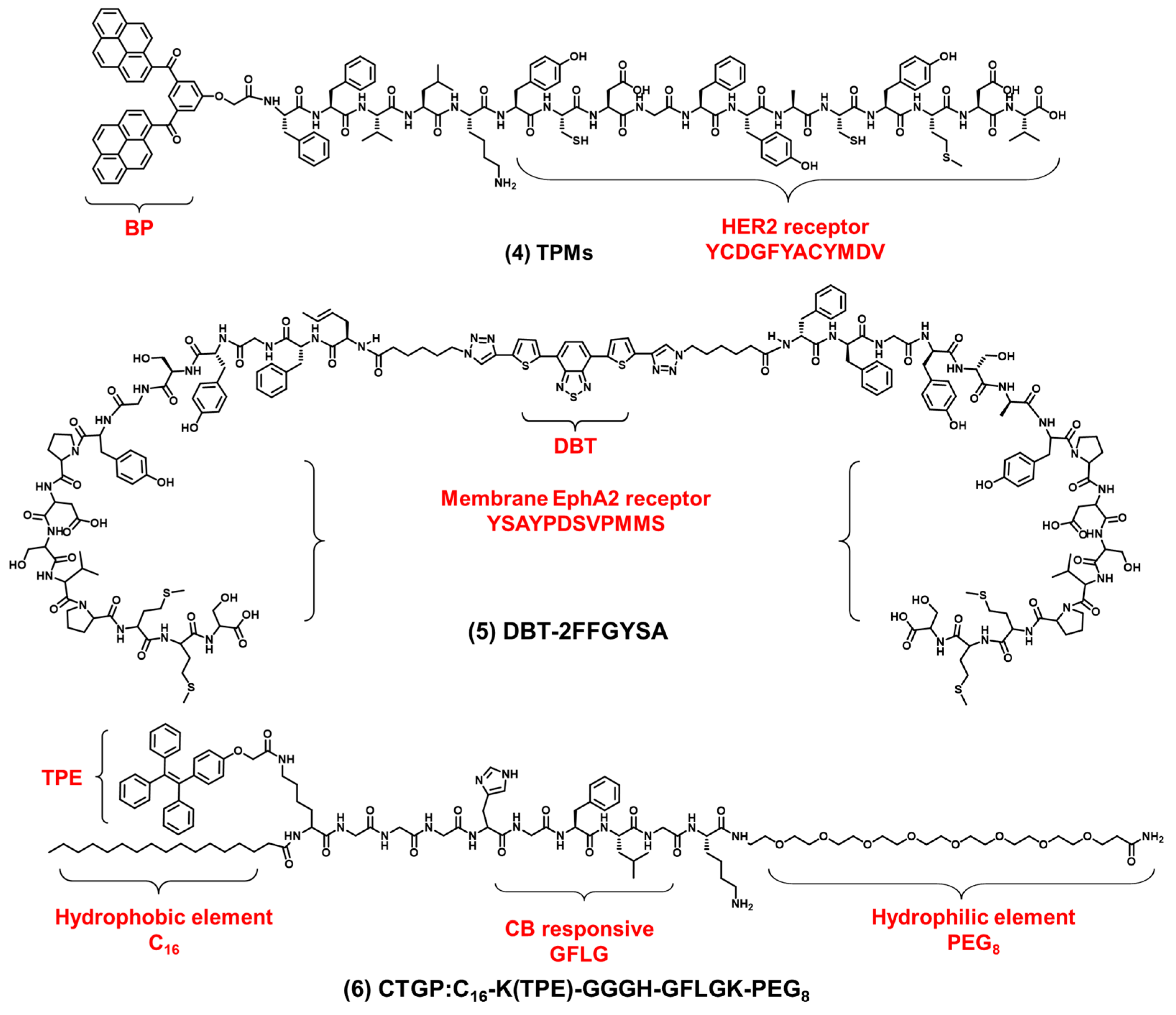
Figure 3. The chemical structures of cell membrane-targeting bioprobes relying on the cell membrane protein.
References
- Tian, M.; Zhan, J.; Lin, W. Single fluorescent probes enabling simultaneous visualization of duple organelles: Design principles, mechanisms, and applications. Coord. Chem. Rev. 2022, 451, 214–266.
- Saminathan, A.; Zajac, M.; Anees, P.; Krishnan, Y. Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater. 2021, 7, 355–371.
- Yu, H.; Guo, Y.; Zhu, W.; Havener, K.; Zheng, X. Recent advances in 1,8-naphthalimide-based small-molecule fluorescent probes for organelles imaging and tracking in living cells. Coord. Chem. Rev. 2021, 444, 214019.
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343.
- Cho, H.; Cho, Y.Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeted drug delivery in cancers. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165808.
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.H. Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy. Cancers 2019, 12, 4.
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal biology and function: Modern view of cellular debris bin. Cells 2020, 9, 1131.
- Perera, R.M.; Zoncu, R. Lysosome as a regulatory hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253.
- Schwarz, D.S.; Blower, M.D. Endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94.
- King, A.P.; Wilson, J.J. Endoplasmic reticulum stress: An arising target for metal-based anticancer agents. Chem. Soc. Rev. 2020, 49, 8113–8136.
- Liu, J.; Zhai, Z.; Niu, H.; Zhang, Y.; Song, X.; Zhang, P.; Ye, Y. Endoplasmic reticulum-targetable fluorescent probe for visualizing HClO in EC1 cells. Tetrahedron Lett. 2020, 61, 152301.
- Klinge, S.; Woolford, J.L., Jr. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 2019, 20, 116–131.
- Qiu, K.; Chen, Y.; Rees, T.W.; Ji, L.; Chao, H. Organelle-targeting metal complexes: From molecular design to bio-applications. Coord. Chem. Rev. 2019, 378, 66–86.
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Research 2017, 6, 2050.
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433.
- Sathasivam, S.; Ince, P.G.; Shaw, P.J. Apoptosis in amyotrophic lateral sclerosis: A review of the evidence. Neuropathology 2010, 27, 257–274.
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 90, 487–498.
- Tian, M.; Ma, Y.; Lin, W. Fluorescent probes for the visualization of cell viability. Acc. Chem. Res. 2019, 52, 2147–2157.
- Wang, Q.; Wang, X.D.; Min, X.; Lou, X.D.; Xia, F. One-dimensional and two-dimensional nanomaterials for the detection of multiple biomolecules. Chin. Chem. Lett. 2019, 30, 1557–1564.
- Gao, P.; Pan, W.; Li, N.; Tang, B.Z. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071.
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940.
- Liu, H.; Xiong, L.H.; Kwok, R.T.K.; He, X.; Lam, J.W.Y.; Tang, B.Z. AIE bioconjugates for biomedical applications. Adv. Opt. Mater. 2020, 8, 2000162.
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile synthesis of AIEgens with wide color tunability for cellular imaging and therapy. Chem. Sci. 2019, 10, 3494–3501.
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-induced emission photosensitizers: From molecular design to photodynamic therapy. J. Med. Chem. 2020, 63, 1996–2012.
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886.
- Xu, S.; Duan, Y.; Liu, B. Precise molecular design for high-performance luminogens with aggregation-induced emission. Adv. Mater. 2020, 32, e1903530.
- Mei, J.; Huang, Y.; Tian, H. Progress and trends in AIE-based bioprobes: A brief overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261.
- Li, Y.; Tang, R.; Liu, X.; Gong, J.; Zhao, Z.; Sheng, Z.; Zhang, J.; Li, X.; Niu, G.; Kwok, R.T.K.; et al. Bright aggregation-induced emission nanoparticles for two-photon imaging and localized compound therapy of cancers. ACS Nano 2020, 14, 16840–16853.
- Wu, W.; Yang, Y.Q.; Yang, Y.; Yang, Y.M.; Wang, H.; Zhang, K.Y.; Guo, L.; Ge, H.F.; Liu, J.; Feng, H. An organic NIR-II nanofluorophore with aggregation-induced emission characteristics for in vivo fluorescence imaging. Int. J. Nanomed. 2019, 14, 3571–3582.
- Wang, Y.; Zhang, Y.; Wang, J.; Liang, X.J. Aggregation-induced emission (AIE) fluorophores as imaging tools to trace the biological fate of nano-based drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 161–176.
- Dong, Y.; Liu, B.; Yuan, Y. AIEgen based drug delivery systems for cancer therapy. J. Control. Release 2018, 290, 129–137.
- Li, H.; Yao, Q.; Xu, F.; Li, Y.; Kim, D.; Chung, J.; Baek, G.; Wu, X.; Hillman, P.F.; Lee, E.Y.; et al. An activatable AIEgen probe for high-fidelity monitoring of overexpressed tumor enzyme activity and its application to surgical tumor excision. Angew. Chem. Int. Ed. 2020, 59, 10186–10195.
- Chen, C.; Ni, X.; Tian, H.W.; Liu, Q.; Guo, D.S.; Ding, D. Calixarene-based supramolecular AIE dots with highly inhibited nonradiative decay and intersystem crossing for ultrasensitive fluorescence image-guided cancer surgery. Angew. Chem. Int. Ed. 2020, 59, 10008–10012.
- He, X.; Yang, Y.; Guo, Y.; Lu, S.; Du, Y.; Li, J.J.; Zhang, X.; Leung, N.L.C.; Zhao, Z.; Niu, G.; et al. Phage-guided targeting, discriminative imaging, and synergistic killing of bacteria by AIE bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969.
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient near-infrared photosensitizer with aggregation-induced emission for imaging-guided photodynamic therapy in multiple xenograft tumor models. ACS Nano 2020, 14, 854–866.
- Yi, X.; Hu, J.J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-guiding polymeric prodrug micelles with two aggregation-induced emission photosensitizers for enhanced chemo-photodynamic therapy. ACS Nano 2021, 15, 3026–3037.
- Jiang, R.; Dai, J.; Dong, X.; Wang, Q.; Meng, Z.; Guo, J.; Yu, Y.; Wang, S.; Xia, F.; Zhao, Z.; et al. Improving image-guided surgical and immunological tumor treatment efficacy by photothermal and photodynamic therapies based on a multifunctional NIR AIEgen. Adv. Mater. 2021, 33, e2101158.
- Wang, Z.; Yu, L.; Wang, Y.; Wang, C.; Mu, Q.; Liu, X.; Yu, M.; Wang, K.N.; Yao, G.; Yu, Z. Dynamic adjust of non-radiative and radiative attenuation of AIE molecules reinforces NIR-II imaging mediated photothermal therapy and immunotherapy. Adv. Sci. 2022, 9, e2104793.
- Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; et al. Brain-targeted aggregation-induced-emission nanoparticles with near-infrared imaging at 1550 nm boosts orthotopic glioblastoma theranostics. Adv. Mater. 2022, 34, e2106082.
- Yan, D.; Wang, M.; Wu, Q.; Niu, N.; Li, M.; Song, R.; Rao, J.; Kang, M.; Zhang, Z.; Zhou, F.; et al. Multimodal imaging-guided photothermal immunotherapy based on a versatile NIR-II aggregation-induced emission luminogen. Angew. Chem. Int. Ed. 2022, 61, e202202614.
- Jia, H.; Ding, D.; Hu, J.; Dai, J.; Yang, J.; Li, G.; Lou, X.; Xia, F. AIEgen-based lifetime-probes for precise furin quantification and identification of cell subtypes. Adv. Mater. 2021, 33, e2104615.
- Dai, J.; Hu, J.J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep downregulation of PD-L1 by caged peptide-conjugated AIEgen/miR-140 nanoparticles for enhanced immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798.
- Zhang, C.; Wu, W.; Li, R.Q.; Qiu, W.X.; Zhuang, Z.N.; Cheng, S.X.; Zhang, X.Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018, 28, 1804492.
- Liu, Z.; Liang, G.; Zhan, W. In situ activatable peptide-based nanoprobes for tumor imaging. Chem. Res. Chin. 2021, 37, 889–899.
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, e1703444.
- Wu, F.; Wu, X.; Duan, Z.; Huang, Y.; Lou, X.; Xia, F. Biomacromolecule-functionalized AIEgens for advanced biomedical studies. Small 2019, 15, e1804839.
- Daniel, L.; Kai, S. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50.
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188.
- Shi, L.; Liu, Y.H.; Li, K.; Sharma, A.; Yu, K.K.; Ji, M.S.; Li, L.L.; Zhou, Q.; Zhang, H.; Kim, J.S.; et al. An AIE-based probe for rapid and ultrasensitive imaging of plasma membranes in biosystems. Angew. Chem. Int. Ed. 2020, 59, 9962–9966.
- Chen, S.; Xu, Z.; Li, S.; Liang, H.; Zhang, C.; Wang, Z.; Li, J.; Li, J.; Yang, H. Systematic interrogation of cellular signaling in live cells using a membrane-anchored DNA multitasking processor. Angew. Chem. Int. Ed. 2022, 61, e202113795.
- Wang, M.D.; Lv, G.T.; An, H.W.; Zhang, N.Y.; Wang, H. In situ self-assembly of bispecific peptide for cancer immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202113649.
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-based detection of circulating targets for precision medicine. Chem. Rev. 2021, 121, 12035–12105.
- Shi, H.; Liu, J.; Geng, J.; Tang, B.Z.; Liu, B. Specific detection of integrin αvβ3 by light-up bioprobe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 9569–9572.
- Zhang, C.; Jin, S.; Yang, K.; Xue, X.; Li, Z.; Jiang, Y.; Chen, W.Q.; Dai, L.; Zou, G.; Liang, X.J. Cell membrane tracker based on restriction of intramolecular rotation. ACS Appl. Mater. Interfaces 2014, 6, 8971–8975.
- Yang, J.; Hu, J.-J.; Wei, J.; Dai, J.; Liu, R.; Xia, F.; Lou, X. Peptide-conjugated aggregation-induced emission fluorogen: Precise and firm cell membrane labeling by multiple weak interactions. CCS Chem. 2022, 4, 464–475.
- Zhang, L.; Jing, D.; Jiang, N.; Rojalin, T.; Baehr, C.M.; Zhang, D.; Xiao, W.; Wu, Y.; Cong, Z.; Li, J.J.; et al. Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo. Nat. Nanotechnol. 2020, 15, 145–153.
- Li, J.; Fang, Y.; Zhang, Y.; Wang, H.; Yang, Z.; Ding, D. Supramolecular self-assembly-facilitated aggregation of tumor-specific transmembrane receptors for signaling activation and converting immunologically cold to hot tumors. Adv. Mater. 2021, 33, e2008518.
- Zhang, C.; Liu, L.H.; Qiu, W.X.; Zhang, Y.H.; Song, W.; Zhang, L.; Wang, S.B.; Zhang, X.Z. A transformable chimeric peptide for cell encapsulation to overcome multidrug resistance. Small 2018, 14, e1703321.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
791
Revisions:
2 times
(View History)
Update Date:
06 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




