Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Afroditi Konsoula | -- | 2434 | 2022-07-21 17:13:38 | | | |
| 2 | Jessie Wu | Meta information modification | 2434 | 2022-09-05 04:48:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Konsoula, A.; Tsioutis, C.; Markaki, I.; Papadakis, M.; Agouridis, A.P.; Spernovasilis, N. Lomentospora prolificans: A Neglected Fungus. Encyclopedia. Available online: https://encyclopedia.pub/entry/26853 (accessed on 07 February 2026).
Konsoula A, Tsioutis C, Markaki I, Papadakis M, Agouridis AP, Spernovasilis N. Lomentospora prolificans: A Neglected Fungus. Encyclopedia. Available at: https://encyclopedia.pub/entry/26853. Accessed February 07, 2026.
Konsoula, Afroditi, Constantinos Tsioutis, Ioulia Markaki, Michail Papadakis, Aris P. Agouridis, Nikolaos Spernovasilis. "Lomentospora prolificans: A Neglected Fungus" Encyclopedia, https://encyclopedia.pub/entry/26853 (accessed February 07, 2026).
Konsoula, A., Tsioutis, C., Markaki, I., Papadakis, M., Agouridis, A.P., & Spernovasilis, N. (2022, September 04). Lomentospora prolificans: A Neglected Fungus. In Encyclopedia. https://encyclopedia.pub/entry/26853
Konsoula, Afroditi, et al. "Lomentospora prolificans: A Neglected Fungus." Encyclopedia. Web. 04 September, 2022.
Copy Citation
Lomentospora prolificans, formerly known as Scedosporium prolificans, is a rare, highly virulent filamentous fungus that has been incriminated for numerous infections in immunocompromised as well as immunocompetent individuals. Disseminated disease is largely confined to immunocompromised patients and has a high mortality rate. Successful recovery from infection is difficult due to high rates of intrinsic resistance to antifungals. Aggressive surgical debridement wherever appropriate, and effective and timely antifungal treatment are the pillars for successful management.
Lomentospora prolificans
fungal infection
opportunistic
immunosupression
1. Pathogenesis and Host Defense
The first step in the pathogenesis of L. prolificans is the introduction of the fungus into the host. There are two well-known routes by which the fungus enters the human body: by inhalation of airborne conidia or by traumatic inoculation of conidial cells from contaminated environmental sources [1]. Depending on the immunologic status of the host, infection may be localized, extend to the surrounding tissues (deep extension), or disseminate hematogenously to distant organs [1]. Patients with impaired bronchopulmonary anatomy, as in cystic fibrosis, bronchiectasis and lung transplantation, are susceptible to chronic airway colonization [2][3].
After infection, a crucial step in both the life cycle and the pathogenesis is the transformation of conidia into hyphae, a process called germination [4]. In healthy individuals, conidia can be cleared by the mucociliary escalator or by pulmonary alveolar macrophages [1]. If these primary defense mechanisms fail and conidia germinate and form hyphae, they can penetrate macrophages and invade cells/tissues, as well as basal membranes/extracellular matrices [4]. One study showed that the in vitro interaction of human lung epithelial cells with L. prolificans resulted in the complete destruction of the monolayer of the epithelial cells and the formation of a biofilm [5]. In addition, hyphae can infiltrate blood vessels, cause extensive tissue infarction, and lead to widespread dissemination [6].
Depending on the site of entry, the immunological response varies, and different cells are challenged to remove the conidia. Initially, the innate immune system recognizes molecular components in the fungal cell wall through pattern recognition receptors (PRRs) [7]. The PRRs induce the synthesis of pro-inflammatory cytokines, phagocytosis, and adaptive immunity mechanisms. Toll-like-receptors (TLRs), especially TLR2 and TLR4, and C-type lectin receptors, such as Dectin-1 and mannose receptors (MR), are mainly investigated as PRRs. Their importance was demonstrated by Lamaris et al.; TLR-deficient Drosophila melanogaster flies were susceptible to infection with L. prolificans and developed acute infections with high mortality rates [8]. In another study, MR and Dectin-1 receptors were found to mediate conidial uptake by central nervous system (CNS) microglial cells [9]. It should be emphasized that blocking each receptor individually successfully inhibited the process of phagocytosis, but simultaneous inhibition of the aforementioned receptors did not result in a synergistic effect [9]. Thus, it is hypothesized that other receptors may also be involved in this process [9].
Potential virulence factors in the fungal cell wall involved in important biological events include peptidorhamnomannan, glucosylceramide, and melanin [10]. Peptidorhamnomannan, especially the O-glycosides of the molecule, is a key determinant for fungal recognition and phagocytosis, and induces killing by macrophages and production of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), and nitric oxide (NO) [11]. Another bioactive molecule present on the surface of conidial and hyphal cells is glucosylceramide (GLcCer) [10]. GLcCer belongs to sphingolipids, which are essential for fungal growth, virulence, and hyphal elongation. Explicitly, purified GLcCer from L. prolificans activates peritoneal macrophages, leading to the production of NO and superoxide and, consequently, to conidial death [12]. Moreover, in vivo experiments have shown that purified GLcCer from L. prolificans was able to increase the production of pro-inflammatory cytokines by splenocytes and induce recruitment of PMNs, eosinophils, small peritoneal macrophages, and mononuclear cells to the peritoneal cavity [10][12]. However, the receptor of GLcCer has not yet been discovered. In addition, L. prolificans produces 1, 8-dihydroxynaphthalene melanin (DHN-melanin), which helps in evading the immune response by masking pathogen-associated molecular patterns (PAMPs), by blocking phagolysosome formation and acidification, and by interfering with host cell apoptotic pathways [10]. Targeted deletion of melanin biosynthetic genes has shown that melanin protects fungi from oxidative killing by H2O2 and UV radiation [13].
Recognition of the fungal molecules triggers the immune system to confront the microbial presence. Phagocytes (monocytes, neutrophils, microglia) are among the most important cells against fungal infections, as they are able to recognize and phagocytose the fungi and act as antigen-presenting cells, bridging innate and adaptive immune responses. In a study conducted by Gil-Lamaignere et al., the innate immune response was compared to the well-studied fungus, Aspergillus fumigatus [14]. Specifically, monocyte-derived macrophages could phagocytose L. prolificans, in a sense proportional to A. fumigatus, despite the larger size of its conidia. In contrast, the germination process of L. prolificans conidia was inhibited less efficiently than that of A. fumigatus [14]. Thus, despite the fact that conidia can be phagocytosed, they can germinate inside macrophages and form germ tube-like projections that can lyse the membrane to reach the extracellular medium.
Neutrophils are an important component of the innate immunity regarding the control of the hyphae. Neutrophils damage hyphae mainly by degranulation by the release of large amounts of reactive oxygen species (ROS) and by the formation of neutrophil extracellular traps (NETs) that enclose microbes in a matrix of DNA and enzymes with antimicrobial activity [14][15][16]. The aforementioned susceptibility of this fungus to the innate immune system may explain its high incidence in neutropenic patients [17].
It has been demonstrated that phagocytes in the CNS respond poorly to this fungus and allow germination and branching of the hyphae [9]. Specifically, phagocytosis is impaired in microglial cells compared with other phagocytes, with lower release of pro-inflammatory cytokines, such as TNF and interleukin-6 (IL-6), and production of ROS [9]. Even in extremely acidic environments, as may be found inside microglial phagolysosomes, L. prolificans cells manage to survive pH stress and maintain high viability levels under both basic and acidic conditions [9]. Given the data above, a weak microglial response against L. prolificans could partially explain the propensity of this fungus to invade and live in the CNS, a phenomenon known as neurotropism [9].
A number of studies have aimed to evaluate the immunomodulatory and therapeutic effect of cytokines against L. prolificans [9][18]. L. prolificans has been shown to elicit higher synthesis of TNF-a and IL-6 by human monocytes in vitro compared with Aspergillus species [18]. This effect may be associated with the differences in the composition of their cell walls [18]. Similarly, Pellon et al. measured the release of these cytokines by peritoneal macrophage-like cells and microglial cells and demonstrated that macrophages produce them faster and at higher concentrations [9]. Therefore, as mentioned above, microglial response to this fungus is impaired.
Since cytokines are produced by immune cells in response to the presence of fungi, they have been studied as therapeutic agents alone or in combination with other drugs. The granulocyte-colony stimulating factor (G-CSF) has been shown to be effective against L. prolificans invasion in neutropenic hosts when combined with antifungal agents [19][20]. G-CSF stimulates proliferation and differentiation of myeloid progenitor cells, resulting in increased numbers of circulating neutrophils and enhanced phagocytic response [21].
Other cytokines that have been studied for therapeutic purposes include granulocyte-macrophage colony stimulating factor (GM-CSF) and interferon gamma (INF-γ). GM-CSF stimulates myeloid hematopoiesis in the early stages of differentiation of myeloid cells to produce more neutrophils, eosinophils and monocytes [21], enhancing their antifungal response and the expression of TLR2 and Dectin-1 [22][23][24]. INF-γ is a crucial cytokine for the innate and adaptive immune response to invasive fungal infections, mainly because it is associated with the migration, adherence and antifungal activity of neutrophils and/or macrophages [21][25]. It has been demonstrated that INF-γ and GM-CSF in combination accelerate the antifungal activity of neutrophils by increasing superoxide production [25]. Likewise, treatment with interleukin-15 has been shown to enhance hyphal damage, release of interleukin-8 and oxidative burst of neutrophils in response to L. prolificans [26]. Therefore, considering the previously mentioned evidence and the susceptibility of this fungus to phagocytosis, the low incidence in immunocompetent individuals can be explained [15].
Some of the antigenic epitopes of L. prolificans have recently been identified and some of the antibodies that recognize them may provide protection against this fungus [7][27][28]. Of note, human saliva containing IgA almost exclusively recognizes L. prolificans conidia [28], whereas serum IgG recognizes both forms of the fungus, hyphae and conidia [27]. This finding is consistent with the hypothesis of fungal invasion of the respiratory tract, in which conidia and not hyphae are inhaled by the host [28]. Saliva and serum from immunocompetent individuals were used in these studies [27][28]. Some of these antibodies produced by healthy populations may provide protection against fungal infections, and their antigenic targets may be investigated as therapeutic agents in the future [7] (Figure 1).
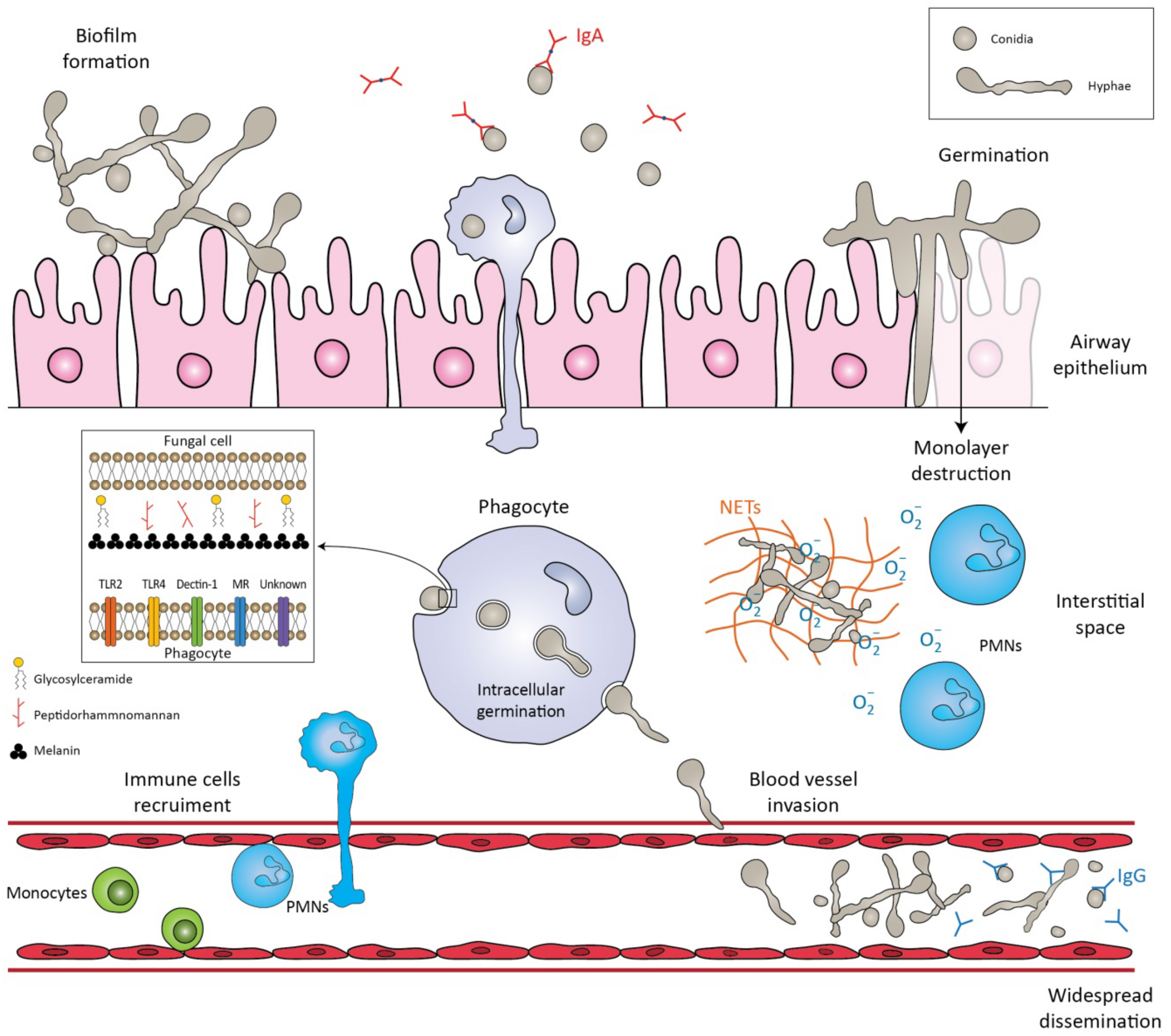
Figure 1. Schematic representation of the immune response against Lomentospora prolificans. The conidia are inhaled by the host. The mucociliary escalator and pulmonary alveolar macrophages can clear the conidia. If these primary mechanisms fail, the conidia transform into hyphae, a process called germination. The hyphae can form a biofilm, invade cell tissues/extracellular matrices, or destroy the monolayer of epithelial cells. Potential virulence factors in the fungal cell wall are peptidorhamnomannan, glycosylceramide, and melanin. Pattern recognition receptors (PRRs) that are involved in the recognition of fungus by phagocytes are TLR2, TLR4, Dectin-1, ΜR and other unknown receptors. Recognition of fungal molecules leads to the activation of immune cells in response to the microbial presence. Polymorphonuclears (PMNs) damage hyphae by degranulating reactive oxygen species (ROS) and by forming neutrophil extracellular traps (NETs). Even if phagocytosed, conidia can germinate inside phagocytes and penetrate their cell membrane. The hyphae can invade blood vessels and sporulate, leading to widespread dissemination. Salivary IgA exclusively recognizes the conidia, whereas serum IgG recognizes both forms of the fungus, conidia and hyphae.
2. Antifungal Therapeutic Strategies
Treatment of deep infections caused by L. prolificans remains a rather challenging aspect, as the pathogen carries intrinsic resistance to most of the antifungal regimens used in clinical practice. The lack of new and effective antifungal agents makes the treatment of such infections even harder.
In further detail, L. prolificans has been described in the current literature as a pan-antifungal resistant species [29][30], with innate resistance to commonly used antifungals [31]. Voriconazole has been proposed as the initial regimen for L. prolificans disseminated infections, complementary to surgical removal of the infected tissue when deemed feasible [30][32]. Such suggestions come from studies that demonstrate that voriconazole has the most robust antifungal effect when compared to other regimens [33], but without offering significant reductions in mortality rates [15][30].
A potential solution is the combination of antifungal agents. In vitro studies have demonstrated that dual therapy with voriconazole and amphotericin B or echinocandins could have a synergistic effect against L. prolificans [31][34], while the same principles apply to combinations of various azoles (voriconazole, itraconazole, miconazole) and terbinafine, which have demonstrated well-documented synergy with beneficial outcomes [33]. The triple combination of voriconazole, amphotericin B and anidulafungin has also been tested in vitro, with reported high synergistic effects [35].
In accordance with the aforementioned, current clinical practice guidelines recommend that treatment, including surgical resection when deemed feasible, should be initiated immediately when L. prolificans invasive infection is confirmed or suspected [36]. Additionally, the first line of antifungal treatment should include combination therapy with voriconazole and terbinafine, whereas other combinations are only moderately or marginally recommended because there are limited data to support such a therapeutic approach [36]. Monotherapy with voriconazole should only be considered as first line treatment in immunocompetent patients with localized infection [36]. The duration of treatment is controversial, with current recommendations suggesting that combination antifungal therapy lasting at least 4 to 6 months is most likely to be associated with favorable outcomes, while it is highly recommended that response to treatment should be frequently assessed [36]. In case of disease progression, salvage treatment should be individualized and tailored to previous regimen administration [36] (Figure 2).
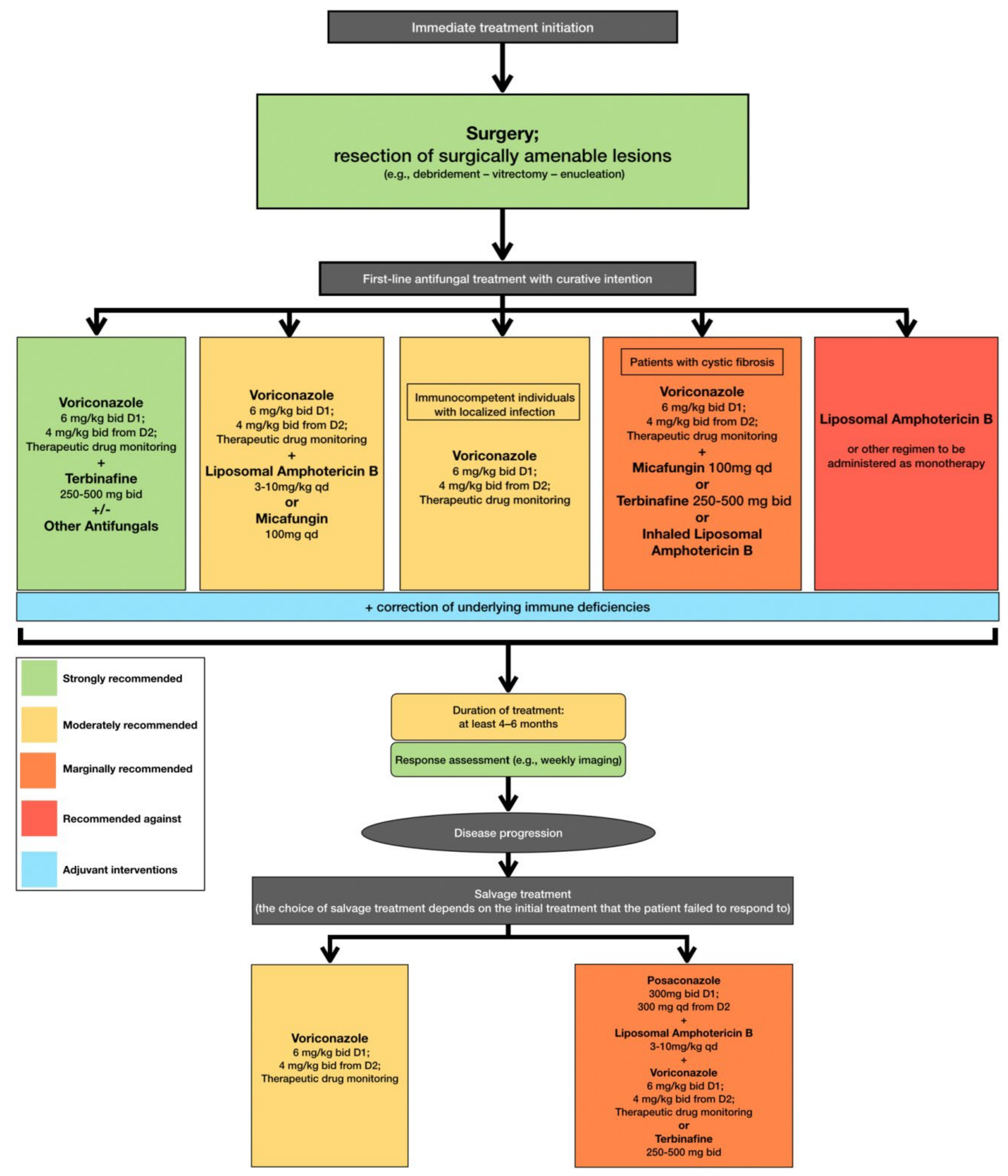
Figure 2. Optimal therapeutical pathway for L. prolificans infections in adults.
Schemuth et al. described the potential role of antibiotics in treating such infections, as colistin was demonstrated to have an antifungal effect when tested in vitro, either as monotherapy or when combined with antifungal regimens [37]. Moreover, Homa et al. studied the use of five antipsychotic regimens (chlorpromazine hydrochloride, trifluoperazine hydrochloride, amantadine hydrochloride, R-(-)-deprenyl hydrochloride, and valproic acid sodium salt) with antifungal activity, and their prospects in fungal infections in vitro [38]. In further detail, phenothiazines could exhibit possible antifungal properties in the treatment of locally invasive L. prolificans infections, while of interest remain possible combinations of antipsychotic and antifungal regimens [38].
Immune modulation interventions also play a key role in treating L. prolificans infections, as available data support that resolution of neutropenia in immunocompromised individuals is associated with a favorable outcome. In fact, in disseminated infection by L. prolificans in an immunocompromised murine model, treatment with G-CSF and liposomal amphotericin B (LAMB) improved survival compared with LAMB alone, but the improvement was not statistically significant [20]. Such data seem to also be supported by clinical experience, as there are case studies where reversion of neutropenia was linked to favorable patient outcome [19][39].
Another approach that needs further investigation is the use of adjunctive hyperbaric oxygen therapy. In a study conducted by Farina et al., in vitro tests showed that all antifungal agents had lower minimum inhibitory concentrations (MICs) when incubated with L. prolificans isolates in a hyperbaric hyperoxide atmosphere (100% O2) [40]. However, when incubated in a normal atmosphere, growth was systematically observed and MICs returned to the expected high levels [40]. Future in vivo studies may provide more information on whether hyperbaric oxygen therapy can potentially increase the antifungal activity of a single antifungal agent in order to replace the use of combination antifungal treatment.
New prospects in the treatment of disseminated resistant fungal infections are focused on the development of new pharmaceutical compounds and on deciphering molecular mechanisms through which L. prolificans responds to antifungal treatment. Miyazaki et al. reported that the compound E1210, a molecule that inhibits the inositol acylation step in glycosylphosphatidylinositol biosynthesis, resulting in defects in fungal cells [41], offered the potential for broader antifungal activity when compared to conventional antifungal medication, presenting with potent activity against L. prolificans when tested in vitro, even against azole and amphotericin B resistant strains [42]. Olorofim, the first member of the orotomide class of antifungals to be clinically tested for the treatment of such infections, has shown promising results. This molecule has the capability to inhibit dihydroorotate dehydrogenase, a key enzyme in the biosynthesis of pyrimidines. The efficacy of olorofim has been demonstrated in in vitro studies, and improved clinical outcomes were observed in two case reports [43][44][45]. The clinical efficacy of this regimen is still being tested, as the medication is currently in Phase IIB clinical trials, with existing published data coming from case reports [44][45].
Additionally, interesting data emerge from studies of the fungal molecular and structural alterations in response to antifungal treatment, as such results could shed light on yet unknown molecular cascades and potential pharmaceutical targets. In this setting, Pellon et al. studied the alterations that occur to L. prolificans after the administration of voriconazole, concluding that the overexpression of certain protein molecules such as heat shock proteins (HSP) could play an important role in the orchestration of antifungal drug resistance, proposing potential molecular targets for novel, more effective, antifungal compounds [46].
References
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197.
- Cooley, L.; Spelman, D.; Thursky, K.; Slavin, M. Infection with Scedosporium apiospermum and S. prolificans, Australia. Emerg. Infect. Dis. 2007, 13, 1170–1177.
- Rougeron, A.; Giraud, S.; Alastruey-Izquierdo, A.; Cano-Lira, J.; Rainer, J.; Mouhajir, A.; Le Gal, S.; Nevez, G.; Meyer, W.; Bouchara, J.P. Ecology of Scedosporium Species: Present Knowledge and Future Research. Mycopathologia 2018, 183, 185–200.
- Mello, T.P.; Aor, A.C.; Oliveira, S.S.; Branquinha, M.H.; Santos, A.L. Conidial germination in Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans: Influence of growth conditions and antifungal susceptibility profiles. Mem. Inst. Oswaldo Cruz 2016, 111, 484–494.
- Mello, T.P.; Aor, A.C.; Goncalves, D.S.; Seabra, S.H.; Branquinha, M.H.; Santos, A.L. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling 2016, 32, 737–749.
- Kauffman, C.A. Fungal infections. Proc. Am. Thorac. Soc. 2006, 3, 35–40.
- Buldain, I.; Martin-Souto, L.; Antoran, A.; Areitio, M.; Aparicio-Fernandez, L.; Rementeria, A.; Hernando, F.L.; Ramirez-Garcia, A. The Host Immune Response to Scedosporium/Lomentospora. J. Fungi 2021, 7, 75.
- Lamaris, G.A.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Virulence studies of Scedosporium and Fusarium species in Drosophila melanogaster. J. Infect. Dis. 2007, 196, 1860–1864.
- Pellon, A.; Ramirez-Garcia, A.; Guruceaga, X.; Zabala, A.; Buldain, I.; Antoran, A.; Anguita, J.; Rementeria, A.; Matute, C.; Hernando, F.L. Microglial immune response is impaired against the neurotropic fungus Lomentospora prolificans. Cell. Microbiol. 2018, 20, e12847.
- Rollin-Pinheiro, R.; da Silva Xisto, M.I.D.; Rochetti, V.P.; Barreto-Bergter, E. Scedosporium Cell Wall: From Carbohydrate-Containing Structures to Host-Pathogen Interactions. Mycopathologia 2020, 185, 931–946.
- Xisto, M.I.; Bittencourt, V.C.; Liporagi-Lopes, L.C.; Haido, R.M.; Mendonca, M.S.; Sassaki, G.; Figueiredo, R.T.; Romanos, M.T.V.; Barreto-Bergter, E. O-glycosylation in cell wall proteins in Scedosporium prolificans is critical for phagocytosis and inflammatory cytokines production by macrophages. PLoS ONE 2015, 10, e0123189.
- Xisto, M.; Henao, J.E.M.; Dias, L.D.S.; Santos, G.M.P.; Calixto, R.O.R.; Bernardino, M.C.; Taborda, C.P.; Barreto-Bergter, E. Glucosylceramides From Lomentospora prolificans Induce a Differential Production of Cytokines and Increases the Microbicidal Activity of Macrophages. Front. Microbiol. 2019, 10, 554.
- Al-Laaeiby, A.; Kershaw, M.J.; Penn, T.J.; Thornton, C.R. Targeted Disruption of Melanin Biosynthesis Genes in the Human Pathogenic Fungus Lomentospora prolificans and Its Consequences for Pathogen Survival. Int. J. Mol. Sci. 2016, 17, 444.
- Gil-Lamaignere, C.; Maloukou, A.; Rodriguez-Tudela, J.L.; Roilides, E. Human phagocytic cell responses to Scedosporium prolificans. Med. Mycol. 2001, 39, 169–175.
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; De Meirelles, J.V.; Xisto, M.I.D.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An updated overview of underrated opportunists. Med. Mycol. 2018, 56 (Suppl. 1), 102–125.
- Brakhage, A.A.; Bruns, S.; Thywissen, A.; Zipfel, P.F.; Behnsen, J. Interaction of phagocytes with filamentous fungi. Curr. Opin. Microbiol. 2010, 13, 409–415.
- Bronnimann, D.; Garcia-Hermoso, D.; Dromer, F.; Lanternier, F.; The French Mycoses Study Group; Characterization of the Isolates at the NRCMA. Scedosporiosis/lomentosporiosis observational study (SOS): Clinical significance of Scedosporium species identification. Med. Mycol. 2021, 59, 486–497.
- Warris, A.; Netea, M.G.; Verweij, P.E.; Gaustad, P.; Kullberg, B.J.; Weemaes, C.M.; Abrahamsen, T.G. Cytokine responses and regulation of interferon-gamma release by human mononuclear cells to Aspergillus fumigatus and other filamentous fungi. Med. Mycol. 2005, 43, 613–621.
- Bouza, E.; Munoz, P.; Vega, L.; Rodriguez-Creixems, M.; Berenguer, J.; Escudero, A. Clinical resolution of Scedosporium prolificans fungemia associated with reversal of neutropenia following administration of granulocyte colony-stimulating factor. Clin. Infect. Dis. 1996, 23, 192–193.
- Ortoneda, M.; Capilla, J.; Pujol, I.; Pastor, F.J.; Mayayo, E.; Fernandez-Ballart, J.; Guarro, J. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 2002, 49, 525–529.
- Antachopoulos, C.; Roilides, E. Cytokines and fungal infections. Br. J. Haematol. 2005, 129, 583–596.
- Armitage, J.O. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 1998, 92, 4491–4508.
- Kurt-Jones, E.A.; Mandell, L.; Whitney, C.; Padgett, A.; Gosselin, K.; Newburger, P.E.; Finberg, R.W. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood 2002, 100, 1860–1868.
- Willment, J.A.; Lin, H.H.; Reid, D.M.; Taylor, P.R.; Williams, D.L.; Wong, S.Y.; Gordon, S.; Brown, G.D. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 2003, 171, 4569–4573.
- Gil-Lamaignere, C.; Winn, R.M.; Simitsopoulou, M.; Maloukou, A.; Walsh, T.J.; Roilides, E. Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: Comparison with Aspergillus spp. Med. Mycol. 2005, 43, 253–260.
- Winn, R.M.; Gil-Lamaignere, C.; Roilides, E.; Simitsopoulou, M.; Lyman, C.A.; Maloukou, A.; Walsh, T.J. Effects of interleukin-15 on antifungal responses of human polymorphonuclear leukocytes against Fusarium spp. and Scedosporium spp. Cytokine 2005, 31, 1–8.
- Pellon, A.; Ramirez-Garcia, A.; Buldain, I.; Antoran, A.; Rementeria, A.; Hernando, F.L. Immunoproteomics-Based Analysis of the Immunocompetent Serological Response to Lomentospora prolificans. J. Proteome Res. 2016, 15, 595–607.
- Pellon, A.; Ramirez-Garcia, A.; Antoran, A.; Fernandez-Molina, J.V.; Abad-Diaz-de-Cerio, A.; Montanez, D.; Sevilla, M.J.; Rementeria, A.; Hernando, F.L. Scedosporium prolificans immunomes against human salivary immunoglobulin A. Fungal Biol. 2014, 118, 94–105.
- Lackner, M.; Rezusta, A.; Villuendas, M.C.; Palacian, M.P.; Meis, J.F.; Klaassen, C.H. Infection and colonisation due to Scedosporium in Northern Spain. An in vitro antifungal susceptibility and molecular epidemiology study of 60 isolates. Mycoses 2011, 54 (Suppl. 3), 12–21.
- Tortorano, A.M.; Richardson, M.; Roilides, E.; Van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp.; Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 27–46.
- Cuenca-Estrella, M.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Gomez-Lopez, A.; Buitrago, M.J.; Mellado, E.; Rodriguez-Tudela, J.L. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob. Agents Chemother. 2008, 52, 1136–1139.
- Pellon, A.; Ramirez-Garcia, A.; Buldain, I.; Antoran, A.; Martin-Souto, L.; Rementeria, A.; Hernando, F.L. Pathobiology of Lomentospora prolificans: Could this species serve as a model of primary antifungal resistance? Int. J. Antimicrob. Agents 2018, 51, 10–15.
- Meletiadis, J.; Meis, J.F.; Mouton, J.W.; Rodriquez-Tudela, J.L.; Donnelly, J.P.; Verweij, P.E.; Eurofung Network. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 2002, 46, 62–68.
- Yustes, C.; Guarro, J. In vitro synergistic interaction between amphotericin B and micafungin against Scedosporium spp. Antimicrob. Agents Chemother. 2005, 49, 3498–3500.
- Martin-Vicente, A.; Guarro, J.; Capilla, J. Does a triple combination have better activity than double combinations against multiresistant fungi? Experimental in vitro evaluation. Int. J. Antimicrob. Agents 2017, 49, 422–426.
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257.
- Schemuth, H.; Dittmer, S.; Lackner, M.; Sedlacek, L.; Hamprecht, A.; Steinmann, E.; Buer, J.; Rath, P.M.; Steinmann, J. In vitro activity of colistin as single agent and in combination with antifungals against filamentous fungi occurring in patients with cystic fibrosis. Mycoses 2013, 56, 297–303.
- Homa, M.; Galgoczy, L.; Toth, E.; Toth, L.; Papp, T.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vagvolgyi, C. In vitro antifungal activity of antipsychotic drugs and their combinations with conventional antifungals against Scedosporium and Pseudallescheria isolates. Med. Mycol. 2015, 53, 890–895.
- Howden, B.P.; Slavin, M.A.; Schwarer, A.P.; Mijch, A.M. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 111–113.
- Farina, C.; Marchesi, G.; Passera, M.; Diliberto, C.; Russello, G. Comparative study of the in vitro activity of various antifungal drugs against Scedosporium spp. in aerobic and hyperbaric atmosphere versus normal atmosphere. J. Mycol. Med. 2012, 22, 142–148.
- Castanheira, M.; Duncanson, F.P.; Diekema, D.J.; Guarro, J.; Jones, R.N.; Pfaller, M.A. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob. Agents Chemother. 2012, 56, 352–357.
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.A.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011, 55, 4652–4658.
- Wiederhold, N.P. Review of the Novel Investigational Antifungal Olorofim. J. Fungi 2020, 6, 122.
- Chen, S.; Rai, N.J.; Cunneen, S.; Cornelissen, K.; Rex, J.H.; Heath, C.H.; Harvey, E. A case of Lomentospora prolificans treated with the novel antifungal olorofim. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, 18–21 April 2020.
- Tio, S.; Thursky, K.; Ng, G.; Rex, J.; Carney, D.; Slavin, M. Olorofim for a case of severe disseminated Lomentospora prolificans infection. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, 18–21 April 2020.
- Pellon, A.; Ramirez-Garcia, A.; Buldain, I.; Antoran, A.; Rementeria, A.; Hernando, F.L. Molecular and cellular responses of the pathogenic fungus Lomentospora prolificans to the antifungal drug voriconazole. PLoS ONE 2017, 12, e0174885.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
05 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




