Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grzegorz Procyk | -- | 2310 | 2022-08-31 17:40:20 | | | |
| 2 | Sirius Huang | + 5 word(s) | 2315 | 2022-09-01 03:28:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Procyk, G.; Bilicki, D.; Balsam, P.; Lodziński, P.; Grabowski, M.; Gąsecka, A. Extracellular Vesicles in Patients with Atrial Fibrillation. Encyclopedia. Available online: https://encyclopedia.pub/entry/26750 (accessed on 07 February 2026).
Procyk G, Bilicki D, Balsam P, Lodziński P, Grabowski M, Gąsecka A. Extracellular Vesicles in Patients with Atrial Fibrillation. Encyclopedia. Available at: https://encyclopedia.pub/entry/26750. Accessed February 07, 2026.
Procyk, Grzegorz, Dominik Bilicki, Paweł Balsam, Piotr Lodziński, Marcin Grabowski, Aleksandra Gąsecka. "Extracellular Vesicles in Patients with Atrial Fibrillation" Encyclopedia, https://encyclopedia.pub/entry/26750 (accessed February 07, 2026).
Procyk, G., Bilicki, D., Balsam, P., Lodziński, P., Grabowski, M., & Gąsecka, A. (2022, August 31). Extracellular Vesicles in Patients with Atrial Fibrillation. In Encyclopedia. https://encyclopedia.pub/entry/26750
Procyk, Grzegorz, et al. "Extracellular Vesicles in Patients with Atrial Fibrillation." Encyclopedia. Web. 31 August, 2022.
Copy Citation
Extracellular vesicles are particles released from cells and delimited by a lipid bilayer. They have been widely studied, including extensive investigation in cardiovascular diseases. The content presented here focuses on their role in atrial fibrillation.
atrial fibrillation
extracellular vesicles
microRNA
ablation
1. Preclinical Studies
It was proven that cardiomyocytes, treated with the Extracellular vesicles (EVs) derived from myofibroblasts, showed the downregulation of L-type calcium channel Cav1.2. This downregulation is considered to be a characteristic feature of the ionic remodeling associated with atrial fibrillation (AF) [1]. Additionally, EVs derived from the angiotensin II-treated human cardiac myocytes encouraged macrophages to conducted M1 polarization and, consequently, proinflammatory state; moreover, it was evidenced that Plasmacytoma Variant Translocation 1 (PVT1) contained in EVs promoted extracellular matrix remodeling in atrial fibroblasts [2].
Another research group established that beagles undergoing rapid atrial pacing for 7 days showed a rise in the atrial and plasma EVs release; moreover, it was effectively hampered by GW4869, a commonly used agent inhibiting EVs generation. It was also suggested that miR-21-5p could play a role, as it was upregulated in both atrial and plasma EVs [3]. EVs originating from mouse adipose-tissue-derived mesenchymal stem cells and transfected with the X-inactive specific transcript (XIST) caused a decrease in inflammation and myocardial pyroptosis [4]. In a recent study, EVs from bone marrow mesenchymal stem cells were transduced with the nuclear factor-erythroid 2-related factor 2 (Nrf2). These EVs injected into rats with AF not only shortened AF duration and reduced cardiomyocyte apoptosis but also minimized atrial fibrosis [5]. It was also proven that EVs derived from bone marrow mesenchymal stem cells overexpressing miR-148a reduced cardiomyocyte apoptosis by inhibiting SPARC-associated modular calcium-binding protein 2 (SMOC2) [6]. The preclinical studies discussed in this section are graphically summarized in Figure 1.
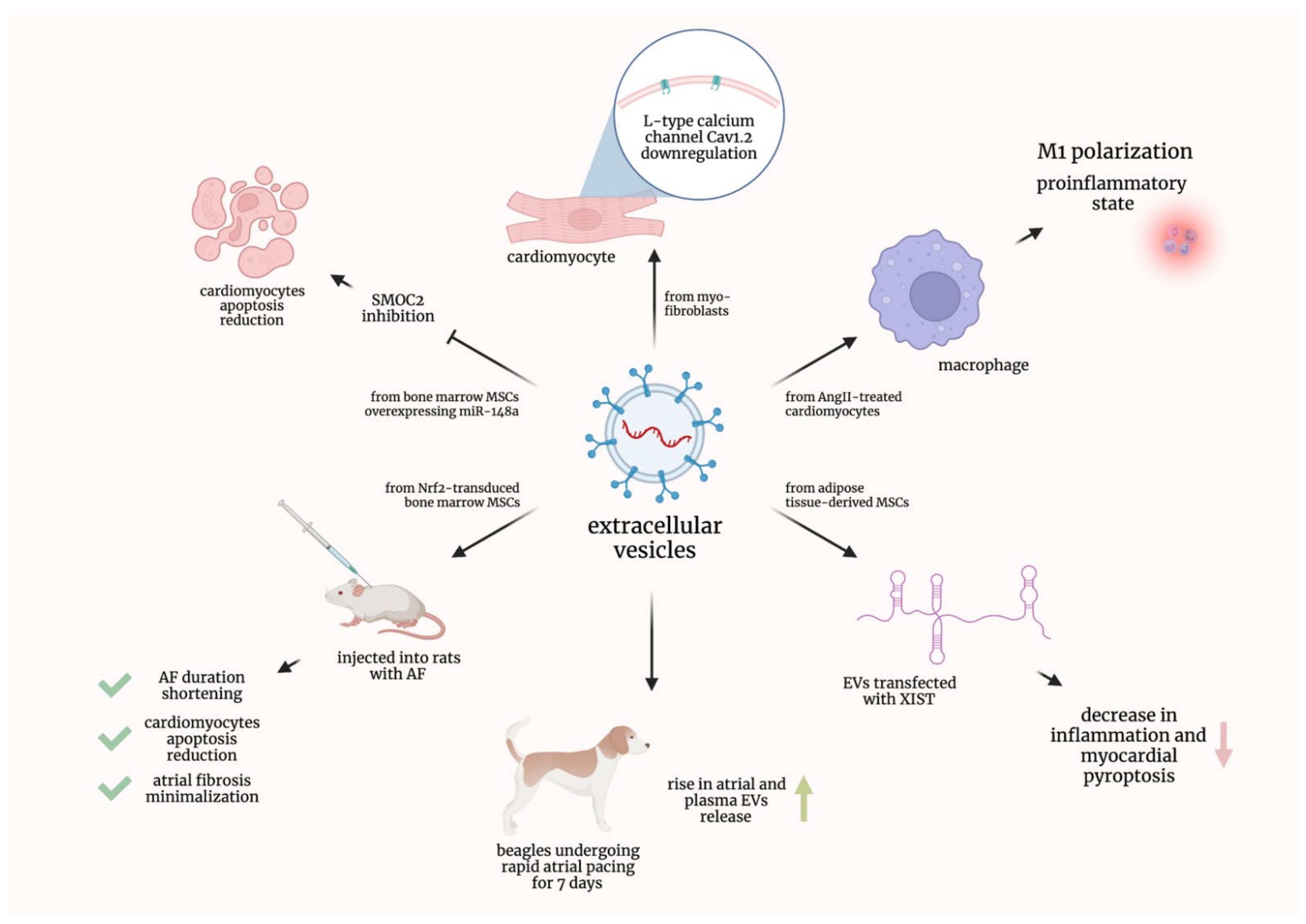
Figure 1. Graphical summarization of the preclinical studies concerning extracellular vesicles in atrial fibrillation. AF—atrial fibrillation; AngII—Angiotensin II; EVs—extracellular vesicles; miR—micro-ribonucleic acid; MSCs—mesenchymal stem cells; Nrf2—nuclear factor-erythroid 2-related factor 2; SMOC2—SPARC-associated modular calcium-binding protein 2; XIST—X-inactive specific transcript.
2. EVs in Atrial Fibrillation Patients Treated with Anticoagulants and Other Drugs
Chirinos et al. investigated the association between digoxin use in patients suffering from non-valvular AF and the level of EVs originating from endothelium and platelets. They showed that patients taking digoxin exhibited increased levels of endothelial EVs [7]. Lau et al. studied the population of AF patients treated with warfarin. It was shown that endothelial and platelet EVs collectively negatively correlated with the estimated glomerular filtration rate (eGFR), making them potential nephrotoxicity biomarkers. However, platelet-derived EVs alone did not present any correlation with the eGFR [8]. Lenart-Migdalska et al. studied the impact of dabigatran intake on platelet and endothelial EV concentration in non-valvular AF patients. Platelet-derived EVs were increased in patients treated with dabigatran; moreover, dabigatran concentration correlated negatively with the concentration change among these EVs. Endothelial-derived EVs did not exhibit such relationships [9]. The same research group evidenced that non-valvular AF patients treated with rivaroxaban presented increased levels of both platelet- and endothelial-derived EVs after drug administration [10]. Moreover, Weiss et al. demonstrated that non-valvular AF patients treated with rivaroxaban had a significantly altered proteomic profile of EVs compared with those treated with warfarin; proinflammatory proteins and complement factors were decreased, whereas negative regulators of inflammatory pathways were elevated in patients treated with rivaroxaban [11]. Complementary to the aforementioned studies, Duarte et al. showed that AF patients treated with rivaroxaban or warfarin presented increased levels of platelet-derived EVs compared with the control subjects with no AF. However, no differences in endothelial-derived EVs were noted [12]. Further improvement in this field may lead to the treatment individualization with EVs being potential predictors of treatment response in AF patients.
3. EVs Containing Nucleic Acids in Atrial Fibrillation Patients
Wang et al. investigated EVs containing micro-ribonucleic acids (miRs). They proved that patients suffering from non-valvular AF presented increased levels of miR-483-5p and decreased levels of miR-142-5p, miR-223-3p, and miR-223-5p compared with controls in sinus rhythm. Moreover, miR-483-5p, miR-142-5p, and miR-223-3p were shown to be related to AF by univariate logistic analysis, whereas multivariate logistic analyses proved miR-483-5p to be independently correlated with AF [13]. Mun et al. researched differences in the expression of miRs in circulating EVs between subjects with supraventricular tachycardia and patients suffering from persistent AF. The study revealed that the latter group presented increased levels of miR-107, miR-320d, miR-103a-3p, miR-486-5p, and let-7b-5p [14]. Soltész et al. proved that there were no differences in the EV-contained mitochondrial deoxyribonucleic acid (mtDNA) copy numbers between AF patients and healthy controls [15]. Additionally, Wei et al. demonstrated that AF patients had increased levels of miR-92b-3p, miR-1306-5p, and miR-let-7b-3p contained in the EVs compared with control patients in sinus rhythm [16].
Liu et al. found that patients suffering from congenital heart disease (CHD) and AF presented different concentrations of miRs associated with EVs when compared with CHD patients in sinus rhythm. Quantitative analysis showed reduced levels of miR-382-3p and miR-450a-2-3p as well as increased levels of miR-3126-5p in AF patients [17]. Wang et al. showed that AF patients had higher expression of EV-contained miR-107 compared with healthy controls [18].
Interestingly, Liu et al. demonstrated that AF patients presented decreased expression levels of EV-incorporated LINC00636 (Long Intergenic Non-Protein Coding RNA) and miR-450a-2-3p compared with non-AF patients. Moreover, the expression levels of these two RNAs positively correlated with each other [19]. Importantly, Chen et al. proved that AF patients showed an increased expression of myocardial infarction associated transcript (MIAT) in serum-derived EVs when compared with healthy individuals. Interestingly, the highest MIAT expression was observed in patients with permanent AF. It was also evidenced that MIAT, abundant in those EVs, promoted atrial fibrosis and thus compounded atrial remodeling and subsequent AF [20].
Siwaponanan et al. evidenced that AF patients presented increased levels of miR-106b-3p, miR-590-5p, miR-339-3p, miR-378-3p, miR-328-3p, and miR-532-3p derived from EVs. These miRs were suggested to be possibly involved in processes such as arrhythmogenesis or structural remodeling in AF [21]. Similarly, Zhu et al. investigated EV-contained miRs expression levels in AF patients. They presented an increase in miR-124-3p, miR-378d, miR-2110, and miR-3180-3p levels as well as a decrease in miR-223-5p, miR-574-3p, miR-125a-3p, and miR-1299 levels when compared with patients in sinus rhythm [22]. Finally, Mun et al. proved that patients with persistent AF showed a significant downregulation of miR-30a-5p in small EVs [23]. Just as EVs alone, miRs and other small molecules contained in EVs can become not only diagnostic but also predictive tools.
4. EVs in Atrial Fibrillation Patients Undergoing Ablation or Other Invasive Procedures
Herrera-Siklódy et al. studied the differences between AF patients undergoing cryoablation and radiofrequency (RF) ablation in terms of EVs as cellular damage markers. In both groups of patients, they observed an increase in platelet- and leukocyte-derived (but not endothelial-derived) EVs after the ablation procedure. However, there were no significant differences between the groups [24]. Jesel et al. compared the concentrations of EVs between the right and the left atria in AF patients undergoing ablation. They investigated procoagulant EVs derived from platelets, leukocytes, and endothelial cells. Interestingly, only endothelial-derived EVs presented atrial-specific differences, being increased in the right atrium [25]. Liles et al. measured the levels of tissue-factor extracellular vesicles (TF-EVs) in non-valvular AF patients prior to ablation surgery. They compared patients and healthy controls and divided patients based on the type of their anticoagulant therapy. It was shown that TF-EVs were increased in AF patients compared with healthy controls; however, after dividing AF patients into two groups, not-treated with anticoagulants and treated with anticoagulants, only the latter showed increased levels of TF-EVs compared with healthy controls. Consistently, this group also presented increased levels of TF-EVs compared with the first group. Interestingly, there were no differences in TF-EV levels between AF patients treated with warfarin and AF patients treated with apixaban or rivaroxaban [26]. Pourtau et al. proved the diminished tissue-factor-dependent procoagulant activity of EVs in both paroxysmal and persistent AF patients undergoing ablation compared with healthy controls; however, only the paroxysmal AF patients showed decreased fibrinolytic activity of EVs compared with controls. Moreover, 10 AF patients in sinus rhythm (for 10 days before ablation) were subjected to induced AF. After 20 minutes of acute AF, these patients presented decreased procoagulant activity of EVs with unaltered fibrinolytic activity [27].
Meng et al. demonstrated that AF patients had elevated levels of TF-EVs and EVs derived from platelets, endothelial cells, and leukocytes (but not from erythrocytes) compared with healthy controls. It was also proven that both endothelial-derived EVs (>355/μL) as well as leukocyte-derived EVs (>639/μL) were risk factors for the early recurrence of atrial fibrillation (ERAF). Additionally, the former were shown to be an independent predictor of the ERAF [28].
Mørk et al. analyzed EVs (particularly TF-EVs) in patients with or without AF undergoing cardiac surgery procedures. Total EVs, as well as TF-EVs, were shown to be increased in patients suffering from AF in all measurements, from venous blood preoperatively/intraoperatively and directly from the left atrial appendage intraoperatively [29]. Amabile et al. showed that non-valvular AF patients who underwent left atrial appendage occlusion presented increased levels of annexin V-positive, and platelet-, erythrocyte-, and leukocyte-derived EVs after the intervention. In patients undergoing coronary angiography, who served as a control group, only annexin V-positive EVs were proven to be increased after the procedure [30]. Shaihov-Teper et al. investigated the population of patients with or without AF undergoing elective heart surgery. Organ cultures, grown from epicardial fat, secreted more EVs in samples from AF patients. Moreover, EV contents, proinflammatory and profibrotic cytokines as well as profibrotic miR, were also elevated in this group [31]. Perhaps ongoing and future research will provide evidence that will lead to the EVs’ inclusion into the qualification criteria for invasive procedures such as ablation.
5. Other Research Studies Concerning EVs in Atrial Fibrillation Patients
Choudhury et al. proved that both patients suffering from AF and patients in sinus rhythm suffering from other cardiovascular diseases had increased levels of platelet EVs compared with healthy controls in sinus rhythm. Moreover, they evidenced that neither AF type (paroxysmal or permanent) nor applied therapy (aspirin or warfarin) influenced the level of platelet EVs [32]. Ederhy et al. showed increased levels of annexin V-positive EVs in AF patients compared with control subjects with or without cardiovascular risk factors. The level of platelet- and endothelial-derived EVs showed no differences between AF patients and control subjects with cardiovascular risk factors; however, they were increased in those two groups as compared with control subjects without cardiovascular risk factors [33]. Azzam et al. proved that patients suffering from valvular AF had increased levels of platelet-derived EVs compared with age-matched healthy volunteers in sinus rhythm. Moreover, it appeared that the severity of mitral stenosis correlated with EV levels [34].
Wang et al. investigated non-valvular AF patients, both with paroxysmal AF and persistent AF. It emerged that the latter group had increased levels of EVs compared with normal controls and paroxysmal AF patients. What is more interesting is it was shown that patients with persistent AF had increased levels of EV-bound interleukin-1β and P-selectin [35]. Hayashi et al. compared the levels of P-selectin-positive EVs in platelet-rich plasma between non-valvular AF patients and control subjects; there were no differences between these groups. However, they proved that the induction of AF in paroxysmal AF patients resulted in increased levels of EVs expressing P-selectin [36]. Idriss et al. proved that both mitral valve disease (MVD) patients with AF and MVD patients in sinus rhythm had increased levels of EVs showing positive binding to anti-CD41a as compared with healthy controls; however, there were no differences between these two groups of MVD patients [37].
Wang et al. studied non-valvular AF patients with or without left atrial thrombi. Both groups showed increased phosphatidyl-serine (PS)-exposing EV levels compared with control subjects; moreover, the patients with thrombi had elevated EV levels compared with the patients without thrombi. Both groups presented an increased procoagulant activity to be effectively inhibited by the addition of lactadherin. Interestingly, the amount of EVs positively correlated with the thrombus diameter [38]. Siwaponanan et al. demonstrated that non-valvular AF patients had increased levels of total EVs and increased levels of platelet- and endothelial-derived EVs compared with healthy controls [39].
Thulin et al. investigated AF patients (some of whom were stroke cases) selected from a large randomized clinical trial and a cohort of the randomly selected control individuals of age 70. It was shown that AF patients presented increased levels of PS-positive EVs and EVs derived from platelets, leukocytes, and erythrocytes (but not from endothelial cells) compared with controls. Moreover, there were no differences in the EV levels among the AF patients when comparing the stroke cases to the others [40]. Wang et al. classified non-valvular AF patients as “low to moderate risk” or “high risk” of stroke using the CHADS2 score. The latter group was evidenced to have increased levels of annexin V-positive and platelet-derived EVs as compared with the lower-risk group. Moreover, it was shown that EVs derived from AF patients bound to platelet receptor CD36 and activated platelets [41].
Voukalis et al. evidenced that AF patients presented increased levels of apoptotic EVs (annexin V-positive) when compared with ischemic disease patients in sinus rhythm; however, both groups had similar levels of platelet-derived EVs [42]. Ni et al. conducted a proteomic analysis of serum EVs comparing paroxysmal AF patients with healthy subjects. These two groups were proven to have different expression levels of many proteins mainly involved in anticoagulation, complement system, and protein folding, as indicated by the bioinformatic analysis [43]. Zietzer et al. proved that patients suffering from AF had higher levels of large EVs derived from platelets in the left atrial appendage than patients with no AF. Moreover, patients with permanent AF presented higher levels of these EVs when compared with non-permanent AF patients [44].
References
- Li, S.; Gao, Y.; Liu, Y.; Li, J.; Yang, X.; Hu, R.; Liu, J.; Zhang, Y.; Zuo, K.; Li, K.; et al. Myofibroblast-Derived Exosomes Contribute to Development of a Susceptible Substrate for Atrial Fibrillation. Cardiology 2020, 145, 324–332.
- Cao, F.; Li, Z.; Ding, W.; Yan, L.; Zhao, Q. Angiotensin II-Treated Cardiac Myocytes Regulate M1 Macrophage Polarization via Transferring Exosomal PVT1. J. Immunol. Res. 2021, 2021, 1994328.
- Yao, Y.; He, S.; Wang, Y.; Cao, Z.; Liu, D.; Fu, Y.; Chen, H.; Wang, X.; Zhao, Q. Blockade of Exosome Release Suppresses Atrial Fibrillation by Alleviating Atrial Fibrosis in Canines With Prolonged Atrial Pacing. Front. Cardiovasc. Med. 2021, 8, 699175.
- Yan, B.; Liu, T.; Yao, C.; Liu, X.; Du, Q.; Pan, L. LncRNA XIST shuttled by adipose tissue-derived mesenchymal stem cell-derived extracellular vesicles suppresses myocardial pyroptosis in atrial fibrillation by disrupting miR-214-3p-mediated Arl2 inhibition. Lab. Investig. 2021, 101, 1427–1438.
- Xu, L.; Fan, Y.; Wu, L.; Zhang, C.; Chu, M.; Wang, Y.; Zhuang, W. Exosomes from Bone Marrow Mesenchymal Stem Cells with Overexpressed Nrf2 Inhibit Cardiac Fibrosis in Rats with Atrial Fibrillation. Cardiovasc. Ther. 2022, 2022, 2687807.
- Zhang, W.; Man, Y.; Chen, Z. microRNA-148a in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Alleviates Cardiomyocyte Apoptosis in Atrial Fibrillation by Inhibiting SMOC2. Mol. Biotechnol. 2022.
- Chirinos, J.A.; Castrellon, A.; Zambrano, J.P.; Jimenez, J.J.; Jy, W.; Horstman, L.L.; Willens, H.J.; Castellanos, A.; Myerburg, R.J.; Ahn, Y.S. Digoxin use is associated with increased platelet and endothelial cell activation in patients with nonvalvular atrial fibrillation. Heart Rhythm. 2005, 2, 525–529.
- Lau, Y.C.; Xiong, Q.; Blann, A.D.; Lip, G.Y. Relationship between renal function and circulating microparticles, soluble P-selectin and E-selectin levels in atrial fibrillation. J. Thromb. Thrombolysis 2017, 43, 18–23.
- Lenart-Migdalska, A.; Drabik, L.; Kaźnica-Wiatr, M.; Tomkiewicz-Pająk, L.; Podolec, P.; Olszowska, M. Flow Cytometric Assessment of Endothelial and Platelet Microparticles in Patients With Atrial Fibrillation Treated With Dabigatran. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620972467.
- Lenart-Migdalska, A.; Drabik, L.; Kaźnica-Wiatr, M.; Tomkiewicz-Pająk, L.; Podolec, P.; Olszowska, M. Increased Levels of Platelets and Endothelial-Derived Microparticles in Patients With Non-Valvular Atrial Fibrillation During Rivaroxaban Therapy. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211019465.
- Weiss, L.; Keaney, J.; Szklanna, P.B.; Prendiville, T.; Uhrig, W.; Wynne, K.; Kelliher, S.; Ewins, K.; Comer, S.P.; Egan, K.; et al. Nonvalvular atrial fibrillation patients anticoagulated with rivaroxaban compared with warfarin exhibit reduced circulating extracellular vesicles with attenuated pro-inflammatory protein signatures. J. Thromb. Haemost. 2021, 19, 2583–2595.
- Duarte, R.C.F.; Rios, D.R.A.; Figueiredo, E.L.; Caiaffa, J.R.S.; Silveira, F.R.; Lanna, R.; Alves, L.C.V.; Martins, G.L.; Reis, H.J.; Reis, E.A.; et al. Thrombin Generation and other hemostatic parameters in patients with atrial fibrillation in use of warfarin or rivaroxaban. J. Thromb. Thrombolysis 2021, 51, 47–57.
- Wang, S.; Min, J.; Yu, Y.; Yin, L.; Wang, Q.; Shen, H.; Yang, J.; Zhang, P.; Xiao, J.; Wang, Z. Differentially expressed miRNAs in circulating exosomes between atrial fibrillation and sinus rhythm. J. Thorac. Dis. 2019, 11, 4337–4348.
- Mun, D.; Kim, H.; Kang, J.Y.; Park, H.; Park, H.; Lee, S.H.; Yun, N.; Joung, B. Expression of miRNAs in circulating exosomes derived from patients with persistent atrial fibrillation. FASEB J. 2019, 33, 5979–5989.
- Soltész, B.; Urbancsek, R.; Pös, O.; Hajas, O.; Forgács, I.N.; Szilágyi, E.; Nagy-Baló, E.; Szemes, T.; Csanádi, Z.; Nagy, B. Quantification of peripheral whole blood, cell-free plasma and exosome encapsulated mitochondrial DNA copy numbers in patients with atrial fibrillation. J. Biotechnol. 2019, 299, 66–71.
- Wei, Z.; Bing, Z.; Shaohuan, Q.; Yanran, W.; Shuo, S.; Bi, T.; Feiyu, Z.; Heng, Z.; Qin, G.; Pinfang, K. Expression of miRNAs in plasma exosomes derived from patients with atrial fibrillation. Clin. Cardiol. 2020, 43, 1450–1459.
- Liu, L.; Chen, Y.; Shu, J.; Tang, C.E.; Jiang, Y.; Luo, F. Identification of microRNAs enriched in exosomes in human pericardial fluid of patients with atrial fibrillation based on bioinformatic analysis. J. Thorac. Dis. 2020, 12, 5617–5627.
- Wang, S.; Li, L.; Hu, X.; Liu, T.; Jiang, W.; Wu, R.; Ren, Y.; Wang, M. Effects of Atrial Fibrillation-Derived Exosome Delivery of miR-107 to Human Umbilical Vein Endothelial Cells. DNA Cell Biol. 2021, 40, 568–579.
- Liu, L.; Luo, F.; Lei, K. Exosomes Containing LINC00636 Inhibit MAPK1 through the miR-450a-2-3p Overexpression in Human Pericardial Fluid and Improve Cardiac Fibrosis in Patients with Atrial Fibrillation. Mediat. Inflamm. 2021, 2021, 9960241.
- Chen, Y.; Chen, X.; Li, H.; Li, Y.; Cheng, D.; Tang, Y.; Sang, H. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin. Transl. Med. 2021, 11, e482.
- Siwaponanan, P.; Kaewkumdee, P.; Phromawan, W.; Udompunturak, S.; Chomanee, N.; Udol, K.; Pattanapanyasat, K.; Krittayaphong, R. Increased expression of six-large extracellular vesicle-derived miRNAs signature for nonvalvular atrial fibrillation. J. Transl. Med. 2022, 20, 4.
- Zhu, P.; Li, H.; Zhang, A.; Li, Z.; Zhang, Y.; Ren, M.; Zhang, Y.; Hou, Y. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J. Physiol. Biochem. 2022, 78, 85–98.
- Mun, D.; Kim, H.; Kang, J.Y.; Yun, N.; Youn, Y.N.; Joung, B. Small extracellular vesicles derived from patients with persistent atrial fibrillation exacerbate arrhythmogenesis via miR-30a-5p. Clin. Sci. 2022, 136, 621–637.
- Herrera Siklódy, C.; Arentz, T.; Minners, J.; Jesel, L.; Stratz, C.; Valina, C.M.; Weber, R.; Kalusche, D.; Toti, F.; Morel, O.; et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: A randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012, 9, 189–196.
- Jesel, L.; Arentz, T.; Herrera-Siklody, C.; Trenk, D.; Zobairi, F.; Abbas, M.; Weber, R.; Minners, J.; Toti, F.; Morel, O. Do atrial differences in endothelial damage, leukocyte and platelet activation, or tissue factor activity contribute to chamber-specific thrombogenic status in patients with atrial fibrillation? J. Cardiovasc. Electrophysiol. 2014, 25, 266–270.
- Liles, J.; Liles, J.; Wanderling, C.; Syed, M.; Hoppensteadt, D.; Fareed, J. Increased Level of Thrombotic Biomarkers in Patients with Atrial Fibrillation Despite Traditional and New Anticoagulant Therapy. Clin. Appl. Thromb. Hemost. 2016, 22, 743–748.
- Pourtau, L.; Sellal, J.M.; Lacroix, R.; Poncelet, P.; Bernus, O.; Clofent-Sanchez, G.; Hocini, M.; Haïssaguerre, M.; Dignat-George, F.; Sacher, F.; et al. Platelet function and microparticle levels in atrial fibrillation: Changes during the acute episode. Int. J. Cardiol. 2017, 243, 216–222.
- Meng, H.; Kou, J.; Ma, R.; Ding, W.; Kou, Y.; Cao, M.; Dong, Z.; Bi, Y.; Thatte, H.S.; Shi, J. Prognostic implications and procoagulant activity of phosphatidylserine exposure of blood cells and microparticles in patients with atrial fibrillation treated with pulmonary vein isolation. Mol. Med. Rep. 2017, 16, 8579–8588.
- Mørk, M.; Andreasen, J.J.; Rasmussen, L.H.; Lip, G.Y.H.; Pedersen, S.; Bæk, R.; Jørgensen, M.M.; Kristensen, S.R. Elevated blood plasma levels of tissue factor-bearing extracellular vesicles in patients with atrial fibrillation. Thromb. Res. 2019, 173, 141–150.
- Amabile, N.; Bagdadi, I.; Armero, S.; Elhadad, S.; Sebag, F.; Landolff, Q.; Saby, L.; Mechulan, A.; Boulanger, C.M.; Caussin, C. Impact of left atrial appendage closure on circulating microvesicles levels: The MICROPLUG study. Int. J. Cardiol. 2020, 307, 24–30.
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.P.; et al. Extracellular Vesicles From Epicardial Fat Facilitate Atrial Fibrillation. Circulation 2021, 143, 2475–2493.
- Choudhury, A.; Chung, I.; Blann, A.D.; Lip, G.Y.H. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: Relationship to p-selectin and antithrombotic therapy. Chest 2007, 131, 809–815.
- Ederhy, S.; Di Angelantonio, E.; Mallat, Z.; Hugel, B.; Janower, S.; Meuleman, C.; Boccara, F.; Freyssinet, J.M.; Tedgui, A.; Cohen, A. Levels of circulating procoagulant microparticles in nonvalvular atrial fibrillation. Am. J. Cardiol. 2007, 100, 989–994.
- Azzam, H.; Zagloul, M. Elevated platelet microparticle levels in valvular atrial fibrillation. Hematology 2009, 14, 357–360.
- Wang, H.; Yan, H.M.; Tang, M.X.; Wang, Z.H.; Zhong, M.; Zhang, Y.; Deng, J.T.; Zhang, W. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determinated by ELISA using a specific monoclonal antibody AD-1. Clin. Chim. Acta 2010, 411, 1700–1704.
- Hayashi, M.; Takeshita, K.; Inden, Y.; Ishii, H.; Cheng, X.W.; Yamamoto, K.; Murohara, T. Platelet activation and induction of tissue factor in acute and chronic atrial fibrillation: Involvement of mononuclear cell-platelet interaction. Thromb. Res. 2011, 128, e113–e118.
- Idriss, N.K.; Blann, A.D.; Sayed, D.M.; Gaber, M.A.; Hassen, H.A.; Kishk, Y.T. Circulating Endothelial Cells and Platelet Microparticles in Mitral Valve Disease With and Without Atrial Fibrillation. Angiology 2015, 66, 631–637.
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 258, 138–143.
- Siwaponanan, P.; Keawvichit, R.; Udompunturak, S.; Hunnangkul, S.; Reesukumal, K.; Sukapirom, K.; Pattanapanyasat, K.; Krittayaphong, R. Altered profile of circulating microparticles in nonvalvular atrial fibrillation. Clin. Cardiol. 2019, 42, 425–431.
- Thulin, Å.; Lindbäck, J.; Granger, C.B.; Wallentin, L.; Lind, L.; Siegbahn, A. Extracellular vesicles in atrial fibrillation and stroke. Thromb. Res. 2020, 193, 180–189.
- Wang, H.; Song, N.P.; Li, J.P.; Wang, Z.H.; Ti, Y.; Li, Y.H.; Zhang, W.; Zhong, M. The microvesicle/CD36 complex triggers a prothrombotic phenotype in patients with non-valvular atrial fibrillation. J. Cell. Mol. Med. 2020, 24, 7331–7340.
- Voukalis, C.; Lip, G.Y.H.; Shantsila, E. Effects of antithrombotic drugs on the prothrombotic state in patients with atrial fibrillation: The west Birmingham atrial fibrillation project. Thromb. Res. 2021, 200, 149–155.
- Ni, H.; Pan, W.; Jin, Q.; Xie, Y.; Zhang, N.; Chen, K.; Lin, T.; Lin, C.; Xie, Y.; Wu, J.; et al. Label-free proteomic analysis of serum exosomes from paroxysmal atrial fibrillation patients. Clin. Proteom. 2021, 18, 1.
- Zietzer, A.; Al-Kassou, B.; Jamme, P.; Rolfes, V.; Steffen, E.; Bulic, M.; Hosen, M.R.; Goody, P.R.; Tiyerili, V.; Zimmer, S.; et al. Large extracellular vesicles in the left atrial appendage in patients with atrial fibrillation-the missing link? Clin. Res. Cardiol. 2022, 111, 34–49.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
827
Revisions:
2 times
(View History)
Update Date:
01 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




