Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, H.; Konja, D.; Wang, L.; Wang, Y. Sex Dimorphism in Body Fat Distribution. Encyclopedia. Available online: https://encyclopedia.pub/entry/26782 (accessed on 07 February 2026).
Li H, Konja D, Wang L, Wang Y. Sex Dimorphism in Body Fat Distribution. Encyclopedia. Available at: https://encyclopedia.pub/entry/26782. Accessed February 07, 2026.
Li, Haoyun, Daniels Konja, Luyao Wang, Yu Wang. "Sex Dimorphism in Body Fat Distribution" Encyclopedia, https://encyclopedia.pub/entry/26782 (accessed February 07, 2026).
Li, H., Konja, D., Wang, L., & Wang, Y. (2022, September 01). Sex Dimorphism in Body Fat Distribution. In Encyclopedia. https://encyclopedia.pub/entry/26782
Li, Haoyun, et al. "Sex Dimorphism in Body Fat Distribution." Encyclopedia. Web. 01 September, 2022.
Copy Citation
Body fat distribution is a well-established predictor of adverse medical outcomes, independent of overall adiposity. Studying body fat distribution sheds insights into the causes of obesity and provides valuable information about the development of various comorbidities. Compared to total adiposity, body fat distribution is more closely associated with risks of cardiovascular diseases.
obesity
adipose tissue
sexual dimorphism
body fat distribution
1. Introduction
Of the world’s population, one-third are currently overweight or obese (https://www.worldometers.info/obesity/, accessed on 1 July 2022). The rapid increase in obesity is threatening public health globally, including in China [1], where the prevalence has risen from approximately 0 to 16.4% (1982–2019) over the past ~40 years [2]. The pandemic of obesity has greatly burdened individuals, society, and the healthcare system. Obesity is responsible for approximately five million premature deaths each year and represents an independent risk factor for cardiovascular disease, the leading cause of global mortality and a major contributor to the disability [3][4]. The global prevalence of obesity in women is higher than in men. In 2020, the overall global obesity rate for women was estimated at 25% (vs. 17% for men), of which 54 million (vs. 22 million for men) are severe (Class III) BMI ≥ 40 kg/m2. By 2030, this number of women could be as high as 30% (vs. 20% for men) and 77 million (vs. 34 million) being severely obese [5]. Under obese conditions, the dysfunctional adipose tissue contributes to various pathologies in the cardiovascular system in a sex-dependent manner [6][7][8]. There is increasing interest in the pathophysiological differences between males and females in the incidence and consequence of obesity [4][9].
2. Adipose Tissue: Classification, Distribution, and Function
Adipose tissues, also known as body fats, are energy-processing endocrine organs that are classically classified by their functions or anatomical distributions. Functionally, while energy-storing white adipose tissues (WAT) are distributed in almost every part of the human body, thermogenic-controlling brown adipose tissues (BAT) are mainly located in the interscapular and mediastinal regions with rich nerves and blood vessels [10][11][12]. Under the condition of an increasing energy intake, excessive triglycerides deposited in WAT lead to obesity. In contrast, BAT oxidises glucose and lipids through uncoupled mitochondrial respiration to generate heat, thus dissipating energy via adaptive heat production [13][14]. Anatomically, adipose tissues are classified as subcutaneous adipose tissues (SAT) making up over 80% of total fat in the body [15], and visceral adipose tissues (VAT) surrounding the different thoracic and abdominal organs. WAT surrounding the heart comprises the epicardial (ECAT) and pericardial adipose tissue (PCAT) [16]. The abdominal VAT, including the omental, mesenteric, and retroperitoneal fat depots, are highly metabolically active. Most blood vessels are surrounded by perivascular adipose tissues (PVAT). Depending on the anatomical positions, the cellular compositions and the properties of PVAT are different. For example, PVAT associated with the thoracic aorta resembles BAT, whereas those surrounding the abdominal aorta exhibit similarities with WAT [17].
Adipose tissue is not only an energy source, but also the largest endocrine organ in the body [18][19]. The protein factors secreted from adipose tissue are collectively referred to as adipokines. Emerging evidence suggests that adipose tissue has more colours. Beige adipocytes are a distinct type of WAT sharing similarities with the classic cells in BAT. Brown adipocytes and myocytes, which are derived from a Myf5-expressing cell lineage, exhibit similar developmental origins [20][21]. The beige adipocytes originate from different and heterogeneous populations of cell lineages and are characteristic of both white and brown fat cells [22]. Pink adipose tissues (PAT) are sex specific. During pregnancy, the female SAT of the mammary gland begins to transform into a reservoir as PAT, which gradually replaces the WAT during the lactation period. PAT turns into WAT again when breastfeeding ends [23][24]. The whole process is referred to as alveolarogenesis, which involves the development of the lobule-alveolar gland structure to produce milk [23][24]. PAT secretes leptin and adiponectin that act to prevent neonatal obesity [25][26]. The yellow adipocytes are related to those of the marrow adipose tissues (MAT) in bone. MAT accounts for more than 10% of the total fat mass in healthy lean people [27][28]. Similar to WAT, MAT also acts as a large endocrine organ that secretes leptin and adiponectin [28][29], which increase or decrease under pathological conditions such as osteoporosis [30][31], diabetes [32], and obesity [33].
Depending on the anatomical locations, adipose tissue depots show different metabolic and endocrine properties. The propensity to generate new adipocytes in different adipose depots varies, thus their expansions are intrinsically different leading to a diversified cellular composition, function, and cardiometabolic consequences [34]. Adipose-derived factors, including adipokines, are key mediators of the alterations in body fat composition with age [35][36]. Different fat depots produce a distinct profile of mediators, which is affected by age and pathophysiological conditions. For example, the VAT expresses a greater amount of inflammatory adipokines [17][37][38]. Even in the same individual, the ob (obese) mRNA level in the adipose tissue varies from region to region [39]. As a result, the production of leptin as well as other inflammatory cytokines such as angiotensinogen, interleukin 6 (IL-6), and plasmin activator inhibitor 1 from SAT and VAT are different [40]. Leptin produced in the SAT is closely related to the circulating concentration [41][42]. Overall, the heterogeneity in the distribution and functions of adipose tissues exerts different effects on body fat distribution (Figure 1).
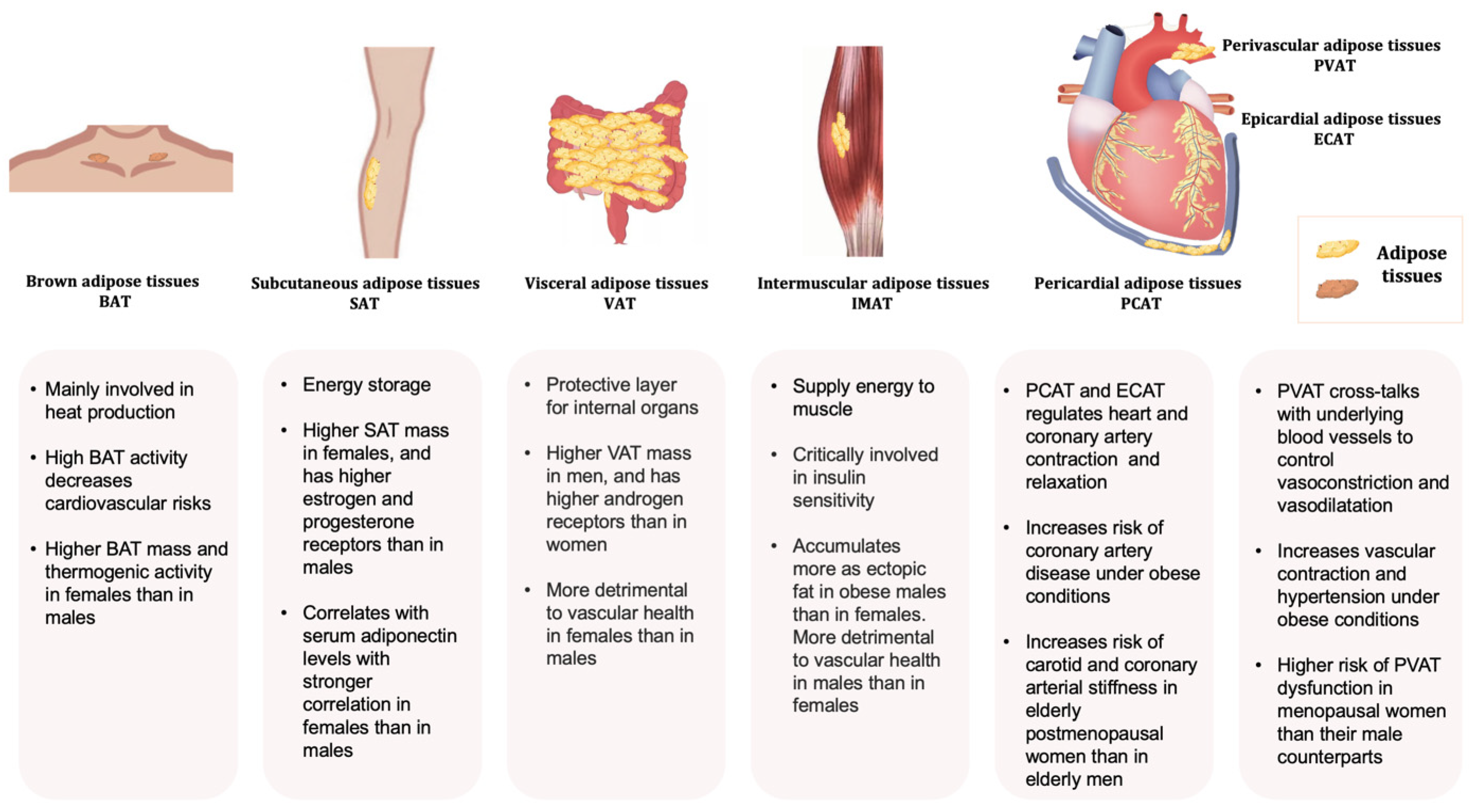
Figure 1. Typical adipose tissues, main functions, and some sex-related differences.
3. Sex Dimorphism in Body Fat Distribution
Sex is defined as the biological feature of males and females determined by genetics, regardless of social or environmental influences. The XX (female) or XY (male) chromosomes make individuals’ distinct sex from each other genetically and physiologically. Sex dimorphism, which refers to the characteristic differences between males and females in a species, helps clinicians and researchers classify, treat, and offer the prognosis of diseases differently. Over the last decade, sex-specific medicine has been drawing great attention as one of the first and foremost advancements of personalised medicine. To date, sex dimorphism has been intensively investigated in obesity, cancers, neurodegenerative disorders, and cardiovascular, bone, and infectious diseases, as well as pain management. The sex disparities in different pathologies, with the development of sex-omics technology, particularly sheds light on personalised management in chronic and severe diseases [43].
Adiposity refers to the distribution of body fat while obesity is a more measurable parameter, emphasising the stratification metrics related to the BMI (ratio of weight to the square of height) and waist circumference [44]. Sexual dimorphism of body fat distribution is subtle in the early stages of life, more distinct in adolescence, and strongly present throughout adult life, but attenuated later in life [45][46][47][48][49]. Of those with the same BMI and similar age, women have a significantly higher amount of adipose tissue deposition, especially the lower extremity fat, than men [50]. By contrast, men often develop central obesity with an increased fat deposition around the abdomen [51]. With advancing age, fat mass increases and peaks around the age of 60–79 years, later in women than men [52]. Age-associated changes in body composition are manifested not only by an increase in VAT, a decrease in SAT, and an accumulation of ectopic fat, but also by a significant reduction in the lean mass [53][54][55][56]. With age, the muscle loses its mass, strength, and physical functionality, leading to a high-risk geriatric syndrome known as sarcopenic obesity (SO), which contributes to various medical complications [57]. SO shows the sex variation and is more prevalent in elderly women [58]. However, the epidemiological findings are heterogeneous due largely to the lack of consensus on a standard definition of SO [59]. The prevalence of SO ranges from 4.4% to 84.0% in men and 3.6% to 94.0% in women when assessed with dual-energy X-ray absorptiometry [60]. In Europe and the US, the prevalence of SO is greater in men than women when using the appendicular lean muscle mass divided by squared height (ALM/h2) to define SO [61][62][63][64][65]. An opposite conclusion is drawn by a study in Korea using ALM/weight (%) as the criterion [66]. A cross-sectional study from China shows men were more likely than women to have sarcopenia and SO, as assessed by the Asian Working Group for Sarcopenia (AWGS) [67]. Women with SO may have higher glucose, while men with SO are more likely to develop osteoporosis and dyslipidaemia [68].
Body fat distribution is modulated by sex hormones and their receptors [69][70][71]. For example, in women, augmented VAT changes the body shape and composition towards a more android type after menopause [69]. The phenomenon is due at least partly to the withdrawal of estrogen levels, which regulate the sexually dimorphic expression of genes involved in adipose tissue development, distribution, and function [72][73][74]. Sex-specific hormonal factors play an important role in the development of SO. In women, the decrease in estrogen levels after menopause leads to an increase in adiposity and a change in the fat distribution pattern, with a shift from subcutaneous to visceral deposits and muscle tissue [75]. In older men, the development of SO is more strongly associated with a decrease in the total testosterone levels, which causes a reduction in both muscle mass and strength [76]. The expression of sex hormone receptors also affects the distribution pattern of adipose depots. Sex hormone-related receptors are differentially expressed in SAT and VAT [77]. The expression levels of estrogen and progesterone receptors are high in SAT, whilst VAT show an increased amount of androgen receptors [78]. Estrogen acts as an antagonist to decrease the expression of androgen receptors [79]. Low total testosterone can also lead to visceral obesity [80]. The decrease in total and bioavailable testosterone is a more direct predictor of VAT accumulation and cardiovascular risk than the decrease in estradiol levels [81]. The sex hormones interact with transcription factors to regulate gene activity in a sex-dependent manner [69][82]. However, animal studies do not support the correlations between circulating sex hormones and obesity-related genes [83].
Intermuscular adipose tissue (IMAT) has been recognised as an independent fat depot in assessing insulin sensitivity, lipid and lipoprotein metabolism, and predicting cardiovascular risk [84][85][86]. Men with overweight and obesity have significantly higher neck IMAT accumulation as an ectopic fat [87]. The ratio between subcutaneous and intramuscular adipose tissue (SAT/(SAT + IMAT) is significantly associated with serum adiponectin levels in both men and women, but more strongly in the latter, while the correlations with SAT or IMAT alone are not significant for both sexes [88]. The different distribution of adipose tissue affects body shape, but not necessarily the overall BMI in women and men. Under thermoneutral conditions, women exhibit more BAT mass and greater thermogenic responses than men [89][90][91]. However, the sex differences in BAT diminish with age or in cold conditions [92][93][94][95][96][97]. Compared with men, PET-CT can identify more UCP1-immunopositive regions, represented as BAT, and higher 18F-fluorodeoxyglucose (18F-FDG) uptake activity in the area extending from the neck to the chest of women [98]. Men display a decreased response to cold exposure due to the lower mitochondrial function [99]. In addition, ageing in men induces a faster functional decline of BAT activity than in women [100]. Fat deposition of the tongue is higher in men than women and associated with decreased upper airway patency [101]. Compared to women, there is a significantly higher amount of PCAT in the men’s [102]. On the contrary, the ECAT volume is significantly increased in middle-aged and older Japanese women [103].
References
- Hemmingsson, E. The unparalleled rise of obesity in China: A call to action. Int. J. Obes. 2021, 45, 921–922.
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Min, Y.I.; Gao, Y.; Anugu, P.; Anugu, A.; Correa, A. Obesity and overall mortality: Findings from the Jackson Heart Study. BMC Public Health 2021, 21, 50.
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642.
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201.
- O’Neil, A.; Scovelle, A.J.; Milner, A.J.; Kavanagh, A. Gender/Sex as a Social Determinant of Cardiovascular Risk. Circulation 2018, 137, 854–864.
- Agarwala, A.; Michos, E.D.; Samad, Z.; Ballantyne, C.M.; Virani, S.S. The Use of Sex-Specific Factors in the Assessment of Women’s Cardiovascular Risk. Circulation 2020, 141, 592–599.
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381.
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790.
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44.
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010, 34 (Suppl. 1), S36–S42.
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376.
- Mulya, A.; Kirwan, J.P. Brown and Beige Adipose Tissue: Therapy for Obesity and Its Comorbidities? Endocrinol. Metab. Clin. 2016, 45, 605–621.
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic resonance imaging of total body fat. J. Appl. Physiol. (1985) 1998, 85, 1778–1785.
- Chaowalit, N.; Lopez-Jimenez, F. Epicardial adipose tissue: Friendly companion or hazardous neighbour for adjacent coronary arteries? Eur. Heart J. 2008, 29, 695–697.
- Zhang, P.; Konja, D.; Wang, Y. Adipose tissue secretory profile and cardiometabolic risk in obesity. Endocr. Metab. Sci. 2020, 1, 100061.
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139.
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 2014, 5, 4099.
- Sanchez-Gurmaches, J.; Hung, C.M.; Sparks, C.A.; Tang, Y.; Li, H.; Guertin, D.A. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012, 16, 348–362.
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508.
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666.
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171.
- Palou, A.; Sanchez, J.; Pico, C. Nutrient-gene interactions in early life programming: Leptin in breast milk prevents obesity later on in life. Adv. Exp. Med. Biol. 2009, 646, 95–104.
- Mohamad, M.; Loy, S.L.; Lim, P.Y.; Wang, Y.; Soo, K.L.; Mohamed, H.J.J. Maternal Serum and Breast Milk Adiponectin: The Association with Infant Adiposity Development. Int. J. Environ. Res. Public Health 2018, 15, 1250.
- Zinngrebe, J.; Debatin, K.M.; Fischer-Posovszky, P. Adipocytes in hematopoiesis and acute leukemia: Friends, enemies, or innocent bystanders? Leukemia 2020, 34, 2305–2316.
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375.
- Margetic, S.; Gazzola, C.; Pegg, G.; Hill, R. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. 2002, 26, 1407–1433.
- Justesen, J.; Stenderup, K.; Ebbesen, E.N.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171.
- Yeung, D.K.; Griffith, J.F.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285.
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Rosen, C.J.; Klibanski, A.; Miller, K.K. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity 2011, 19, 49–53.
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219.
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218.
- Fei, J.; Cook, C.; Blough, E.; Santanam, N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis 2010, 212, 488–494.
- Karelis, A.D.; St-Pierre, D.H.; Conus, F.; Rabasa-Lhoret, R.; Poehlman, E.T. Metabolic and body composition factors in subgroups of obesity: What do we know? J. Clin. Endocrinol. Metab. 2004, 89, 2569–2575.
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282.
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19.
- Masuzaki, H.; Ogawa, Y.; Isse, N.; Satoh, N.; Okazaki, T.; Shigemoto, M.; Mori, K.; Tamura, N.; Hosoda, K.; Yoshimasa, Y.; et al. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes 1995, 44, 855–858.
- Arner, P. Regional differences in protein production by human adipose tissue. Biochem. Soc. Trans. 2001, 29, 72–75.
- Montague, C.T.; Prins, J.B.; Sanders, L.; Digby, J.E.; O’Rahilly, S. Depot- and sex-specific differences in human leptin mRNA expression: Implications for the control of regional fat distribution. Diabetes 1997, 46, 342–347.
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917.
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296.
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383.
- Wells, J.C.; Treleaven, P.; Cole, T.J. BMI compared with 3-dimensional body shape: The UK National Sizing Survey. Am. J. Clin. Nutr. 2007, 85, 419–425.
- Jackson, A.S.; Stanforth, P.R.; Gagnon, J.; Rankinen, T.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C.; Wilmore, J.H. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 789–796.
- Taylor, R.W.; Grant, A.M.; Williams, S.M.; Goulding, A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity 2010, 18, 1410–1416.
- Cartwright, M.J.; Schlauch, K.; Lenburg, M.E.; Tchkonia, T.; Pirtskhalava, T.; Cartwright, A.; Thomou, T.; Kirkland, J.L. Aging, depot origin, and preadipocyte gene expression. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 242–251.
- Silventoinen, K.; Jelenkovic, A.; Sund, R.; Hur, Y.M.; Yokoyama, Y.; Honda, C.; Hjelmborg, J.; Möller, S.; Ooki, S.; Aaltonen, S.; et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: An individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am. J. Clin. Nutr. 2016, 104, 371–379.
- Schorr, M.; Dichtel, L.E.; Gerweck, A.V.; Valera, R.D.; Torriani, M.; Miller, K.K.; Bredella, M.A. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex Differ. 2018, 9, 28.
- Pi-Sunyer, F.X. The epidemiology of central fat distribution in relation to disease. Nutr. Rev. 2004, 62, S120–S126.
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. (Lausanne) 2019, 10, 861.
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395.
- Arner, P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann. Med. 1995, 27, 435–438.
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18.
- Wollina, U.; Wetzker, R.; Abdel-Naser, M.B.; Kruglikov, I.L. Role of adipose tissue in facial aging. Clin. Interv. Aging 2017, 12, 2069.
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700.
- Chung, J.Y.; Kang, H.T.; Lee, D.C.; Lee, H.R.; Lee, Y.J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278.
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537.
- Batsis, J.A.; Barre, L.K.; Mackenzie, T.A.; Pratt, S.I.; Lopez-Jimenez, F.; Bartels, S.J. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: Dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J. Am. Geriatr. Soc. 2013, 61, 974–980.
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763.
- von Berens, Å.; Obling, S.R.; Nydahl, M.; Koochek, A.; Lissner, L.; Skoog, I.; Frändin, K.; Skoglund, E.; Rothenberg, E.; Cederholm, T. Sarcopenic obesity and associations with mortality in older women and men—A prospective observational study. BMC Geriatr. 2020, 20, 199.
- Daskalopoulou, C.; Wu, Y.-T.; Pan, W.; Giné Vázquez, I.; Prince, M.; Prina, M.; Tyrovolas, S. Factors related with sarcopenia and sarcopenic obesity among low- and middle-income settings: The 10/66 DRG study. Sci. Rep. 2020, 10, 20453.
- Bahat, G.; Kilic, C.; Topcu, Y.; Aydin, K.; Karan, M. Fat percentage cutoff values to define obesity and prevalence of sarcopenic obesity in community-dwelling older adults in Turkey. Aging Male 2018, 23, 477–482.
- Santos, V.R.D.; Gomes, I.C.; Bueno, D.R.; Christofaro, D.G.D.; Freitas, I.F., Jr.; Gobbo, L.A. Obesity, sarcopenia, sarcopenic obesity and reduced mobility in Brazilian older people aged 80 years and over. Einstein (Sao Paulo) 2017, 15, 435–440.
- Oh, C.; Jho, S.; No, J.K.; Kim, H.S. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr. Res. 2015, 35, 1–6.
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 615.
- Du, Y.; Wang, X.; Xie, H.; Zheng, S.; Wu, X.; Zhu, X.; Zhang, X.; Xue, S.; Li, H.; Hong, W.; et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr. Disord. 2019, 19, 109.
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958.
- Liedtke, S.; Schmidt, M.E.; Vrieling, A.; Lukanova, A.; Becker, S.; Kaaks, R.; Zaineddin, A.K.; Buck, K.; Benner, A.; Chang-Claude, J.; et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity 2012, 20, 1088–1095.
- Haffner, S.M. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: Epidemiological and clinical correlation. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. 2), S56–S58.
- van Nas, A.; Guhathakurta, D.; Wang, S.S.; Yehya, N.; Horvath, S.; Zhang, B.; Ingram-Drake, L.; Chaudhuri, G.; Schadt, E.E.; Drake, T.A.; et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology 2009, 150, 1235–1249.
- Gesta, S.; Bluher, M.; Yamamoto, Y.; Norris, A.W.; Berndt, J.; Kralisch, S.; Boucher, J.; Lewis, C.; Kahn, C.R. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA 2006, 103, 6676–6681.
- Grove, K.L.; Fried, S.K.; Greenberg, A.S.; Xiao, X.Q.; Clegg, D.J. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int. J. Obes. 2010, 34, 989–1000.
- Petroni, M.L.; Caletti, M.T.; Dalle Grave, R.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302.
- Frank, A.P.; de Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719.
- Crandall, D.L.; Busler, D.E.; Novak, T.J.; Weber, R.V.; Kral, J.G. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochem. Biophys. Res. Commun. 1998, 248, 523–526.
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69.
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33.
- Allan, C.A.; Strauss, B.J.; Burger, H.G.; Forbes, E.A.; McLachlan, R.I. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J. Clin. Endocrinol. Metab. 2008, 93, 139–146.
- Nielsen, T.L.; Hagen, C.; Wraae, K.; Brixen, K.; Petersen, P.H.; Haug, E.; Larsen, R.; Andersen, M. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J. Clin. Endocrinol. Metab. 2007, 92, 2696–2705.
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430.
- Kocher, C.; Christiansen, M.; Martin, S.; Adams, C.; Wehner, P.; Gress, T.; Santanam, N. Sexual dimorphism in obesity-related genes in the epicardial fat during aging. J. Physiol. Biochem. 2017, 73, 215–224.
- Boettcher, M.; Machann, J.; Stefan, N.; Thamer, C.; Häring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Intermuscular adipose tissue (IMAT): Association with other adipose tissue compartments and insulin sensitivity. J. Magn. Reson. Imaging 2009, 29, 1340–1345.
- Bergia, R.E., 3rd; Kim, J.E.; Campbell, W.W. Differential Relationship between Intermuscular Adipose Depots with Indices of Cardiometabolic Health. Int. J. Endocrinol. 2018, 2018, 2751250.
- Gallagher, D.; Kuznia, P.; Heshka, S.; Albu, J.; Heymsfield, S.B.; Goodpaster, B.; Visser, M.; Harris, T.B. Adipose tissue in muscle: A novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 2005, 81, 903–910.
- Torriani, M.; Gill, C.M.; Daley, S.; Oliveira, A.L.; Azevedo, D.C.; Bredella, M.A. Compartmental neck fat accumulation and its relation to cardiovascular risk and metabolic syndrome. Am. J. Clin. Nutr. 2014, 100, 1244–1251.
- Hassler, E.M.; Deutschmann, H.; Almer, G.; Renner, W.; Mangge, H.; Herrmann, M.; Leber, S.; Michenthaler, M.; Staszewski, A.; Gunzer, F.; et al. Distribution of subcutaneous and intermuscular fatty tissue of the mid-thigh measured by MRI-A putative indicator of serum adiponectin level and individual factors of cardio-metabolic risk. PLoS ONE 2021, 16, e0259952.
- Fuller-Jackson, J.P.; Dordevic, A.L.; Clarke, I.J.; Henry, B.A. Effect of sex and sex steroids on brown adipose tissue heat production in humans. Eur. J. Endocrinol. 2020, 183, 343–355.
- Brendle, C.; Werner, M.K.; Schmadl, M.; la Fougère, C.; Nikolaou, K.; Stefan, N.; Pfannenberg, C. Correlation of brown adipose tissue with other body fat compartments and patient characteristics: A retrospective analysis in a large patient cohort using PET/CT. Acad. Radiol. 2018, 25, 102–110.
- Ouellet, V.; Routhier-Labadie, A.; Bellemare, W.; Lakhal-Chaieb, L.; Turcotte, E.; Carpentier, A.C.; Richard, D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 2011, 96, 192–199.
- Fletcher, L.A.; Kim, K.; Leitner, B.P.; Cassimatis, T.M.; O’Mara, A.E.; Johnson, J.W.; Halprin, M.S.; McGehee, S.M.; Brychta, R.J.; Cypess, A.M.; et al. Sexual Dimorphisms in Adult Human Brown Adipose Tissue. Obesity 2020, 28, 241–246.
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408.
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. (Lausanne) 2021, 12, 652444.
- Cedikova, M.; Kripnerová, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016, 2016, 6067349.
- Bahler, L.; Verberne, H.J.; Admiraal, W.M.; Stok, W.J.; Soeters, M.R.; Hoekstra, J.B.; Holleman, F. Differences in sympathetic nervous stimulation of brown adipose tissue between the young and old, and the lean and obese. J. Nucl. Med. 2016, 57, 372–377.
- Valle, A.; Santandreu, F.; García-Palmer, F.; Roca, P.; Oliver, J. The serum levels of 17β-estradiol, progesterone and triiodothyronine correlate with brown adipose tissue thermogenic parameters during aging. Cell. Physiol. Biochem. 2008, 22, 337–346.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517.
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Häring, H.U.; Claussen, C.D.; Stefan, N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 2010, 59, 1789–1793.
- Valencak, T.G.; Hoffman, J.M. Sex differences in brown adipose tissue. Mol. Cell. Endocrinol. 2021, 536, 111413.
- Godoy, I.R.; Martinez-Salazar, E.L.; Eajazi, A.; Genta, P.R.; Bredella, M.A.; Torriani, M. Fat accumulation in the tongue is associated with male gender, abnormal upper airway patency and whole-body adiposity. Metabolism 2016, 65, 1657–1663.
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613.
- Sugita, Y.; Ito, K.; Sakurai, S.; Sakai, S.; Kuno, S. Epicardial adipose tissue is associated with cardiorespiratory fitness and hemodynamics among Japanese individuals of various ages and of both sexes. PLoS ONE 2021, 16, e0254733.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
2 times
(View History)
Update Date:
01 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




