Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Radu Nartita and Version 3 by Beatrix Zheng.
Dental materials used for reconstructing tooth defects can be improved with graphene oxide (GO), dental implants can be coated using GO, it can be used in tissue engineering in order to repair bone defects and it can also be used to suppress cariogenic biofilm formation. Additionally, GO has also been promoted as a good candidate for neural implants, not only because it provides outstanding resistance to corrosion, but also because it promotes the growth of neuronal cells and reduces ROS expression.
- graphene oxide
- graphene oxide biofilm
- implant biofilm formation
- graphene oxide peri-implantitis
1. Introduction
Due to its valency, carbon can be present in a great number of different allotropes with remarkable properties and thus it can be used in challenging industrial applications [1][2][3][4][5][1,2,3,4,5]. Based on traditionally known carbon allotropes structures [6][7][8][9][10][11][12][13][14][15][6,7,8,9,10,11,12,13,14,15] such as diamond, graphite and amorphous carbon, micro and nano forms have been elaborated and investigated as nanoparticles [16], fibers [17], films [18], complex heterostructures [19], coatings [20] and 3D structures [21], starting from the middle of the last century. This has also led to the elaboration of new complex materials and the identification of new correlations between structure, properties and applications [22][23][24][25][26][27][28][29][30][31][32][33][22,23,24,25,26,27,28,29,30,31,32,33] as it can be seen in Figure 1. Following the synthesis of molecular carbon in the form of C60 and other fullerenes [2], the preparation of a new type of structure consisting of needle-like tubes was a significant achievement in the material carbon world [3]. Although H.P. Boehm reduced graphite oxide with a thin film formation in the last century, the serious investigation of chemically modified graphene started much later, in 2004 [5]. In fact, the remarkable properties of the carbon family [22][23][26][22,23,26] with an accent on graphene [27], enlarged the application fields from electronics and energy to biomedicine [28]. With a positive answer to the question “Is it toxic or is it safe?” and the discovery of the antibacterial effect of carbonic materials [34][35][34,35], including graphene and its composites, graphene was accepted as a biomaterial for tissue engineering, bioimaging and drug delivery [28][36][28,36]. Moreover, coatings with graphene and graphene oxide (GO) are intensively studied as sustainable coatings in biomedical applications and especially in the dental industry [37].
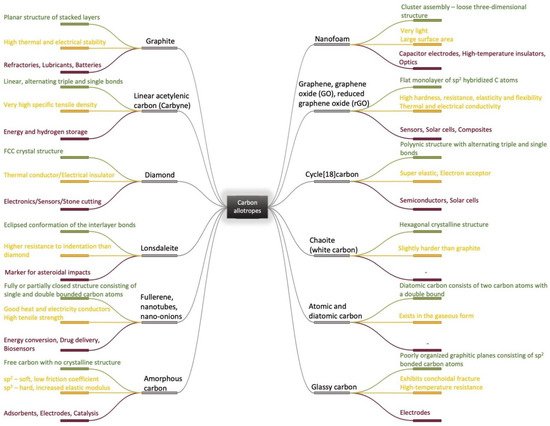
Figure 1.
Carbon allotropes (
—structure
,
—physicochemical properties
,
The dental implant industry was quantified at about USD 3.7 billion in 2015 and is estimated to almost double its value by 2023 [38]. To overcome the existing problems, multiple materials are being researched for use as implant coatings: proteins, peptides, microRNA, hydroxyapatite, calcium phosphate, antibiotics, silver nanoparticles, carbon-based nanomaterials, etc. or various combinations of these. From these, GO has already proved beneficial in multiple applications and the number of articles that study graphene and its derivatives as a reinforcing phase in composite coatings is exponentially increasing in recent years [39][40][41][42][43][44][45][46][39,40,41,42,43,44,45,46].
Dental materials used for reconstructing tooth defects can be improved with GO, dental implants can be coated using GO, it can be used in tissue engineering in order to repair bone defects and it can also be used to suppress cariogenic biofilm formation [47][48][49][50][51][52][47,48,49,50,51,52]. Additionally, GO has also been promoted as a good candidate for neural implants, not only because it provides outstanding resistance to corrosion, but also because it promotes the growth of neuronal cells and reduces ROS expression [53].
The implant failure rate is nowadays approximately 11% and considering that 100,000–300,000 dental implants are inserted yearly, an 11% implant failure rate indicates that there are between 11,000 and 33,000 patients affected per year. Moreover, even when the implant is successful, a full recovery is achieved in 3–6 months; until then, patients may experience masticatory difficulties [54][55][54,55].
There are various types of materials studied in dentistry regarding implant osseointegration [56], from which the use of GO in implant coatings seems promising for mitigating the main problems. Firstly, one of the principal causes of implant failure is represented by the bacteria on peri-implant tissue and graphene oxide’s antimicrobial activity, as well as its ability to promote osseointegration which has been proven in numerous studies. Secondly, due to its structure and physicochemical properties, GO can bind different substances which could speed up the recovery process [47][57][58][59][60][61][62][47,57,58,59,60,61,62].
2. Graphene Oxide as an Implant Coating
The industry concerning the products of graphene was estimated to reach USD 1.3 billion in 2023 [63]. Graphene, as well as GO and reduced graphene oxide (rGO), have multiple applications in the fields of science and engineering, due to its physicochemical properties such as their high surface area, biocompatibility, mechanical strength, etc. [64][65][66][64,65,66] Regarding the synthesis of GO, it is important to note that different starting materials, as well as different method parameters, could lead to a structure with different functional groups and properties. The same applies to rGO, when due to different reduction conditions the product obtained might display slightly different properties [67][68][69][70][67,68,69,70].
Additionally, due to the high surface area and multiple functional groups, such as hydroxyl, epoxy, carbonyl, carboxyl, phenol, quinone and lactone, GO forms a stable aqueous dispersion that is able to also bind polymers, biomolecules and active substances [47][71][72][73][74][75][76][47,71,72,73,74,75,76]. Although both forms present π-π stacking between the aromatic rings that promotes protein adsorption and implicit cell attachment and proliferation, rGO exhibits somewhat different properties than GO. Compared with the traditional surface modifications such as grit-blasting, acid etching, micro arc oxidation, etc., both GO and rGO seem to naturally stimulate osteogenic differentiation, which is crucial for the success rate of dental implants [77].
In a study conducted by Yadav et al., the effect of two GO-coated surfaces created through two distinct methods was studied, further highlighting the influence of the preparation method. One method created a rougher surface, with a non-uniform thickness, where GO was carboxylic rich and displayed an increased inhibitory effect against Staphylococcus aureus, while the other method created a smoother surface, where GO presented more epoxy and hydroxyl groups and showed a more selective inhibiting effect against Escherichia coli [78].
When negatively charged, the carboxyl groups prevent anion adsorption, increasing the resistance to corrosion, while the aromatic ring enhances protein adsorption through a non-covalent interaction. Moreover, the hydroxyl and epoxy groups beside carboxyl groups, in combination with a large surface area, enable the possible multi-functionalization with various bioactive substances [79][80][79,80]. Such an example was provided by the coating of an Mg alloy by GO-chitosan (CS), that contained heparin and the bone morphogenetic protein 2 (BMP-2) incorporated within it and that was sustainably released over a period of14 days [79]. In another study on rats, rGO was used as a coating for CP Ti that showed an increased cell attachment and cell viability. Additionally, dexamethasone was loaded and the implant was placed on calvarial bone defects for bone tissue regeneration [81]. The dexamethasone-loaded rGO was also studied for dental applications and showed a significant increase in cell growth and differentiation. By comparing the bare substrate with the coated one, with and without dexamethasone, it became clear that rGO alone had a beneficial effect, which was further increased by the gene regulating drug [82]. The incorporation of BMP-2 in the GO coating was also studied on Ti substrates along with Substance P (SP), which was incorporated in order to help recruit stem cells. The study evaluated the bone formation, in vivo, and showed that the GO coated implants with BMP-2 and SP induced extensive bone formation [83].
The hydrophobic/hydrophilic character, in combination with the surface roughness, dictates the interaction with the proteins and subsequently with the cells, the goal being to promote cell growth, differentiation and anchorage for the implant. An interesting material that lacks these surface properties, but has a closer elastic modulus to the natural bone, is the polyether ether ketone (PEEK) [84]. Taking advantage of the GO structure, a functional modification was proposed on sulfonated PEEK, reinforced with carbon fibers, that showed promising results in vitro and in vivo regarding cell adhesion and proliferation, as well as promoting new bone formation [85].
Moreover, the rough surface that favors cell attachment, the π-π stacking, as well as the hydrogen bonds, allow the GO to absorb osteoinductive factors by non-covalent binding, thus accelerating the osteogenic differentiation of stem cells [86].
One of the substances with great biocompatibility that could be used in dental implant coatings is hydroxyapatite. However, hydroxyapatite alone is not suitable because of its low stability on long-term contact with tissue/biological fluids, even though it has great biocompatibility. Moreover, the acidic environment created through the fermentation of sugars that accumulate on teeth further accelerates the corrosion of hydroxyapatite. To deal with this issue, GO was researched as a reinforcement material and this showed the homogeneous dispersion and fluoride ions were also introduced by partially substituting the hydroxyl ions from hydroxyapatite. The resulting composite presents an increased biocompatibility, an increase in corrosion resistance and promotion of cell proliferation and differentiation [87].
A cathodic electrophoretic procedure was first mentioned for the fabrication of a GO/hydroxyapatite coating on a pure Ti substrate, in the employment of the GO at nanoscale in a bioactive coating [88]. Later, the GO was also used in combination with hydroxyapatite as a coating for 316L stainless steel. The functional groups of GO bind the calcium ions from the hydroxyapatite, thereby improving the strength of the material and also acting as anchors for the metallic surface and increasing the adhesion of the coating [89]. To further improve the biocompatibility and other parameters, polymers can also be used in combination with the hydroxyapatite and the GO. It was shown on a Ti alloy that the hydroxyapatite, doped with Mg and Zn and topped with polycaprolactone-GO, can establish direct bonds with the bone tissue, while also having bactericidal properties [90]. Many other combinations of GO with bioactive materials have been recently investigated, introducing more applications using new technologies [91][92][91,92].
Furthermore, it was proved in a series of studies that graphene-based coatings can improve the corrosion resistance of different substrates, and this was further challenged by Malhotra R et al., who performed corrosion tests over a period of 240 days, with the use of a very acidic solution. The coated alloy, Ti-6Al-4V, showed negligible signs of corrosion after 240 days, thereby maintaining its structural integrity, while the uncoated alloy presented a high corrosion rate, resulting in a 12-fold increase in surface roughness [93]. Another study on the same alloy that used a bioglass/graphene oxide coating, showed improved antibacterial activity that increased along with the content of the GO [94].
Of course, other factors contributed to the outcome, such as the geometry of the implant. Different alterations regarding the helix angle, the thread pitch and the dimensions also produced different results, due to stress distribution [95].
