Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radu Nartita | -- | 1705 | 2022-09-05 16:05:24 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1705 | 2022-09-06 03:01:29 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1705 | 2022-09-06 03:07:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nartita, R.; Andrei, M.; Ionita, D.; Didilescu, A.C.; Demetrescu, I. Graphene Oxide as an Implant Coating in Dentistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/26889 (accessed on 07 February 2026).
Nartita R, Andrei M, Ionita D, Didilescu AC, Demetrescu I. Graphene Oxide as an Implant Coating in Dentistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/26889. Accessed February 07, 2026.
Nartita, Radu, Mihai Andrei, Daniela Ionita, Andreea Cristiana Didilescu, Ioana Demetrescu. "Graphene Oxide as an Implant Coating in Dentistry" Encyclopedia, https://encyclopedia.pub/entry/26889 (accessed February 07, 2026).
Nartita, R., Andrei, M., Ionita, D., Didilescu, A.C., & Demetrescu, I. (2022, September 05). Graphene Oxide as an Implant Coating in Dentistry. In Encyclopedia. https://encyclopedia.pub/entry/26889
Nartita, Radu, et al. "Graphene Oxide as an Implant Coating in Dentistry." Encyclopedia. Web. 05 September, 2022.
Copy Citation
Dental materials used for reconstructing tooth defects can be improved with graphene oxide (GO), dental implants can be coated using GO, it can be used in tissue engineering in order to repair bone defects and it can also be used to suppress cariogenic biofilm formation. Additionally, GO has also been promoted as a good candidate for neural implants, not only because it provides outstanding resistance to corrosion, but also because it promotes the growth of neuronal cells and reduces ROS expression.
graphene oxide
graphene oxide biofilm
implant biofilm formation
graphene oxide peri-implantitis
1. Introduction
Due to its valency, carbon can be present in a great number of different allotropes with remarkable properties and thus it can be used in challenging industrial applications [1][2][3][4][5]. Based on traditionally known carbon allotropes structures [6][7][8][9][10][11][12][13][14][15] such as diamond, graphite and amorphous carbon, micro and nano forms have been elaborated and investigated as nanoparticles [16], fibers [17], films [18], complex heterostructures [19], coatings [20] and 3D structures [21], starting from the middle of the last century. This has also led to the elaboration of new complex materials and the identification of new correlations between structure, properties and applications [22][23][24][25][26][27][28][29][30][31][32][33] as it can be seen in Figure 1. Following the synthesis of molecular carbon in the form of C60 and other fullerenes [2], the preparation of a new type of structure consisting of needle-like tubes was a significant achievement in the material carbon world [3]. Although H.P. Boehm reduced graphite oxide with a thin film formation in the last century, the serious investigation of chemically modified graphene started much later, in 2004 [5]. In fact, the remarkable properties of the carbon family [22][23][26] with an accent on graphene [27], enlarged the application fields from electronics and energy to biomedicine [28]. With a positive answer to the question “Is it toxic or is it safe?” and the discovery of the antibacterial effect of carbonic materials [34][35], including graphene and its composites, graphene was accepted as a biomaterial for tissue engineering, bioimaging and drug delivery [28][36]. Moreover, coatings with graphene and graphene oxide (GO) are intensively studied as sustainable coatings in biomedical applications and especially in the dental industry [37].
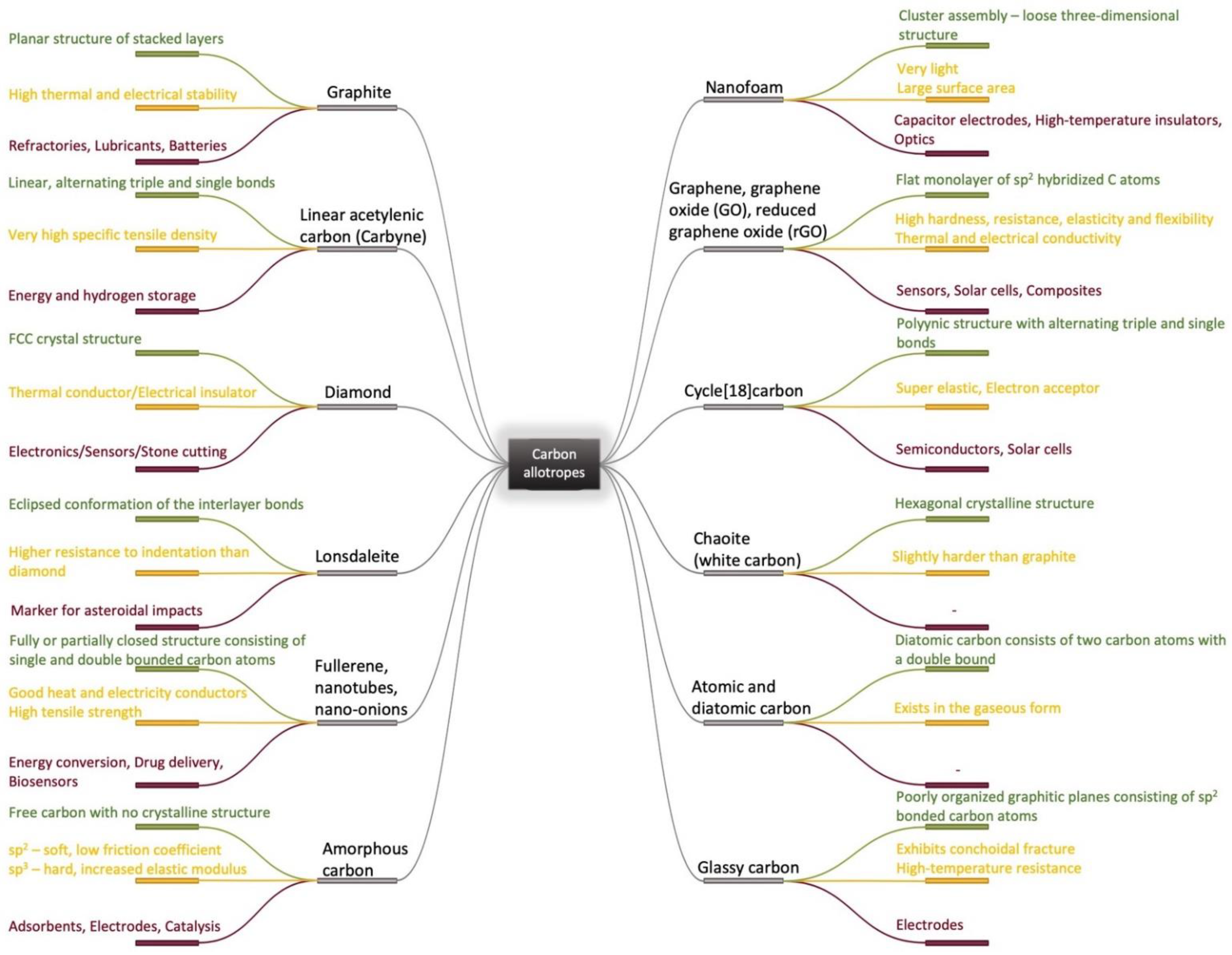
The dental implant industry was quantified at about USD 3.7 billion in 2015 and is estimated to almost double its value by 2023 [38]. To overcome the existing problems, multiple materials are being researched for use as implant coatings: proteins, peptides, microRNA, hydroxyapatite, calcium phosphate, antibiotics, silver nanoparticles, carbon-based nanomaterials, etc. or various combinations of these. From these, GO has already proved beneficial in multiple applications and the number of articles that study graphene and its derivatives as a reinforcing phase in composite coatings is exponentially increasing in recent years [39][40][41][42][43][44][45][46].
Dental materials used for reconstructing tooth defects can be improved with GO, dental implants can be coated using GO, it can be used in tissue engineering in order to repair bone defects and it can also be used to suppress cariogenic biofilm formation [47][48][49][50][51][52]. Additionally, GO has also been promoted as a good candidate for neural implants, not only because it provides outstanding resistance to corrosion, but also because it promotes the growth of neuronal cells and reduces ROS expression [53].
The implant failure rate is nowadays approximately 11% and considering that 100,000–300,000 dental implants are inserted yearly, an 11% implant failure rate indicates that there are between 11,000 and 33,000 patients affected per year. Moreover, even when the implant is successful, a full recovery is achieved in 3–6 months; until then, patients may experience masticatory difficulties [54][55].
There are various types of materials studied in dentistry regarding implant osseointegration [56], from which the use of GO in implant coatings seems promising for mitigating the main problems. Firstly, one of the principal causes of implant failure is represented by the bacteria on peri-implant tissue and graphene oxide’s antimicrobial activity, as well as its ability to promote osseointegration which has been proven in numerous studies. Secondly, due to its structure and physicochemical properties, GO can bind different substances which could speed up the recovery process [47][57][58][59][60][61][62].
2. Graphene Oxide as an Implant Coating
The industry concerning the products of graphene was estimated to reach USD 1.3 billion in 2023 [63]. Graphene, as well as GO and reduced graphene oxide (rGO), have multiple applications in the fields of science and engineering, due to its physicochemical properties such as their high surface area, biocompatibility, mechanical strength, etc. [64][65][66] Regarding the synthesis of GO, it is important to note that different starting materials, as well as different method parameters, could lead to a structure with different functional groups and properties. The same applies to rGO, when due to different reduction conditions the product obtained might display slightly different properties [67][68][69][70].
Additionally, due to the high surface area and multiple functional groups, such as hydroxyl, epoxy, carbonyl, carboxyl, phenol, quinone and lactone, GO forms a stable aqueous dispersion that is able to also bind polymers, biomolecules and active substances [47][71][72][73][74][75][76]. Although both forms present π-π stacking between the aromatic rings that promotes protein adsorption and implicit cell attachment and proliferation, rGO exhibits somewhat different properties than GO. Compared with the traditional surface modifications such as grit-blasting, acid etching, micro arc oxidation, etc., both GO and rGO seem to naturally stimulate osteogenic differentiation, which is crucial for the success rate of dental implants [77].
In a study conducted by Yadav et al., the effect of two GO-coated surfaces created through two distinct methods was studied, further highlighting the influence of the preparation method. One method created a rougher surface, with a non-uniform thickness, where GO was carboxylic rich and displayed an increased inhibitory effect against Staphylococcus aureus, while the other method created a smoother surface, where GO presented more epoxy and hydroxyl groups and showed a more selective inhibiting effect against Escherichia coli [78].
When negatively charged, the carboxyl groups prevent anion adsorption, increasing the resistance to corrosion, while the aromatic ring enhances protein adsorption through a non-covalent interaction. Moreover, the hydroxyl and epoxy groups beside carboxyl groups, in combination with a large surface area, enable the possible multi-functionalization with various bioactive substances [79][80]. Such an example was provided by the coating of an Mg alloy by GO-chitosan (CS), that contained heparin and the bone morphogenetic protein 2 (BMP-2) incorporated within it and that was sustainably released over a period of14 days [79]. In another study on rats, rGO was used as a coating for CP Ti that showed an increased cell attachment and cell viability. Additionally, dexamethasone was loaded and the implant was placed on calvarial bone defects for bone tissue regeneration [81]. The dexamethasone-loaded rGO was also studied for dental applications and showed a significant increase in cell growth and differentiation. By comparing the bare substrate with the coated one, with and without dexamethasone, it became clear that rGO alone had a beneficial effect, which was further increased by the gene regulating drug [82]. The incorporation of BMP-2 in the GO coating was also studied on Ti substrates along with Substance P (SP), which was incorporated in order to help recruit stem cells. The study evaluated the bone formation, in vivo, and showed that the GO coated implants with BMP-2 and SP induced extensive bone formation [83].
The hydrophobic/hydrophilic character, in combination with the surface roughness, dictates the interaction with the proteins and subsequently with the cells, the goal being to promote cell growth, differentiation and anchorage for the implant. An interesting material that lacks these surface properties, but has a closer elastic modulus to the natural bone, is the polyether ether ketone (PEEK) [84]. Taking advantage of the GO structure, a functional modification was proposed on sulfonated PEEK, reinforced with carbon fibers, that showed promising results in vitro and in vivo regarding cell adhesion and proliferation, as well as promoting new bone formation [85].
Moreover, the rough surface that favors cell attachment, the π-π stacking, as well as the hydrogen bonds, allow the GO to absorb osteoinductive factors by non-covalent binding, thus accelerating the osteogenic differentiation of stem cells [86].
One of the substances with great biocompatibility that could be used in dental implant coatings is hydroxyapatite. However, hydroxyapatite alone is not suitable because of its low stability on long-term contact with tissue/biological fluids, even though it has great biocompatibility. Moreover, the acidic environment created through the fermentation of sugars that accumulate on teeth further accelerates the corrosion of hydroxyapatite. To deal with this issue, GO was researched as a reinforcement material and this showed the homogeneous dispersion and fluoride ions were also introduced by partially substituting the hydroxyl ions from hydroxyapatite. The resulting composite presents an increased biocompatibility, an increase in corrosion resistance and promotion of cell proliferation and differentiation [87].
A cathodic electrophoretic procedure was first mentioned for the fabrication of a GO/hydroxyapatite coating on a pure Ti substrate, in the employment of the GO at nanoscale in a bioactive coating [88]. Later, the GO was also used in combination with hydroxyapatite as a coating for 316L stainless steel. The functional groups of GO bind the calcium ions from the hydroxyapatite, thereby improving the strength of the material and also acting as anchors for the metallic surface and increasing the adhesion of the coating [89]. To further improve the biocompatibility and other parameters, polymers can also be used in combination with the hydroxyapatite and the GO. It was shown on a Ti alloy that the hydroxyapatite, doped with Mg and Zn and topped with polycaprolactone-GO, can establish direct bonds with the bone tissue, while also having bactericidal properties [90]. Many other combinations of GO with bioactive materials have been recently investigated, introducing more applications using new technologies [91][92].
Furthermore, it was proved in a series of studies that graphene-based coatings can improve the corrosion resistance of different substrates, and this was further challenged by Malhotra R et al., who performed corrosion tests over a period of 240 days, with the use of a very acidic solution. The coated alloy, Ti-6Al-4V, showed negligible signs of corrosion after 240 days, thereby maintaining its structural integrity, while the uncoated alloy presented a high corrosion rate, resulting in a 12-fold increase in surface roughness [93]. Another study on the same alloy that used a bioglass/graphene oxide coating, showed improved antibacterial activity that increased along with the content of the GO [94].
Of course, other factors contributed to the outcome, such as the geometry of the implant. Different alterations regarding the helix angle, the thread pitch and the dimensions also produced different results, due to stress distribution [95].
References
- Hoffmann, R.; Kabanov, A.A.; Golov, A.A.; Proserpio, D.M. Homo Citans and Carbon Allotropes: For an Ethics of Citation. Angew. Chem. Int. Ed. 2016, 55, 10962–10976.
- Kroto, H.W.; Heath, J.R.; O Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Boehm, H.-P. Graphene-How a Laboratory Curiosity Suddenly Became Extremely Interesting. Angew. Chem. Int. Ed. 2010, 49, 9332–9335.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Karthik, P.; Himaja, A.; Singh, S.P. Carbon-allotropes: Synthesis methods, applications and future perspectives. Carbon Lett. 2014, 15, 219–237.
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871.
- Loos, M. Allotropes of Carbon and Carbon Nanotubes. In Carbon Nanotube Reinforced Composites. CNT Polymer Science and Technology; Elsevier: Amsterdam, Netherlands, 2015; pp. 73–101.
- Falcao, E.H.; Wudl, F. Carbon allotropes: Beyond graphite and diamond. J. Chem. Technol. Biotechnol. 2007, 82, 524–531.
- Zhang, R.-S.; Jiang, J.-W. The art of designing carbon allotropes. Front. Phys. 2019, 14, 13401.
- Lagow, R.J.; Kampa, J.J.; Wei, H.-C.; Battle, S.L.; Genge, J.W.; Laude, D.A.; Harper, C.J.; Bau, R.; Stevens, R.C.; Haw, J.F.; et al. Synthesis of Linear Acetylenic Carbon: The “sp” Carbon Allotrope. Science 1995, 267, 362–367.
- Dekanski, A.; Stevanović, J.; Stevanović, R.; Nikolić, B.; Jovanović, V.M. Glassy carbon electrodes: I. Characterization and electrochemical activation. Carbon 2001, 39, 1195–1205.
- Parigger, C.; Hornkohl, J.O.; Keszler, A.M.; Nemes, L. Measurement and analysis of atomic and diatomic carbon spectra from laser ablation of graphite. Appl. Opt. 2003, 42, 6192–6198.
- Simakov, S.K.; Kalmykov, A.E.; Sorokin, L.M.; Novikov, M.P.; Drozdova, I.A.; Yagovkina, M.A.; Grebenshchikova, E.A. Chaoite formation at low PT parameters from fluid phases. Dokl. Akad. Nauk 2004, 399, 671–672.
- Kaiser, K.; Scriven, L.M.; Schulz, F.; Gawel, P.; Gross, L.; Anderson, H.L. An sp-hybridized molecular carbon allotrope, cyclocarbon. Science 2019, 365, 1299–1301.
- Mondal, R.; Yilmaz, M.D.; Mandal, A.K. Green synthesis of carbon nanoparticles: Characterization and their biocidal properties. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, Netherlands, 2021; pp. 277–306.
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Šťastná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials 2021, 14, 1428.
- DU, Z.-M.; Lei, Z.-P.; Yu, W.-H.; Yan, J.-C.; Li, Z.-K.; Shui, H.-F.; Ren, S.-B.; Wang, Z.-C.; Kang, S.-G. Growth of high performance coal tar-based carbon film and its application in Joule heating. J. Fuel Chem. Technol. 2021, 49, 1599–1607.
- Sobola, D.; Ramazanov, S.; Konečný, M.; Orudzhev, F.; Kaspar, P.; Papež, N.; Knápek, A.; Potoček, M. Complementary SEM-AFM of Swelling Bi-Fe-O Film on HOPG Substrate. Materials 2020, 13, 2402.
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 1–16.
- Gao, Y.; Zhou, Z.; Hu, H.; Xiong, J. New concept of carbon fiber reinforced composite 3D auxetic lattice structures based on stretching-dominated cells. Mech. Mater. 2020, 152, 103661.
- Song, G.-L.; Zhang, C.; Chen, X.; Zheng, D. Galvanic activity of carbon fiber reinforced polymers and electrochemical behavior of carbon fiber. Corros. Commun. 2021, 1, 26–39.
- Ollik, K.; Lieder, M. Review of the Application of Graphene-Based Coatings as Anticorrosion Layers. Coatings 2020, 10, 883.
- Miyamoto, K.; Narita, S.; Masumoto, Y.; Hashishin, T.; Osawa, T.; Kimura, M.; Ochiai, M.; Uchiyama, M. Room-temperature chemical synthesis of C2. Nat. Commun. 2020, 11, 2134.
- Uskoković, V. A historical review of glassy carbon: Synthesis, structure, properties and applications. Carbon Trends 2021, 5, 100116.
- Jiang, J.; Yao, X.; Xu, C.; Su, Y.; Zhou, L.; Deng, C. Influence of electrochemical oxidation of carbon fiber on the mechanical properties of carbon fiber/graphene oxide/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 248–256.
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344.
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547.
- Pan, Z.; Sun, H.; Zhang, Y.; Chen, C. Harder than Diamond: Superior Indentation Strength of Wurtzite BN and Lonsdaleite. Phys. Rev. Lett. 2009, 102, 055503.
- Chen, L.; Zhao, S.; Hasi, Q.; Luo, X.; Zhang, C.; Li, H.; Li, A. Porous Carbon Nanofoam Derived from Pitch as Solar Receiver for Efficient Solar Steam Generation. Glob. Challenges 2020, 4, 1900098.
- Castelvecchi, D. Chemists make first-ever ring of pure carbon. Nature 2019, 572, 426.
- Narayan, J.; Bhaumik, A.; Gupta, S.; Haque, A.; Sachan, R. Progress in Q-carbon and related materials with extraordinary properties. Mater. Res. Lett. 2018, 6, 353–364.
- Warr, L.N. IMA–CNMNC approved mineral symbols. Miner. Mag. 2021, 85, 291–320.
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617.
- Ionita, D.; Golgovici, F.; Mazare, A.; Badulescu, M.; Demetrescu, I.; Pandelea-Dobrovicescu, G.-R. Corrosion and antibacterial characterization of Ag-DLC coatingon a new CoCrNbMoZr dental alloy. Mater. Corros. 2018, 69, 1403–1411.
- Syama, S.; Mohanan, P. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555.
- Nartita, R.; Ionita, D.; Demetrescu, I. Sustainable Coatings on Metallic Alloys as a Nowadays Challenge. Sustainability 2021, 13, 10217.
- Li, H.; Gao, C.; Tang, L.; Wang, C.; Chen, Q.; Zheng, Q.; Yang, S.; Sheng, S.; Zan, X. Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Appl. Bio Mater. 2020, 3, 673–684.
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201.
- Dutta, S.; Gupta, S.; Roy, M. Recent Developments in Magnesium Metal–Matrix Composites for Biomedical Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 4748–4773.
- Zhang, C.; Jiang, Z.; Zhao, L.; Liu, W.; Si, P.; Lan, J. Synthesis and characterization of multilayer graphene oxide on yttria-zirconia ceramics for dental implant. J. Mater. Res. 2020, 35, 2466–2477.
- Salehi, S.; Kharaziha, M.; Salehi, M. Multifunctional plasma-sprayed nanocomposite coating based on FA-ZnO-GO with improved bioactivity and wear behaviour. Surf. Coatings Technol. 2020, 404, 126472.
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946.
- Catt, K.; Li, H.; Cui, X.T. Poly (3,4-ethylenedioxythiophene) graphene oxide composite coatings for controlling magnesium implant corrosion. Acta Biomater. 2016, 48, 530–540.
- Prema, D.; Prakash, J.; Vignesh, S.; Veluchamy, P.; Ramachandran, C.; Samal, D.B.; Oh, D.-H.; Sahabudeen, S.; Venkatasubbu, G.D. Mechanism of inhibition of graphene oxide/zinc oxide nanocomposite against wound infection causing pathogens. Appl. Nanosci. 2020, 10, 827–849.
- Ali, F.A.A.; Alam, J.; Shukla, A.K.; Alhoshan, M.; Ansari, M.A.; Al-Masry, W.A.; Rehman, S.; Alam, M. Evaluation of antibacterial and antifouling properties of silver-loaded GO polysulfone nanocomposite membrane against Escherichia coli, Staphylococcus aureus, and BSA protein. React. Funct. Polym. 2019, 140, 136–147.
- Qi, X.; Jiang, F.; Zhou, M.; Zhang, W.; Jiang, X. Graphene oxide as a promising material in dentistry and tissue regeneration: A review. Smart Mater. Med. 2021, 2, 280–291.
- Martini, C.; Longo, F.; Castagnola, R.; Marigo, L.; Grande, N.M.; Cordaro, M.; Cacaci, M.; Papi, M.; Palmieri, V.; Bugli, F.; et al. Antimicrobial and Antibiofilm Properties of Graphene Oxide on Enterococcus faecalis. Antibiotics 2020, 9, 692.
- Murugesan, B.; Arumugam, M.; Pandiyan, N.; Veerasingam, M.; Sonamuthu, J.; Samayanan, S.; Mahalingam, S. Ornamental morphology of ionic liquid functionalized ternary doped N, P, F and N, B, F-reduced graphene oxide and their prevention activities of bacterial biofilm-associated with orthopedic implantation. Mater. Sci. Eng. C 2019, 98, 1122–1132.
- Mao, M.; Zhang, W.; Huang, Z.; Huang, J.; Wang, J.; Li, W.; Gu, S. Graphene Oxide-Copper Nanocomposites Suppress Cariogenic Streptococcus mutans Biofilm Formation. Int. J. Nanomed. 2021, 16, 7727–7739.
- He, J.; Zhu, X.; Qi, Z.; Wang, L.; Aldalbahi, A.; Shi, J.; Song, S.; Fan, C.; Lv, M.; Tang, Z. The Inhibition Effect of Graphene Oxide Nanosheets on the Development of Streptococcus mutans Biofilms. Part. Part. Syst. Charact. 2017, 34, 1700001.
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179.
- Tasnim, N.; Kumar, A.; Joddar, B. Attenuation of the in vitro neurotoxicity of 316L SS by graphene oxide surface coating. Mater. Sci. Eng. C 2017, 73, 788–797.
- Park, C.; Park, S.; Lee, D.; Choi, K.S.; Lim, H.-P.; Kim, J. Graphene as an Enabling Strategy for Dental Implant and Tissue Regeneration. Tissue Eng. Regen. Med. 2017, 14, 481–493.
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60.
- Andrei, M.; Dinischiotu, A.; Didilescu, A.C.; Ionita, D.; Demetrescu, I. Periodontal materials and cell biology for guided tissue and bone regeneration. Ann. Anat. 2018, 216, 164–169.
- Eivazzadeh-Keihan, R.; Alimirzaloo, F.; Aliabadi, H.A.M.; Noruzi, E.B.; Akbarzadeh, A.R.; Maleki, A.; Madanchi, H.; Mahdavi, M. Functionalized graphene oxide nanosheets with folic acid and silk fibroin as a novel nanobiocomposite for biomedical applications. Sci. Rep. 2022, 12, 6205.
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.; Eldesoukey, I.; Masoud, M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: Fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019, 9, 3704–3714.
- Park, S.; Kim, H.; Choi, K.S.; Ji, M.-K.; Kim, S.; Gwon, Y.; Park, C.; Kim, J.; Lim, H.-P. Graphene–Chitosan Hybrid Dental Implants with Enhanced Antibacterial and Cell-Proliferation Properties. Appl. Sci. 2020, 10, 4888.
- Prusty, S.; Pal, K.; Bera, D.; Paul, A.; Mukherjee, M.; Khan, F.; Dey, A.; Das, S. Enhanced antibacterial activity of a novel biocompatible triarylmethane based ionic liquid-graphene oxide nanocomposite. Colloids Surfaces B Biointerfaces 2021, 203, 111729.
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense walR RNA inhibits the pathogenicity of Enterococcus faecalis in periapical periodontitis. J. Dent. Sci. 2020, 15, 65–74.
- Ramalingam, V.; Raja, S.; Sundaramahalingam, S.; Rajaram, R. Chemical fabrication of graphene oxide nanosheets attenuates biofilm formation of human clinical pathogens. Bioorg. Chem. 2019, 83, 326–335.
- Zhu, C.; Mahmood, Z.; Zhang, W.; Akram, M.W.; Ainur, D.; Ma, H. In situ investigation of acute exposure of graphene oxide on activated sludge: Biofilm characteristics, microbial activity and cytotoxicity. Ecotoxicol. Environ. Saf. 2020, 199, 110639.
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayeva, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated graphene as a material for supercapacitor electrodes: Effects of surface area, pore size distribution and hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912.
- Boulanger, N.; Kuzenkova, A.S.; Iakunkov, A.; Nordenström, A.; Romanchuk, A.Y.; Trigub, A.L.; Zasimov, P.V.; Prodana, M.; Enachescu, M.; Bauters, S.; et al. High Surface Area “3D Graphene Oxide” for Enhanced Sorption of Radionuclides. Adv. Mater. Interfaces 2022, 9, 2200510.
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576.
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185.
- Narayanan, K.B.; Choi, S.M.; Han, S.S. Biofabrication of Lysinibacillus sphaericus-reduced graphene oxide in three-dimensional polyacrylamide/carbon nanocomposite hydrogels for skin tissue engineering. Colloids Surfaces B Biointerfaces 2019, 181, 539–548.
- Ali, N.H.; Amin, M.C.I.M.; Ng, S.-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 629–645.
- Narayanan, K.B.; Kim, H.D.; Han, S.S. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surfaces B Biointerfaces 2020, 185, 110579.
- Bregnocchi, A.; Chandraiahgari, C.; Zanni, E.; De Bellis, G.; Uccelletti, D.; Sarto, M. PVDF composite films including graphene/ZnO nanostructures and their antimicrobial activity. In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016; pp. 907–910.
- Guo, J.; Cao, G.; Wang, X.; Tang, W.; Diwu, W.; Yan, M.; Yang, M.; Bi, L.; Han, Y. Coating cocrmo alloy with graphene oxide and ε-poly-l-lysine enhances its antibacterial and antibiofilm properties. Int. J. Nanomed. 2021, 16, 7249–7268.
- de Faria, A.F.; de Moraes, A.C.M.; Andrade, P.F.; da Silva, D.; Gonçalves, M.D.C.; Alves, O.L. Cellulose acetate membrane embedded with graphene oxide-silver nanocomposites and its ability to suppress microbial proliferation. Cellulose 2017, 24, 781–796.
- Zuo, P.-P.; Feng, H.-F.; Xu, Z.-Z.; Zhang, L.-F.; Zhang, Y.-L.; Xia, W.; Zhang, W.-Q. Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem. Cent. J. 2013, 7, 39.
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide improved the antibacterial property of antisense yycG RNA on Staphylococcus aureus. J. Orthop. Surg. Res. 2019, 14, 305.
- Ying, Y.; Wu, Y.; Huang, J. Preparation and characterization of chitosan/poly(vinyl alcohol)/graphene oxide films and studies on their antibiofilm formation activity. J. Biomed. Mater. Res. Part A 2020, 108, 2015–2022.
- Li, X.; Lin, K.; Wang, Z. Enhanced growth and osteogenic differentiation of MC3T3-E1 cells on Ti6Al4V alloys modified with reduced graphene oxide. RSC Adv. 2017, 7, 14430–14437.
- Yadav, N.; Dubey, A.; Shukla, S.; Saini, C.P.; Gupta, G.; Priyadarshini, R.; Lochab, B. Graphene Oxide-Coated Surface: Inhibition of Bacterial Biofilm Formation due to Specific Surface–Interface Interactions. ACS Omega 2017, 2, 3070–3082.
- Lin, Y.; Yang, Y.; Zhao, Y.; Gao, F.; Guo, X.; Yang, M.; Hong, Q.; Yang, Z.; Dai, J.; Pan, C. Incorporation of heparin/BMP2 complex on GOCS-modified magnesium alloy to synergistically improve corrosion resistance, anticoagulation, and osteogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 24.
- Khosravi, F.; Khorasani, S.N.; Khalili, S.; Neisiany, R.E.; Ghomi, E.R.; Ejeian, F.; Das, O.; Nasr-Esfahani, M.H. Development of a Highly Proliferated Bilayer Coating on 316L Stainless Steel Implants. Polymers 2020, 12, 1022.
- Jung, H.S.; Choi, Y.-J.; Jeong, J.; Lee, Y.; Hwang, B.; Jang, J.; Shim, J.-H.; Kim, Y.S.; Choi, H.S.; Oh, S.H.; et al. Nanoscale graphene coating on commercially pure titanium for accelerated bone regeneration. RSC Adv. 2016, 6, 26719–26724.
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface Modification of Multipass Caliber-Rolled Ti Alloy with Dexamethasone-Loaded Graphene for Dental Applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607.
- Kim, B.-S.; La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.H.; Park, H.; Char, K. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116.
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Antibacterial activity, bio-compatibility and osteogenic differentiation of graphene oxide coating on 3D-network poly-ether-ether-ketone for orthopaedic implants. J. Mater. Sci. Mater. Med. 2021, 32, 135.
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and biosafety of graphene oxide wrapped porous CF/PEEK composites as implantable materials: The role of surface structure and chemistry. Dent. Mater. 2020, 36, 1289–1302.
- Wu, M.; Zou, L.; Jiang, L.; Zhao, Z.; Liu, J. Osteoinductive and antimicrobial mechanisms of graphene-based materials for enhancing bone tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 915–935.
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloy. Compd. 2016, 688, 657–667.
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197.
- Sebastin, A.X.S.; Uthirapathy, V. In Vitro Electrochemical Behavior of Sol-Gel Derived Hydroxyapatite/Graphene Oxide Composite Coatings on 316L SS for Biomedical Applications. ChemistrySelect 2020, 5, 12140–12147.
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arab. J. Chem. 2018, 11, 959–969.
- Lee, H.; Yoo, J.M.; Ponnusamy, N.K.; Nam, S.Y. 3D-printed hydroxyapatite/gelatin bone scaffolds reinforced with graphene oxide: Optimized fabrication and mechanical characterization. Ceram. Int. 2022, 48, 10155–10163.
- Shadianlou, F.; Foorginejad, A.; Yaghoubinezhad, Y. Fabrication of zirconia/reduced graphene oxide/hydroxyapatite scaffold by rapid prototyping method and its mechanical and biocompatibility properties. Ceram. Int. 2022, 48, 7031–7044.
- Malhotra, R.; Han, Y.; Nijhuis, C.A.; Silikas, N.; Neto, A.C.; Rosa, V. Graphene nanocoating provides superb long-lasting corrosion protection to titanium alloy. Dent. Mater. 2021, 37, 1553–1560.
- Bahrami, M.S.; Eshghinejad, P.; Bakhsheshi-Rad, H.R.; Karamian, E.; Chen, X.B. Electrophoretic deposition of bioglass/graphene oxide composite on Ti-alloy implants for improved antibacterial and cytocompatible properties. Mater. Technol. 2020, 35, 69–74.
- Patil, V.; Naik, N.; Gadicherla, S.; Smriti, K.; Raju, A.; Rathee, U. Biomechanical Behavior of Bioactive Material in Dental Implant: A Three-Dimensional Finite Element Analysis. Sci. World J. 2020, 2020, 2363298.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
06 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




