Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Lomentospora prolificans, formerly known as Scedosporium prolificans, is a rare, highly virulent filamentous fungus that has been incriminated for numerous infections in immunocompromised as well as immunocompetent individuals. Disseminated disease is largely confined to immunocompromised patients and has a high mortality rate. Successful recovery from infection is difficult due to high rates of intrinsic resistance to antifungals. Aggressive surgical debridement wherever appropriate, and effective and timely antifungal treatment are the pillars for successful management.
- Lomentospora prolificans
- fungal infection
- opportunistic
- immunosupression
1. Pathogenesis and Host Defense
The first step in the pathogenesis of L. prolificans is the introduction of the fungus into the host. There are two well-known routes by which the fungus enters the human body: by inhalation of airborne conidia or by traumatic inoculation of conidial cells from contaminated environmental sources [18]. Depending on the immunologic status of the host, infection may be localized, extend to the surrounding tissues (deep extension), or disseminate hematogenously to distant organs [18]. Patients with impaired bronchopulmonary anatomy, as in cystic fibrosis, bronchiectasis and lung transplantation, are susceptible to chronic airway colonization [14,19].
After infection, a crucial step in both the life cycle and the pathogenesis is the transformation of conidia into hyphae, a process called germination [20]. In healthy individuals, conidia can be cleared by the mucociliary escalator or by pulmonary alveolar macrophages [18]. If these primary defense mechanisms fail and conidia germinate and form hyphae, they can penetrate macrophages and invade cells/tissues, as well as basal membranes/extracellular matrices [20]. One study showed that the in vitro interaction of human lung epithelial cells with L. prolificans resulted in the complete destruction of the monolayer of the epithelial cells and the formation of a biofilm [21]. In addition, hyphae can infiltrate blood vessels, cause extensive tissue infarction, and lead to widespread dissemination [22].
Depending on the site of entry, the immunological response varies, and different cells are challenged to remove the conidia. Initially, the innate immune system recognizes molecular components in the fungal cell wall through pattern recognition receptors (PRRs) [23]. The PRRs induce the synthesis of pro-inflammatory cytokines, phagocytosis, and adaptive immunity mechanisms. Toll-like-receptors (TLRs), especially TLR2 and TLR4, and C-type lectin receptors, such as Dectin-1 and mannose receptors (MR), are mainly investigated as PRRs. Their importance was demonstrated by Lamaris et al.; TLR-deficient Drosophila melanogaster flies were susceptible to infection with L. prolificans and developed acute infections with high mortality rates [24]. In another study, MR and Dectin-1 receptors were found to mediate conidial uptake by central nervous system (CNS) microglial cells [25]. It should be emphasized that blocking each receptor individually successfully inhibited the process of phagocytosis, but simultaneous inhibition of the aforementioned receptors did not result in a synergistic effect [25]. Thus, it is hypothesized that other receptors may also be involved in this process [25].
Potential virulence factors in the fungal cell wall involved in important biological events include peptidorhamnomannan, glucosylceramide, and melanin [26]. Peptidorhamnomannan, especially the O-glycosides of the molecule, is a key determinant for fungal recognition and phagocytosis, and induces killing by macrophages and production of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), and nitric oxide (NO) [27]. Another bioactive molecule present on the surface of conidial and hyphal cells is glucosylceramide (GLcCer) [26]. GLcCer belongs to sphingolipids, which are essential for fungal growth, virulence, and hyphal elongation. Explicitly, purified GLcCer from L. prolificans activates peritoneal macrophages, leading to the production of NO and superoxide and, consequently, to conidial death [28]. Moreover, in vivo experiments have shown that purified GLcCer from L. prolificans was able to increase the production of pro-inflammatory cytokines by splenocytes and induce recruitment of PMNs, eosinophils, small peritoneal macrophages, and mononuclear cells to the peritoneal cavity [26,28]. However, the receptor of GLcCer has not yet been discovered. In addition, L. prolificans produces 1, 8-dihydroxynaphthalene melanin (DHN-melanin), which helps in evading the immune response by masking pathogen-associated molecular patterns (PAMPs), by blocking phagolysosome formation and acidification, and by interfering with host cell apoptotic pathways [26]. Targeted deletion of melanin biosynthetic genes has shown that melanin protects fungi from oxidative killing by H2O2 and UV radiation [29].
Recognition of the fungal molecules triggers the immune system to confront the microbial presence. Phagocytes (monocytes, neutrophils, microglia) are among the most important cells against fungal infections, as they are able to recognize and phagocytose the fungi and act as antigen-presenting cells, bridging innate and adaptive immune responses. In a study conducted by Gil-Lamaignere et al., the innate immune response was compared to the well-studied fungus, Aspergillus fumigatus [30]. Specifically, monocyte-derived macrophages could phagocytose L. prolificans, in a sense proportional to A. fumigatus, despite the larger size of its conidia. In contrast, the germination process of L. prolificans conidia was inhibited less efficiently than that of A. fumigatus [30]. Thus, despite the fact that conidia can be phagocytosed, they can germinate inside macrophages and form germ tube-like projections that can lyse the membrane to reach the extracellular medium.
Neutrophils are an important component of the innate immunity regarding the control of the hyphae. Neutrophils damage hyphae mainly by degranulation by the release of large amounts of reactive oxygen species (ROS) and by the formation of neutrophil extracellular traps (NETs) that enclose microbes in a matrix of DNA and enzymes with antimicrobial activity [8,30,31]. The aforementioned susceptibility of this fungus to the innate immune system may explain its high incidence in neutropenic patients [32].
It has been demonstrated that phagocytes in the CNS respond poorly to this fungus and allow germination and branching of the hyphae [25]. Specifically, phagocytosis is impaired in microglial cells compared with other phagocytes, with lower release of pro-inflammatory cytokines, such as TNF and interleukin-6 (IL-6), and production of ROS [25]. Even in extremely acidic environments, as may be found inside microglial phagolysosomes, L. prolificans cells manage to survive pH stress and maintain high viability levels under both basic and acidic conditions [25]. Given the data above, a weak microglial response against L. prolificans could partially explain the propensity of this fungus to invade and live in the CNS, a phenomenon known as neurotropism [25].
A number of studies have aimed to evaluate the immunomodulatory and therapeutic effect of cytokines against L. prolificans [25,33]. L. prolificans has been shown to elicit higher synthesis of TNF-a and IL-6 by human monocytes in vitro compared with Aspergillus species [33]. This effect may be associated with the differences in the composition of their cell walls [33]. Similarly, Pellon et al. measured the release of these cytokines by peritoneal macrophage-like cells and microglial cells and demonstrated that macrophages produce them faster and at higher concentrations [25]. Therefore, as mentioned above, microglial response to this fungus is impaired.
Since cytokines are produced by immune cells in response to the presence of fungi, they have been studied as therapeutic agents alone or in combination with other drugs. The granulocyte-colony stimulating factor (G-CSF) has been shown to be effective against L. prolificans invasion in neutropenic hosts when combined with antifungal agents [34,35]. G-CSF stimulates proliferation and differentiation of myeloid progenitor cells, resulting in increased numbers of circulating neutrophils and enhanced phagocytic response [36].
Other cytokines that have been studied for therapeutic purposes include granulocyte-macrophage colony stimulating factor (GM-CSF) and interferon gamma (INF-γ). GM-CSF stimulates myeloid hematopoiesis in the early stages of differentiation of myeloid cells to produce more neutrophils, eosinophils and monocytes [36], enhancing their antifungal response and the expression of TLR2 and Dectin-1 [37,38,39]. INF-γ is a crucial cytokine for the innate and adaptive immune response to invasive fungal infections, mainly because it is associated with the migration, adherence and antifungal activity of neutrophils and/or macrophages [36,40]. It has been demonstrated that INF-γ and GM-CSF in combination accelerate the antifungal activity of neutrophils by increasing superoxide production [40]. Likewise, treatment with interleukin-15 has been shown to enhance hyphal damage, release of interleukin-8 and oxidative burst of neutrophils in response to L. prolificans [41]. Therefore, considering the previously mentioned evidence and the susceptibility of this fungus to phagocytosis, the low incidence in immunocompetent individuals can be explained [8].
Some of the antigenic epitopes of L. prolificans have recently been identified and some of the antibodies that recognize them may provide protection against this fungus [23,42,43]. Of note, human saliva containing IgA almost exclusively recognizes L. prolificans conidia [43], whereas serum IgG recognizes both forms of the fungus, hyphae and conidia [42]. This finding is consistent with the hypothesis of fungal invasion of the respiratory tract, in which conidia and not hyphae are inhaled by the host [43]. Saliva and serum from immunocompetent individuals were used in these studies [42,43]. Some of these antibodies produced by healthy populations may provide protection against fungal infections, and their antigenic targets may be investigated as therapeutic agents in the future [23] (Figure 1).
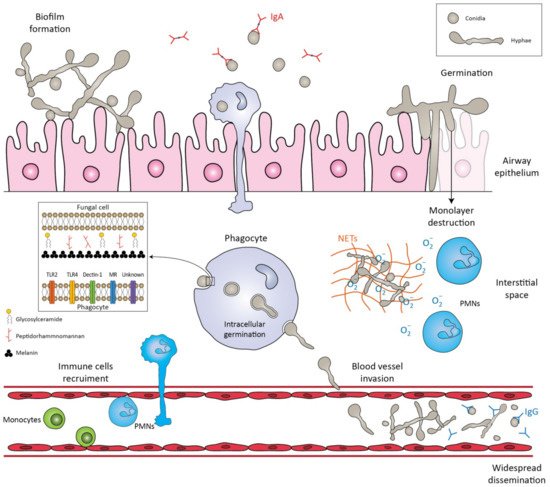
Figure 1. Schematic representation of the immune response against Lomentospora prolificans. The conidia are inhaled by the host. The mucociliary escalator and pulmonary alveolar macrophages can clear the conidia. If these primary mechanisms fail, the conidia transform into hyphae, a process called germination. The hyphae can form a biofilm, invade cell tissues/extracellular matrices, or destroy the monolayer of epithelial cells. Potential virulence factors in the fungal cell wall are peptidorhamnomannan, glycosylceramide, and melanin. Pattern recognition receptors (PRRs) that are involved in the recognition of fungus by phagocytes are TLR2, TLR4, Dectin-1, ΜR and other unknown receptors. Recognition of fungal molecules leads to the activation of immune cells in response to the microbial presence. Polymorphonuclears (PMNs) damage hyphae by degranulating reactive oxygen species (ROS) and by forming neutrophil extracellular traps (NETs). Even if phagocytosed, conidia can germinate inside phagocytes and penetrate their cell membrane. The hyphae can invade blood vessels and sporulate, leading to widespread dissemination. Salivary IgA exclusively recognizes the conidia, whereas serum IgG recognizes both forms of the fungus, conidia and hyphae.
2. Antifungal Therapeutic Strategies
Treatment of deep infections caused by L. prolificans remains a rather challenging aspect, as the pathogen carries intrinsic resistance to most of the antifungal regimens used in clinical practice. The lack of new and effective antifungal agents makes the treatment of such infections even harder.
In further detail, L. prolificans has been described in the current literature as a pan-antifungal resistant species [11,15], with innate resistance to commonly used antifungals [112]. Voriconazole has been proposed as the initial regimen for L. prolificans disseminated infections, complementary to surgical removal of the infected tissue when deemed feasible [15,113]. Such suggestions come from studies that demonstrate that voriconazole has the most robust antifungal effect when compared to other regimens [16], but without offering significant reductions in mortality rates [8,15].
A potential solution is the combination of antifungal agents. In vitro studies have demonstrated that dual therapy with voriconazole and amphotericin B or echinocandins could have a synergistic effect against L. prolificans [112,114], while the same principles apply to combinations of various azoles (voriconazole, itraconazole, miconazole) and terbinafine, which have demonstrated well-documented synergy with beneficial outcomes [16]. The triple combination of voriconazole, amphotericin B and anidulafungin has also been tested in vitro, with reported high synergistic effects [115].
In accordance with the aforementioned, current clinical practice guidelines recommend that treatment, including surgical resection when deemed feasible, should be initiated immediately when L. prolificans invasive infection is confirmed or suspected [95]. Additionally, the first line of antifungal treatment should include combination therapy with voriconazole and terbinafine, whereas other combinations are only moderately or marginally recommended because there are limited data to support such a therapeutic approach [95]. Monotherapy with voriconazole should only be considered as first line treatment in immunocompetent patients with localized infection [95]. The duration of treatment is controversial, with current recommendations suggesting that combination antifungal therapy lasting at least 4 to 6 months is most likely to be associated with favorable outcomes, while it is highly recommended that response to treatment should be frequently assessed [95]. In case of disease progression, salvage treatment should be individualized and tailored to previous regimen administration [95] (Figure 2).
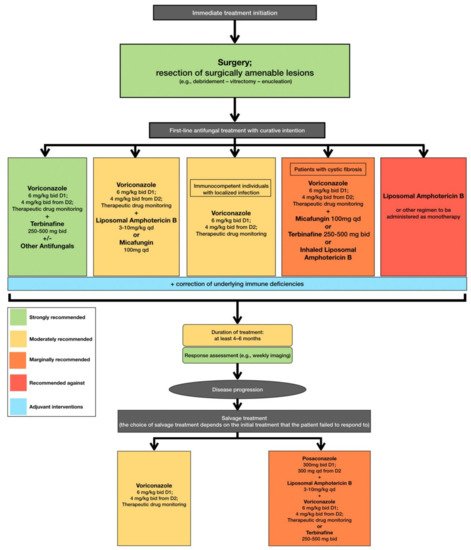
Figure 2. Optimal therapeutical pathway for L. prolificans infections in adults.
Schemuth et al. described the potential role of antibiotics in treating such infections, as colistin was demonstrated to have an antifungal effect when tested in vitro, either as monotherapy or when combined with antifungal regimens [116]. Moreover, Homa et al. studied the use of five antipsychotic regimens (chlorpromazine hydrochloride, trifluoperazine hydrochloride, amantadine hydrochloride, R-(-)-deprenyl hydrochloride, and valproic acid sodium salt) with antifungal activity, and their prospects in fungal infections in vitro [117]. In further detail, phenothiazines could exhibit possible antifungal properties in the treatment of locally invasive L. prolificans infections, while of interest remain possible combinations of antipsychotic and antifungal regimens [117].
Immune modulation interventions also play a key role in treating L. prolificans infections, as available data support that resolution of neutropenia in immunocompromised individuals is associated with a favorable outcome. In fact, in disseminated infection by L. prolificans in an immunocompromised murine model, treatment with G-CSF and liposomal amphotericin B (LAMB) improved survival compared with LAMB alone, but the improvement was not statistically significant [35]. Such data seem to also be supported by clinical experience, as there are case studies where reversion of neutropenia was linked to favorable patient outcome [34,118].
Another approach that needs further investigation is the use of adjunctive hyperbaric oxygen therapy. In a study conducted by Farina et al., in vitro tests showed that all antifungal agents had lower minimum inhibitory concentrations (MICs) when incubated with L. prolificans isolates in a hyperbaric hyperoxide atmosphere (100% O2) [119]. However, when incubated in a normal atmosphere, growth was systematically observed and MICs returned to the expected high levels [119]. Future in vivo studies may provide more information on whether hyperbaric oxygen therapy can potentially increase the antifungal activity of a single antifungal agent in order to replace the use of combination antifungal treatment.
New prospects in the treatment of disseminated resistant fungal infections are focused on the development of new pharmaceutical compounds and on deciphering molecular mechanisms through which L. prolificans responds to antifungal treatment. Miyazaki et al. reported that the compound E1210, a molecule that inhibits the inositol acylation step in glycosylphosphatidylinositol biosynthesis, resulting in defects in fungal cells [120], offered the potential for broader antifungal activity when compared to conventional antifungal medication, presenting with potent activity against L. prolificans when tested in vitro, even against azole and amphotericin B resistant strains [121]. Olorofim, the first member of the orotomide class of antifungals to be clinically tested for the treatment of such infections, has shown promising results. This molecule has the capability to inhibit dihydroorotate dehydrogenase, a key enzyme in the biosynthesis of pyrimidines. The efficacy of olorofim has been demonstrated in in vitro studies, and improved clinical outcomes were observed in two case reports [122,123,124]. The clinical efficacy of this regimen is still being tested, as the medication is currently in Phase IIB clinical trials, with existing published data coming from case reports [123,124].
Additionally, interesting data emerge from studies of the fungal molecular and structural alterations in response to antifungal treatment, as such results could shed light on yet unknown molecular cascades and potential pharmaceutical targets. In this setting, Pellon et al. studied the alterations that occur to L. prolificans after the administration of voriconazole, concluding that the overexpression of certain protein molecules such as heat shock proteins (HSP) could play an important role in the orchestration of antifungal drug resistance, proposing potential molecular targets for novel, more effective, antifungal compounds [125].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10071317
This entry is offline, you can click here to edit this entry!
