Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yu Wang and Version 2 by Rita Xu.
Body fat distribution is a well-established predictor of adverse medical outcomes, independent of overall adiposity. Studying body fat distribution sheds insights into the causes of obesity and provides valuable information about the development of various comorbidities. Compared to total adiposity, body fat distribution is more closely associated with risks of cardiovascular diseases.
- obesity
- adipose tissue
- sexual dimorphism
- body fat distribution
1. Introduction
Of the world’s population, one-third are currently overweight or obese (https://www.worldometers.info/obesity/, accessed on 1 July 2022). The rapid increase in obesity is threatening public health globally, including in China [1], where the prevalence has risen from approximately 0 to 16.4% (1982–2019) over the past ~40 years [2]. The pandemic of obesity has greatly burdened individuals, society, and the healthcare system. Obesity is responsible for approximately five million premature deaths each year and represents an independent risk factor for cardiovascular disease, the leading cause of global mortality and a major contributor to the disability [3][4][3,4]. The global prevalence of obesity in women is higher than in men. In 2020, the overall global obesity rate for women was estimated at 25% (vs. 17% for men), of which 54 million (vs. 22 million for men) are severe (Class III) BMI ≥ 40 kg/m2. By 2030, this number of women could be as high as 30% (vs. 20% for men) and 77 million (vs. 34 million) being severely obese [5]. Under obese conditions, the dysfunctional adipose tissue contributes to various pathologies in the cardiovascular system in a sex-dependent manner [6][7][8][6,7,8]. There is increasing interest in the pathophysiological differences between males and females in the incidence and consequence of obesity [4][9][4,9].
2. Adipose Tissue: Classification, Distribution, and Function
Adipose tissues, also known as body fats, are energy-processing endocrine organs that are classically classified by their functions or anatomical distributions. Functionally, while energy-storing white adipose tissues (WAT) are distributed in almost every part of the human body, thermogenic-controlling brown adipose tissues (BAT) are mainly located in the interscapular and mediastinal regions with rich nerves and blood vessels [10][11][12][10,11,12]. Under the condition of an increasing energy intake, excessive triglycerides deposited in WAT lead to obesity. In contrast, BAT oxidises glucose and lipids through uncoupled mitochondrial respiration to generate heat, thus dissipating energy via adaptive heat production [13][14][13,14]. Anatomically, adipose tissues are classified as subcutaneous adipose tissues (SAT) making up over 80% of total fat in the body [15], and visceral adipose tissues (VAT) surrounding the different thoracic and abdominal organs. WAT surrounding the heart comprises the epicardial (ECAT) and pericardial adipose tissue (PCAT) [16]. The abdominal VAT, including the omental, mesenteric, and retroperitoneal fat depots, are highly metabolically active. Most blood vessels are surrounded by perivascular adipose tissues (PVAT). Depending on the anatomical positions, the cellular compositions and the properties of PVAT are different. For example, PVAT associated with the thoracic aorta resembles BAT, whereas those surrounding the abdominal aorta exhibit similarities with WAT [17]. Adipose tissue is not only an energy source, but also the largest endocrine organ in the body [18][19][18,19]. The protein factors secreted from adipose tissue are collectively referred to as adipokines. Emerging evidence suggests that adipose tissue has more colours. Beige adipocytes are a distinct type of WAT sharing similarities with the classic cells in BAT. Brown adipocytes and myocytes, which are derived from a Myf5-expressing cell lineage, exhibit similar developmental origins [20][21][20,21]. The beige adipocytes originate from different and heterogeneous populations of cell lineages and are characteristic of both white and brown fat cells [22]. Pink adipose tissues (PAT) are sex specific. During pregnancy, the female SAT of the mammary gland begins to transform into a reservoir as PAT, which gradually replaces the WAT during the lactation period. PAT turns into WAT again when breastfeeding ends [23][24][23,24]. The whole process is referred to as alveolarogenesis, which involves the development of the lobule-alveolar gland structure to produce milk [23][24][23,24]. PAT secretes leptin and adiponectin that act to prevent neonatal obesity [25][26][25,26]. The yellow adipocytes are related to those of the marrow adipose tissues (MAT) in bone. MAT accounts for more than 10% of the total fat mass in healthy lean people [27][28][27,28]. Similar to WAT, MAT also acts as a large endocrine organ that secretes leptin and adiponectin [28][29][28,29], which increase or decrease under pathological conditions such as osteoporosis [30][31][30,31], diabetes [32], and obesity [33]. Depending on the anatomical locations, adipose tissue depots show different metabolic and endocrine properties. The propensity to generate new adipocytes in different adipose depots varies, thus their expansions are intrinsically different leading to a diversified cellular composition, function, and cardiometabolic consequences [34]. Adipose-derived factors, including adipokines, are key mediators of the alterations in body fat composition with age [35][36][35,36]. Different fat depots produce a distinct profile of mediators, which is affected by age and pathophysiological conditions. For example, the VAT expresses a greater amount of inflammatory adipokines [17][37][38][17,37,38]. Even in the same individual, the ob (obese) mRNA level in the adipose tissue varies from region to region [39]. As a result, the production of leptin as well as other inflammatory cytokines such as angiotensinogen, interleukin 6 (IL-6), and plasmin activator inhibitor 1 from SAT and VAT are different [40]. Leptin produced in the SAT is closely related to the circulating concentration [41][42][41,42]. Overall, the heterogeneity in the distribution and functions of adipose tissues exerts different effects on body fat distribution (Figure 1).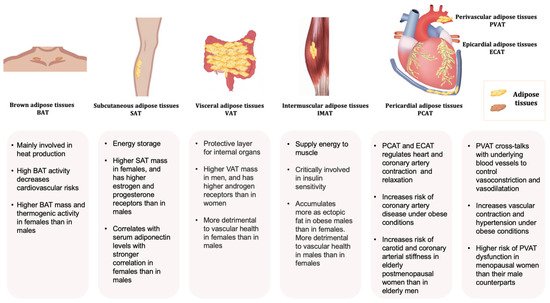
Figure 1. Typical adipose tissues, main functions, and some sex-related differences.
