1. Adsorptive Membrane Technology
An adsorptive membrane is considered as a hybrid technology due to its dual functionality of adsorbing and filtering out pollutants. This membrane relies on a mass transfer process whereby the pollutants will get attached on the solid surface via chemical or physical interactions. Apart from offering flexibility in the design, this membrane is perceived to be cost-efficient due to its ability to regenerate adsorbents. The term “adsorption” was first coined in 1881 by a German physicist called Henrich Kayser
[1]. It was further expanded in the mid-1980s
[2]. Adsorption happens when separated or targeted ions are adsorbed on the membranes, when the solvent permeates through the pores of membranes that provide high flow rates and low internal diffusion along with faster adsorption and desorption rates
[3]. Adsorptive membranes are highly utilized for the purpose of heavy metal removal
[4], biotechnology-related separations
[5] and other water and wastewater treatment applications
[6]. This method is said to be simple and effective, as the adsorbent incorporated into the membrane matrix has the capability of capturing pollutants via chemical or physical relation.
Hybrid adsorptive membranes should be able to have an exclusive morphological structure and chemical structure in order for them to withstand the combined wastewater treatment applications. Therefore, their surface area and active sites in charge of adsorption must be very well preserved by adapting suitable fabrication techniques. As shown in Table 1, various fabrication techniques have been utilized in previous studies to attach multifunctional groups in a membrane framework in order to produce a highly adsorptive hybrid membrane for various contaminants. Each of the techniques has its own uniqueness in providing improved performance.
Table 1. Preparation of hybrid adsorptive membranes based on previous studies.
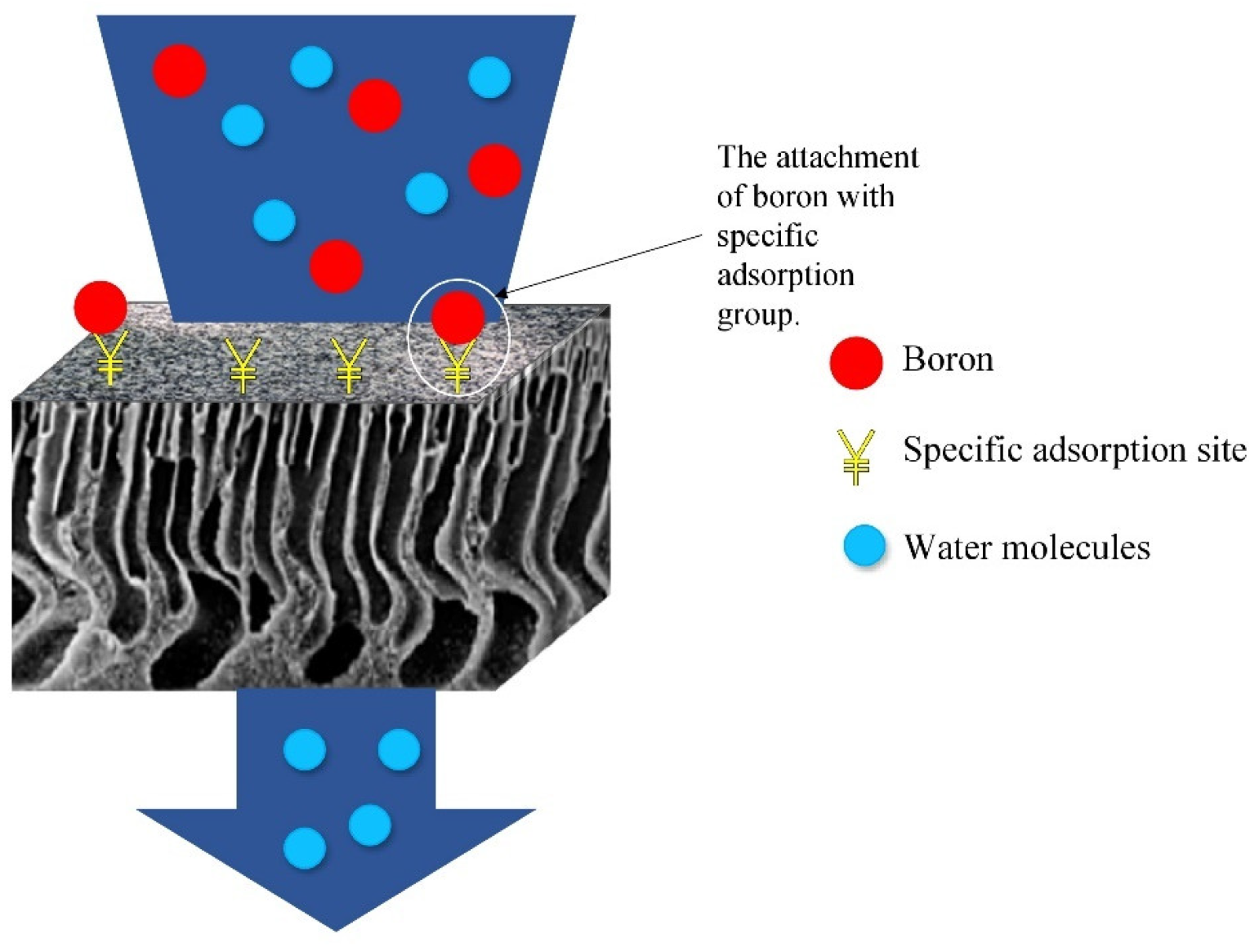
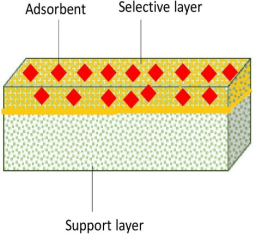
Adsorptive membranes are suitable to be used for boron removal as they retain boron molecules faster and provide a lower energy consumption and higher permeate flux. These membranes are easily functionalized to attach multiple functional groups as adsorption sites for favorable binding in order to maximize the adsorptive efficiency, as shown in
Figure 1. The attachment of boron with a specific adsorbent can be described by a possible binding mechanism proposed in
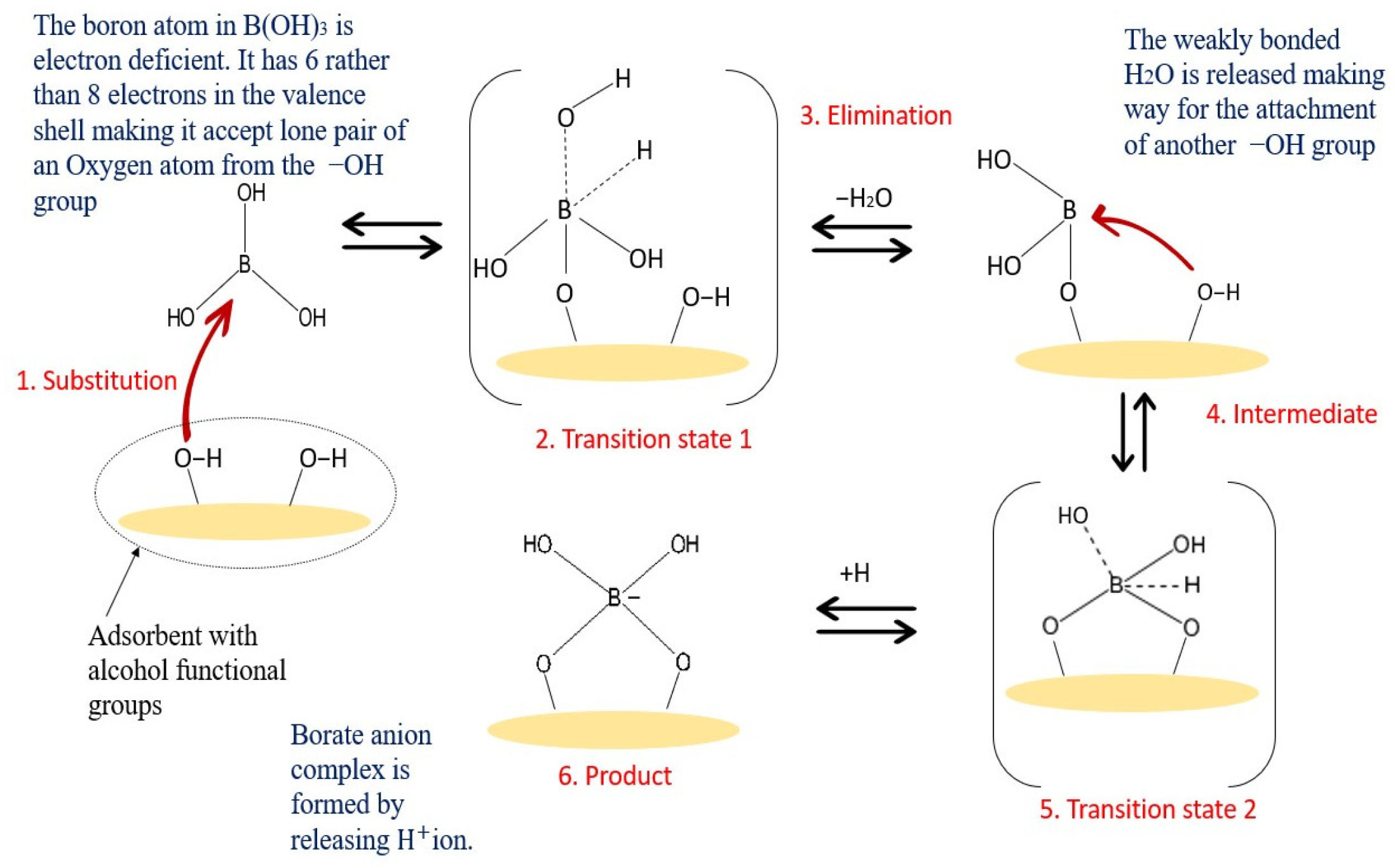
Figure 2. As shown in
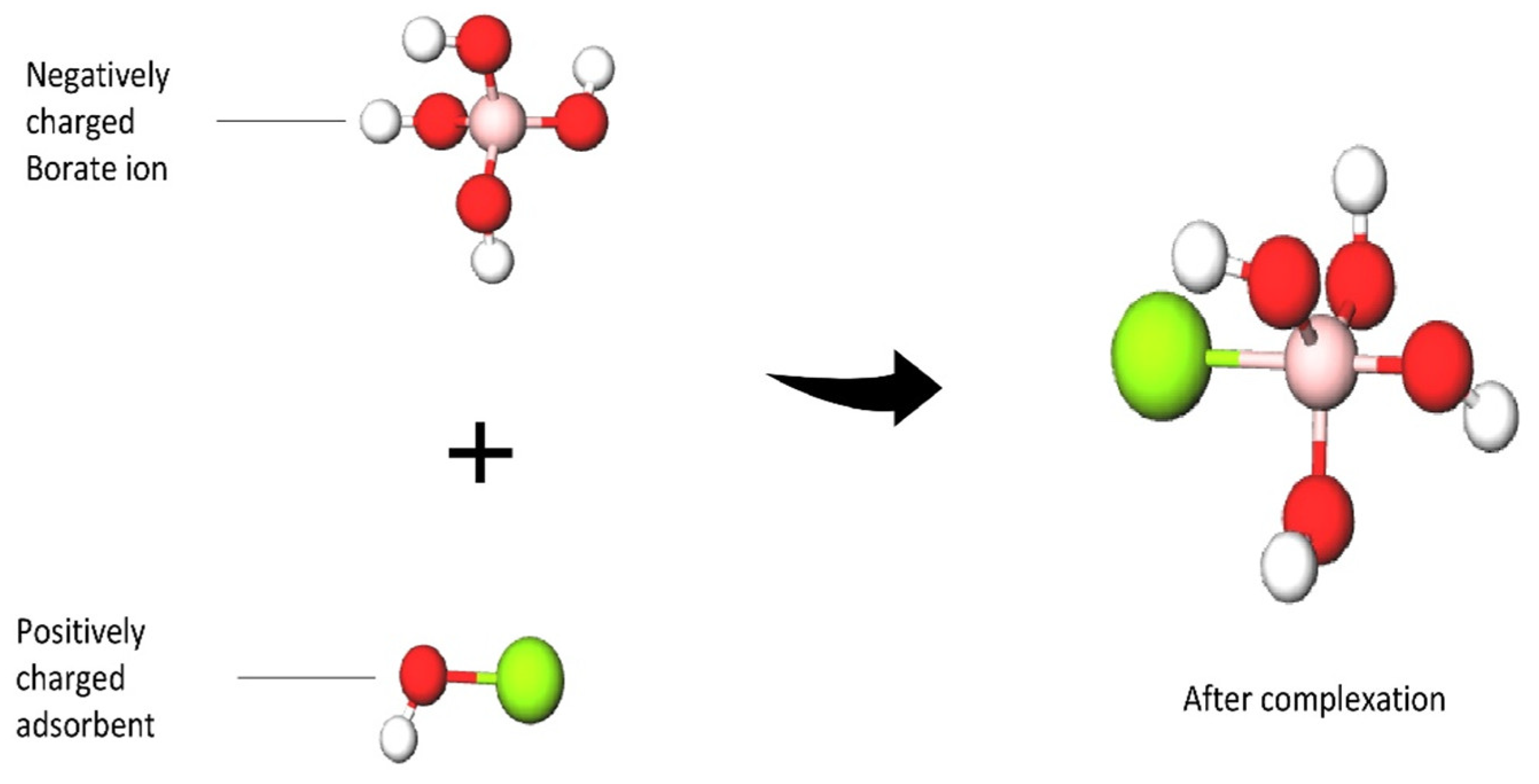
Figure 2, the -OH groups show a high affinity to boron, while these groups are not reactive to other elements. Adsorbents with these alcohol groups tend to bond through the formation of boric acid esters of boron or borate anion complexes with a proton as a counterion. Therefore, boron forms a coordination complex through a covalent attachment. As for the boron that exists as borate anions in water, it will be bonded with a positively charged adsorbent through ion exchange, and the structures are shown in
Figure 3. Due to the release of acidic protons during complexation, the pH of the solution decreases. This complexation reaction also plays a vital role in boron uptake from water. The stability of the borate complex depends on the type of diol in the adsorbent. If the diol involves -OH groups being oriented in such a way that they accurately match the structural parameters required by tetrahedrally coordinated boron, a strong complex will be formed
[14]. A stronger and stable complex will be able to provide efficient boron uptake.
Figure 1. Overall illustration about capturing boron using an adsorptive membrane.
Figure 2. Possible binding mechanism of boric acid and adsorbent with -OH functional groups.
Figure 3. Illustration of the view of the structure after the complexation reaction.
2. Single-Layered Adsorptive Membranes
Single-layered adsorptive membranes have always been the top choice in removing boron since its fabrication process is easier. It is the idea of filling a whole membrane matrix with adsorbents to be used for further rejection testing. This method has been developed in order to solve the hydrophobicity issue that has been faced by most polymeric membranes. The failure of possessing a low affinity towards water makes most of the membranes unfavorable for any water treatment applications. Regardless of the materials used, the overall aim of an adsorptive membrane is to have an excellent rejection without compromising the stable flux and mechanical strength of the membranes. Pore size is one of the important factors that contributes to the aforementioned standards. Incorporating adsorbents into a polymer material can contribute to the narrow pore distribution, which is needed in industrial application nowadays. However, the loading of the adsorbent in an adsorptive membrane must be carefully studied, as too many particles in a polymer matrix can cause aggregation, which can further deteriorate the membrane performance through a cakey layer formation. Due to these reasons, single-layered adsorptive membranes tend to have a shorter lifespan, as they need to be replaced frequently with other membranes for better membrane performance. Therefore, an upgraded version that focuses more on the mechanical strength, which is the double-layer adsorptive membrane, is developed without giving up any of the qualities exhibited by a single-layered membrane. Table 2 shows the overall comparison of both single-layered and double-layered adsorptive membranes in terms of structure, benefits and common issues.
Table 2. Comparison of single-layered and double-layered adsorptive membranes.
2.1. Methods of Incorporating Adsorbents
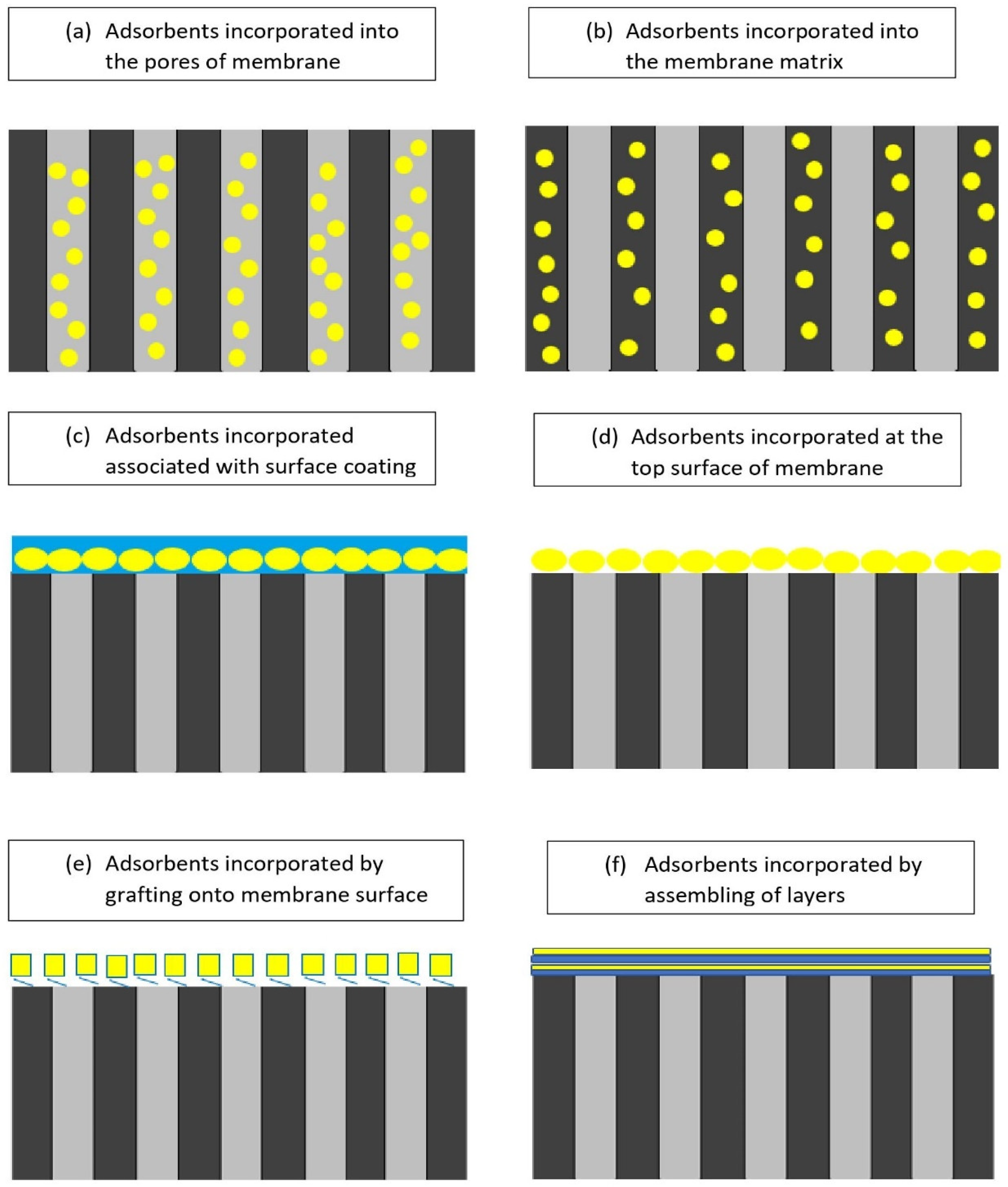
According to the preparation method, there are various ways in which adsorbents can be inserted into a membrane matrix, as shown in
Figure 4. Positioning plays an important role in the adsorptive process of an adsorptive membrane. Single-layered membranes are one of the most common adsorption membranes. Their preparation process is simple, but it is easy to agglomerate when the adsorbent content is high. Adsorbents that are embedded inside the polymer matrix have a lower adsorption capacity and a longer adsorption equilibrium time
[15]. There are several fabrication methods that are already in practice for adsorptive membranes such as coating, grafting, assembling, composite membranes, imprinting, phase inversion/solution casting and electrospinning.
Figure 4. Positioning of adsorbents in the polymer matrix
[15]. (
a) Adsorbents incorporated into the pores of membrane. (
b) Adsorbents incorporated into the membrane matrix. (
c) Adsorbents incorporated associated with surface grafting. (
d) Adsorbents incorporated at the top surface of membrane. (
e) Adsorbents incorporated by grafting onto membrane surface. (
f) Adsorbents incorporated by assembling of layers.
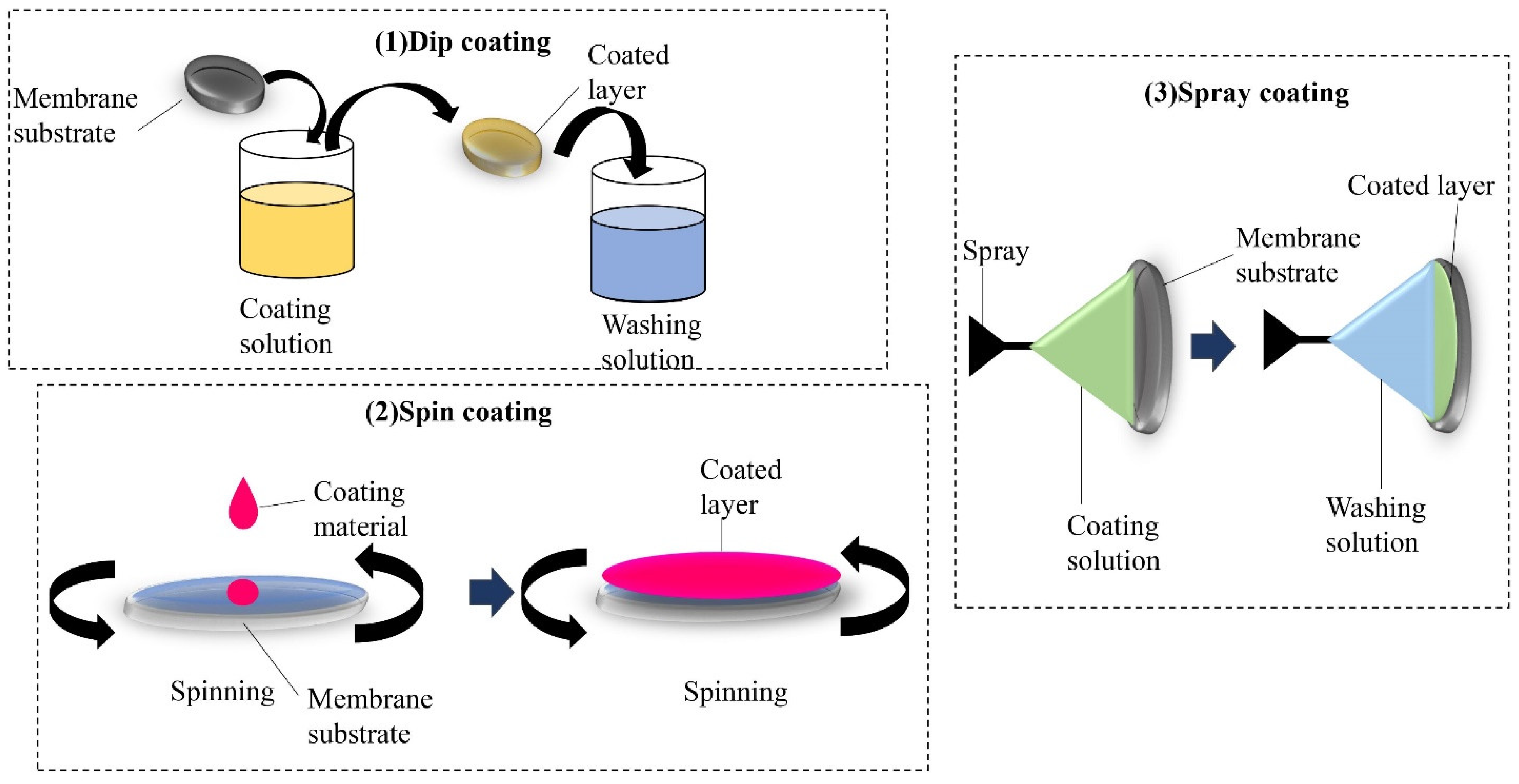
One of the techniques to come up with a highly adsorptive membrane is through modifying the surface of a membrane, as its exposure to pollutants plays a vital role in the process. Blending and coating provide physical adsorption whereby layers with increasing affinity towards adsorbates are formed without any chemical interaction. It is done by dispersing the adsorbents into the solvent via an ultrasonic bath or by stirring where the polymer is added. Then, the solution is cast on a flat surface and dried by an evaporating solvent. The coating process can be categorized as dip coating, spin coating and spray coating. This method can promote a layer-by-layer assembly for the adsorption process.
Figure 5 shows the illustration of various coating techniques, which are (1) dip coating, (2) spin coating and (3) spray coating. Dip coating is a method of depositing functional material on a membrane substrate. It is done by immersing the membrane substrate into a coating solution for an effective formation of layers
[16][17]. Spin coating is a technique of spreading a uniform layer onto a solid surface by a centrifugal force and requires a liquid vapor interface
[18]. Additionally, the coating that is applied on an object with a liquid spray is called spray coating
[19]. One of the studies involved modifying a polysulfone (PSF) membrane via co-depositing polyethyleneimine (PEI) with dopamine (DA) in one step to produce amine-rich surfaces. The functionalized membranes further react with glycidol for boron removal applications
[20]. The motive of this research was to introduce hydrophilic properties on the membrane surface, which could aid in the eliminating process. The fabricated membranes had a very strong hydrophilicity, but the water flux decreased in the process. Therefore, this research suggested this method as a potential attempt to be applied in boron removal applications by improving the permeability factor. Other reported drawbacks of this method include the longer process time for dip coating due to diffusional resistance and rinsing for the elimination of polyelectrolyte complex formation, which can lead to flocculation. Although spin coating is claimed to be the fastest, the surface area of a material to be coated is not sufficient. For spray coating, the possibility of the coating material being drained due to gravity is higher
[21].
Figure 5. Different coating techniques to form adsorptive membranes.
Another method of surface modification is grafting, whereby a pollutant is adsorbed onto the surface that is functionalized with an adsorbent by chemical adsorption such as ion exchanging or electrostatic reaction. Du et al. synthesized a series of polysulfone membranes that were grafted with polyol polymers in order to test the removal of boric acid
[22]. The attached ligand structure with a high vicinal diol content and the presence of the amino group was able to increase the complexing efficiency for boron uptake. Although the surface hydrophilicity was enhanced, the decrease in water flux was very obvious in this research. Apart from that, the poly(amic-acid) electrospun nanofiber membranes that were grafted with hyperbranched polyols were able to provide good regeneration as an adsorbent, besides showing an outstanding removal of boron
[23]. Surface grafting has several advantages, which include the ease of the modification process, the higher chemical stability and the low delamination of grafts
[24]. However, there were some studies that showed changes in the pore structure that could disturb the water movement in membrane-based water applications
[25][26].
Boron removal has been widely tackled using composite membranes. There has been significant research on whether micro and nanoparticles are suitable to be used for boron adsorption and on their potential when being used as composite membranes
[27][28][29]. A composite membrane is the combination of immiscible additives with a polymeric substance. They are used to produce membranes with a high adsorption capacity, fast kinetics, reduced fouling, promising reactivity and flexibility. Composite membranes are fabricated by adding micromaterials or nanomaterials in the structure of a membrane on the surface or dispersing in the matrix. In that context, thin-film composite membranes have received increased attention in water desalination applications since the scope can always be widened with available materials in nanotechnology. Thin-film membranes are able to perform well compared to asymmetric membranes in desalination applications
[30]. Thin-film composites have an active layer which is rich in nanoparticles to provide easier adsorption, whereas the classic composite membranes tend to have agglomerated particles in the polymer matrix
[31]. Kumar et al. functionalized titanium dioxide (TiO
2) nanoparticles with phosphonic acid to remove boron from seawater
[32]. Apart from having a higher boron rejection than commercial membranes, the research of removing neutral boric acid was a success due to the pore narrowing effect.
The limited affinity of removing metals towards adsorbents is one of the critical issues that is raised during the fabrication process of adsorptive membranes. The method of imprinting can be solved with the application of specific binding sites that are incorporated into the membrane matrix. Imprinting is about introducing synthetic receptor locations in the membrane matrix that recognize, remember and identify the target species among others in a solution. This technique is used to obtain selective membrane adsorbents in order to overcome the issues in selectivity that result from the limited specific binding capacity. It is carried out via the addition of specialized configured voids to the polymer by inserting the target when preparing the membrane and then immediately leaching it to vacate the active sites. Imprinted membranes are created in both flat sheet and hollow fiber configurations
[33]. So far, this method has not been applied for boron removal; however, this has already been in practice for other metal removal applications
[34][35].
Phase inversion/solution casting is the most common method of membrane fabrication nowadays. It is a process of membrane synthesis using a polymer-solvent mixture to form a homogeneous solution at specific conditions of temperature and composition, which can be differentiated with a slight change in the conditions. This method can give a better dispersion of fillers, an excellent interaction between the matrix and the filler and a uniform merging of the polymer and adsorbent. It can be done by evaporating a volatile solvent from the homogenous solution or cooling a casting solution. Phase inversion can entrap nanomaterials within the matrix, where they can get blended and dispersed in a polymer dope solution. Shi et al. prepared an amphiphilic graft glycopolymer and used it as membrane additives to prepare hydrophilic polysulfone membranes
[36]. They were prepared using phase inversion with different weight ratios of additives and were then cast as flat sheet membranes. The hydrophilic glycopolymer segments were observed to be accumulated at the membrane surface in phase inversion when blending with polysulfones. This method was able to provide good antifouling of the membrane and boron complexing properties
[36]. The main drawback of this technique is the huge amount of solvent wastage during preparation. Further, it is difficult to control the precision and the uniformity of the prepared membranes
[37].
Furthermore, electrospinning is a high voltage-driven process that creates an electric field to induce electrostatic repulsion forces, which shatter the polymer surface tension and stretch its droplets to form solid continuous nanofibers. This method is used to synthesize nanofibrous membranes with an improved efficiency and excellent removal capacity for heavy metals and organic pollutants. This method was prepared by using a pump that was equipped with a nozzle-fitted syringe, a spinneret, an electric current source and a counter electrode or grounded target. The use of high voltage creates an electric field and droplets at the nozzle. When the charged jet accelerates towards the collector, the solvent evaporates and forms the nanofiber. Ozbey-Unal et al. fabricated a hydrophobic nanofiber membrane via electrospinning to be used for removing boron from geothermal water
[38]. The motive of the aforementioned research is to prevent membrane wetting by narrowing the open fiber structure of the membrane and improving its hydrophobicity via coupling heat treatment. The driving force is also enhanced to aid boron and salt removal.
Single-layered adsorptive membranes have been the new-generation technology in water treatment applications since they combine the inherent characteristics of polymers and adsorbents. Most of the aforementioned methods have been focusing on these types of membranes. Previous research focused on the chemical and structural form of adsorbents. There are very limited studies regarding the placement of adsorbents in the membrane. Surface-functionalized adsorptive membranes tend to have a high adsorptive capacity and a short equilibrium time. However, surface-deposited adsorptive and surface-assembled adsorptive membranes have the detachment risk of deposition and assembly layers during application and regeneration. However, these detachment issues can be easily tackled by several techniques such as the co-casting method when fabricating by the dual-layered membrane.
2.2. Issues/Problems Found in Single-Layered Adsorptive Membranes
Studies regarding single-layered adsorptive membranes have raised some serious issues associated with membrane fouling. One of the prominent limiting factors is the dispersion of the adsorbent onto a polymer matrix. Studies suggested that the aggregation or dispersion behavior control, which is the first process for the preparation of new functional materials that incorporate nanoparticles, is very difficult for nanoparticles that are less than 100 nm in diameter due to surface interactions
[39]. Several studies have shown agglomeration issues, whereby adding more adsorbents into a single-layer polymer matrix will cause more agglomeration, which can lead to membrane fouling
[40]. The over-attachment of solutes onto the membrane surface or into the internal structure of the membrane could form an additional barrier or block the membrane pores by preventing the solvent from transporting through the membrane, which could raise the trans-membrane pressure and lower the permeate productivity
[39]. Some fouling materials even destroy the membrane and shorten its service life. Several techniques should be tackled from time to time in order to prevent this phenomenon from happening
[41].
3. Dual-Layered Adsorptive Membranes
The difficulty and higher cost of merging the advances of materials with the fabrication of the membrane have created the idea of dual-layered membranes. Dual-layered membranes are membranes with two layers which consist of different materials. The sub-layer is a porous structure which is responsible for providing mechanical support, while the top layer is made from a high performance which has a selective dense material.
3.1. Various Methods of Fabricating Dual-Layered Membranes
Asymmetric membranes are characterized by a thin and dense skin layer on top of a porous structure. The thin layer acts as a barrier for substances to move in and out of the membranes, despite providing a stronger mechanical strength. Dual-layered membranes fall under thin-film composite membranes. Recently, the development of this type of asymmetric membrane has been at its peak due to its interesting properties. It must be mentioned that each layer (the top selective layer and the bottom porous substrate) of this type of membrane can be independently controlled and optimized to achieve the desired selectivity and permeability while offering excellent mechanical strength and compression resistance. There are several fabrication methods that are applied in order to come up with high-performing dual-layered flat sheet-type membranes such as the interfacial polymerization
[42], layer-by-layer
[43], coating, cross linking
[44] and co-casting techniques
[45].
Table 3 shows the various methods utilized in developing dual-layered membranes with their respective application in treating water. Dual-layered flat sheet membranes have also been fabricated in previous studies to remove boron, as listed in
Table 4. So far, there are very limited studies that have been attempted for boron removal, especially for double-layered flat sheet adsorptive membranes for boron removal. Boron has a special chemistry, and, thus, it mostly differs from that of other trace elements. In this regard, boron chemistry depends strongly on pH and ionic strength. Therefore, boron studies are mostly focused on having good adsorbents, which could satisfy these parameters because their high surface-to-volume ratio provides higher adsorption. The possibility of having a reduced adsorption of boron in adsorbents when they are being utilized as double-layered membranes could be something researchers fear
[46]. This is because of the probability of the functional groups of the adsorbents reacting with the polymer, especially where polar functional groups are present. This might be a stumbling block for them progressing further as adsorptive membranes.
Table 2. Methods of fabricating dual-layered flat sheet membranes.
| Method |
Feature |
Material |
Application |
Output |
References |
| 1. Interfacial polymerization |
Interfacial polymerization is a type of step-growth polymerization in which polymerization occurs at the interface between two immiscible phases (generally, two liquids), resulting in a polymer that is constrained to the interface. |
Active layer: Polyamide (PA)
Sub-layer: Polysulfone/graphene oxide |
Forward osmosis (FO), salt rejection |
Enhanced water permeability, higher selectivity, improved performance as a TFC-FO membrane |
[42] |
| 2. Layer-by-layer |
Deposition of thin films and coatings with a precisely controlled composition and thickness (can be used for multilayer films too) |
Polyethylenimine (PEI) and trimesoyl chloride (TMC) on a microporous polyethersulfone (PES) substrate. |
Nanofiltration (NF) |
Increased permeability, stable and higher salt rejection, more compact structure |
[43] |
| 3. Coating and cross-linking |
Process of chemically joining two or more molecules by covalent bonding to be spread on the surface of the membrane. |
Polyamide reverse osmosis membrane modified through coating a surface layer of natural polymer sericin |
Reverse osmosis (RO) |
Increased antifouling ability, decreased pure water permeability, increased salt rejection, capability of decreasing the foulant attachment on the membrane surface |
[44] |
| 4. Co-casting technique |
Simultaneous casting of two dope solutions on a casting plate by controlling several parameters |
Silica-impregnated porous bottom layer nano-particle-devoid top surface-interface PA-active layer |
Forward osmosis (FO) |
Defect-free structure and increased water flux without compromising on the reverse salt flux |
[45] |
Table 4. Studies about dual-layered flat sheet membranes for boron removal.
3.2. The Proposed Co-Casting Technique
Co-casting is proven as an effective one-step membrane fabrication technique where the active top layer is extruded simultaneously with a supporting layer, which results in a composite double-layered membrane structure
[50]. Commonly, two different polymers are employed in the preparation of composite membranes so as to improve the membrane permeability and selectivity as well as to decrease the cost in membrane materials. It is well known that the miscibility of the two membrane materials plays an important role in their adhesion or delamination. For example, well-miscible polymer pairs often lead to good adhesion; therefore, composite membranes have been prepared with the same or similar polymers in both coating and support
[51][52].
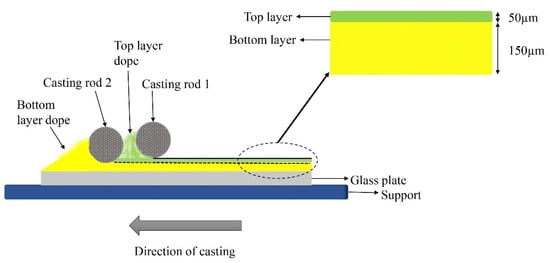
Co-casting is a method whereby a coating solution is casted simultaneously with a support solution, resulting in a DL membrane structure. The universal co-casting method utilizes two individual cylindrical-shape casting knives. Each side of the knife can cast membranes with different thicknesses (100, 150, 200, 250 μm)
[53]. As a result, the DL membrane thickness can be adjusted, and the interface between the two layers can be improved. Therefore, the delamination/adhesion effects can be improved using the co-casting technique, which can result in stable membranes. Surprisingly, the co-casting method for fabricating membranes for boron removal has never been attempted so far. The illustration of this method is shown in
Figure 6.
Figure 6. Illustration of the co-casting technique.
3.2.1. Parameters Involved in the Process of Co-Casting
When it comes to the co-casting technique, there are several factors that affect the process, as listed below.
Solvent and Non-Solvent Selection
The first step was to form a homogeneous solution by choosing the solvent to dissolve or easily disperse the polymer. The quality of the solvent has a direct impact on the membrane morphology. A weak solvent produced a sponge-like porous structure
[54], whereas a stronger solvent led to the formation of macrovoids
[55]. It was also important to make sure that the solvent and non-solvent were miscible such that a more porous membrane could be formed when there was a higher affinity between these two solvents. A low relative miscibility between the solvent and non-solvent could delay the de-mixing process between these two solvents.
Polymer Concentration and Properties
Since the polymer is the component that forms the membrane matrix, the polymer concentration in the casting solution will influence the final membrane morphology. Generally, a higher polymer concentration generates a lower gravimetric porosity. When the polymer concentration is above a certain threshold, there is not enough solvent and non-solvent exchange in the dope solution to form the pores during the phase separation and solidification process. Therefore, the membrane gravimetric porosity is lowered, and the permeability is lost. The blending process supports the formation of macrovoids, which results in a much better performance.
Additives in the Polymer Concentration
Additives clearly improve the pore formation and structure, enhance the pore interconnectivity and increase the hydrophilicity, which has direct impact on the delamination phenomenon of the dual-layered membranes
[56]. Several works of research revealed that adding hydrophilic polymers such as PVP led to a thicker membrane skin layer, whereas the sub-layers of membranes had dense structures with fewer macrovoids, and the presence of finger-like-structures also disappeared
[57][58]. The addition of surfactants such as sulfonic groups or carboxyl groups can produce large pores on the top surface, and a more porous structure in the sub-layer leads to thicker membranes. The delamination issue can be resolved by adding additives
[51].
Film Casting Conditions
The main film casting conditions are the composition and temperature of the coagulation bath. The addition of small amounts of solvent in the coagulation bath is an effective method to prepare a non-porous membrane because it can reduce the rate of mass transfer between the non-solvent and the casting solution, which can provide instant de-mixing
[59]. The addition of a very high concentration of solvent will inhibit the dilution effect from forming a good polymeric membrane
[56]. Apart from that, the casting temperature should be taken into consideration too. This is because the viscosity, µ, will be affected, and this can cause changes in the membrane structure and internal morphology
[60]. There are some conditions analyzed by Hashemifard et al. to enhance a better adhesion between the top and bottom layers: (1) casting the sub-layer (SL) dope with a concentration that is high enough to satisfy the condition, µSL > µTL (µ = viscosity); (2) casting the top layer (TL) at a temperature higher than the sub-layer temperature; (3) incorporating a certain amount of filler in the sub-layer dope. All these conditions were able to avoid the detachment between the two layers
[50].
Li et al. investigated the factors that control the adhesion or delamination in the following aspects
[52]: the coagulation value, the interpenetration of polymer chains, the shrinkage difference in layers and the adhesive components. It was found that the shrinkage values are important in determining adhesion and delamination. At higher coagulation bath temperatures, the membrane shrinkage of PEI and Polysulfone (PSf) membranes in the thickness was much closer, and a good adhesion was observed at temperatures above 65 °C.
So far, co-casting methods have not been introduced in applications of boron removal, but this technique has been already attempted in gas adsorption
[61]. The defect-free selective layer formed in this method providing high permeability and selectivity is the perk of this method for higher boron adsorption. This research serves as a stepping stone in generating more creative ideas associated with co-casting adsorptive membranes for boron removal. This is because these parameters can be easily controlled, and the possibility to generate a denser layer of adsorbents for boron adsorption is higher.
3.3. The Advantages of Bilayer Membranes by the Co-Casting Technique in Ensuring the Boron Adsorption
Bilayer membranes come up with the porous substrate film layered beneath a dense boron-rejecting layer. The major advantage of having the two layers made of different chemicals is that each layer can be individually synthesized or customized so as to optimize the overall performance of the membrane. Compared to the common mixed matrix membranes for the adsorption process, these bilayer membranes are said to have better salt rejection, water flux and resistance to biological attacks, apart from being able to operate at wider range of pH values and temperatures
[62]. Boron, as a unique element, has different behaviors according to different pH values. Having a membrane with a better tolerance towards different pH values could aid better in the boron adsorption process without causing fouling issues.
In terms of the size of boron as a very small component in water (~0.098 µm), it is very much convenient and possible for it to be removed using double-layered membranes. For water treatment applications, efforts have been attempted in fabricating membranes with a higher permeability together with a higher selectivity. Most commercial or existing membranes have continuous and interconnected pores which provide the rapid transport of water, but the broad pore size distribution has limited the selectivity when it comes to the precise separation of contaminants from the water. Therefore, the independently functionable layers of bilayer membranes can be tailored chemically by forming a denser active layer followed by a porous substrate layer. Adding pore-forming agents only at the bottom layer during fabrication could be very much helpful in opening water channels without restricting the positioning of smaller boron molecules on narrow-sized pores for further adsorption. By this method, apart from removing the boron through size exclusion, the boron will also have enough time to get chemically adsorbed by adsorbents on the selective layer through methods such as ion exchange.
Furthermore, in terms of operating pressure, it was observed that boron elimination has been increased with increasing feed pressures due to the dilution effect of the permeate water at a higher flux
[63]. Therefore, it is very important to have a membrane structure with enough tolerance at higher operating pressures. For these reasons, double-layered membranes are very much better in performing at higher operating pressures due to their outstanding mechanical strength provided by the sub-layer compared to the monolayered membranes. In addition, enhancing the hydrophilicity could help to improve the antifouling behavior of a membrane
[64]. Since hydrophilicity is one of the important parameters in ensuring the aligning of layers for co-casting, the antifouling properties of dual-layered membranes will be expected to be very well maintained by the structure provided by the co-casting method.