
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charalampos Proestos | -- | 2718 | 2022-08-29 08:47:15 | | | |
| 2 | Vivi Li | -5 word(s) | 2713 | 2022-08-30 04:01:24 | | |
Video Upload Options
Obesity is arising as a global pandemic throughout the world. Obesity has tripled worldwide, creating an alarming situation. The majority of people nowadays are suffering from obesity and overweight. It affects health of people of all age groups, ethnicity, gender, and sex, and is linked to a sedentary lifestyle of people, poor eating habits, and disturbed sleeping patterns. It causes several diseases such as diabetes mellitus type 2, hypertension, cardiovascular diseases, asthma, gallstones, and colon cancer. Many synthetic anti-obesity drugs such as orlistat, lorcaserin, phentermine, bupropion, and liraglutide are already available on the market. However, these drugs have side effects, including dry mouth and sleeping disorders, dizziness, blood pressure, heart rate elevation, constipation, and headache. Humans have a long and ancient history of dependency on traditional medicinal plants and their major bioactive antioxidant components, such as quercetin, anthocyanins, and ellagic acid, for treating such diseases and disorders.
1. Introduction
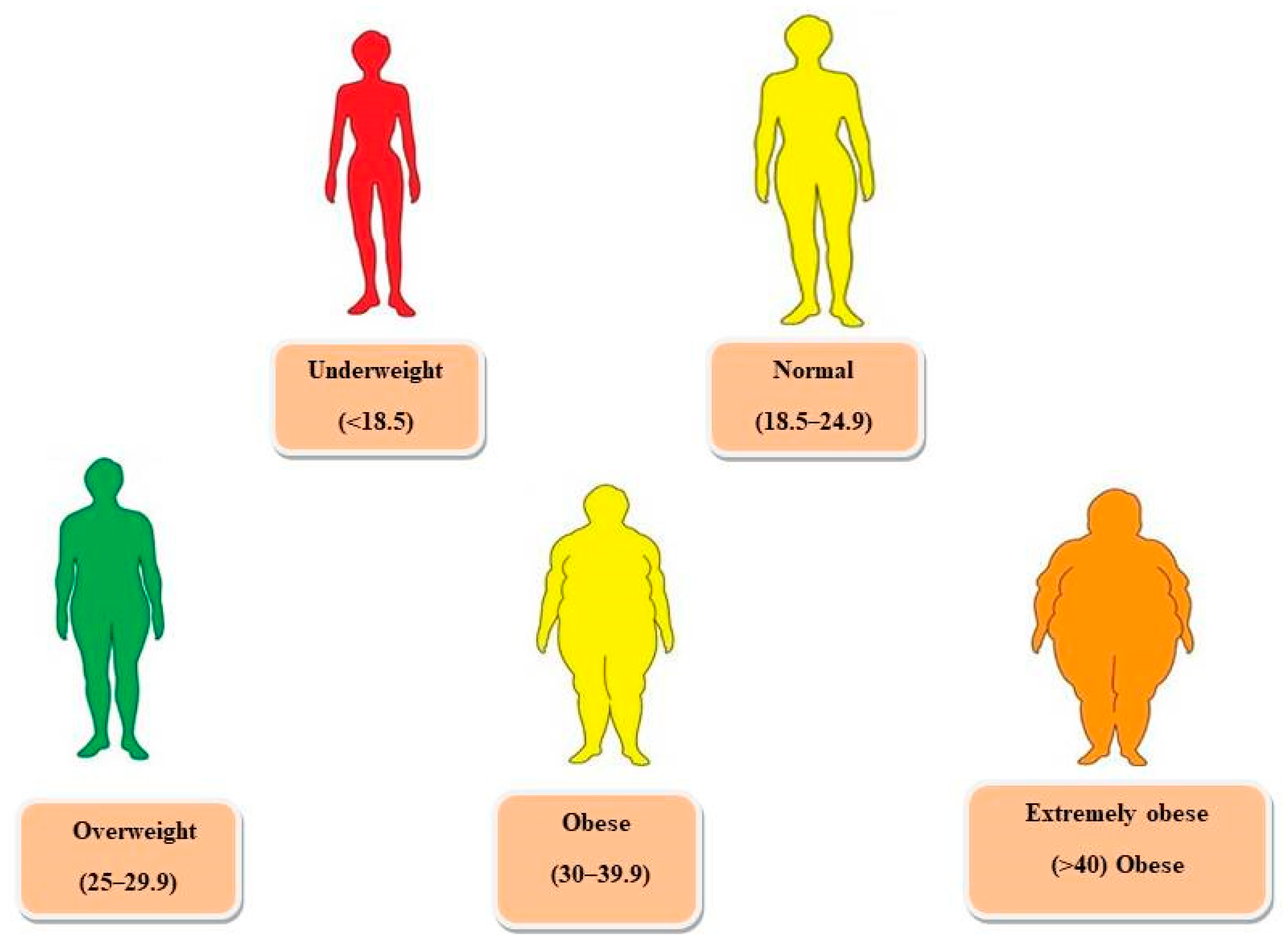
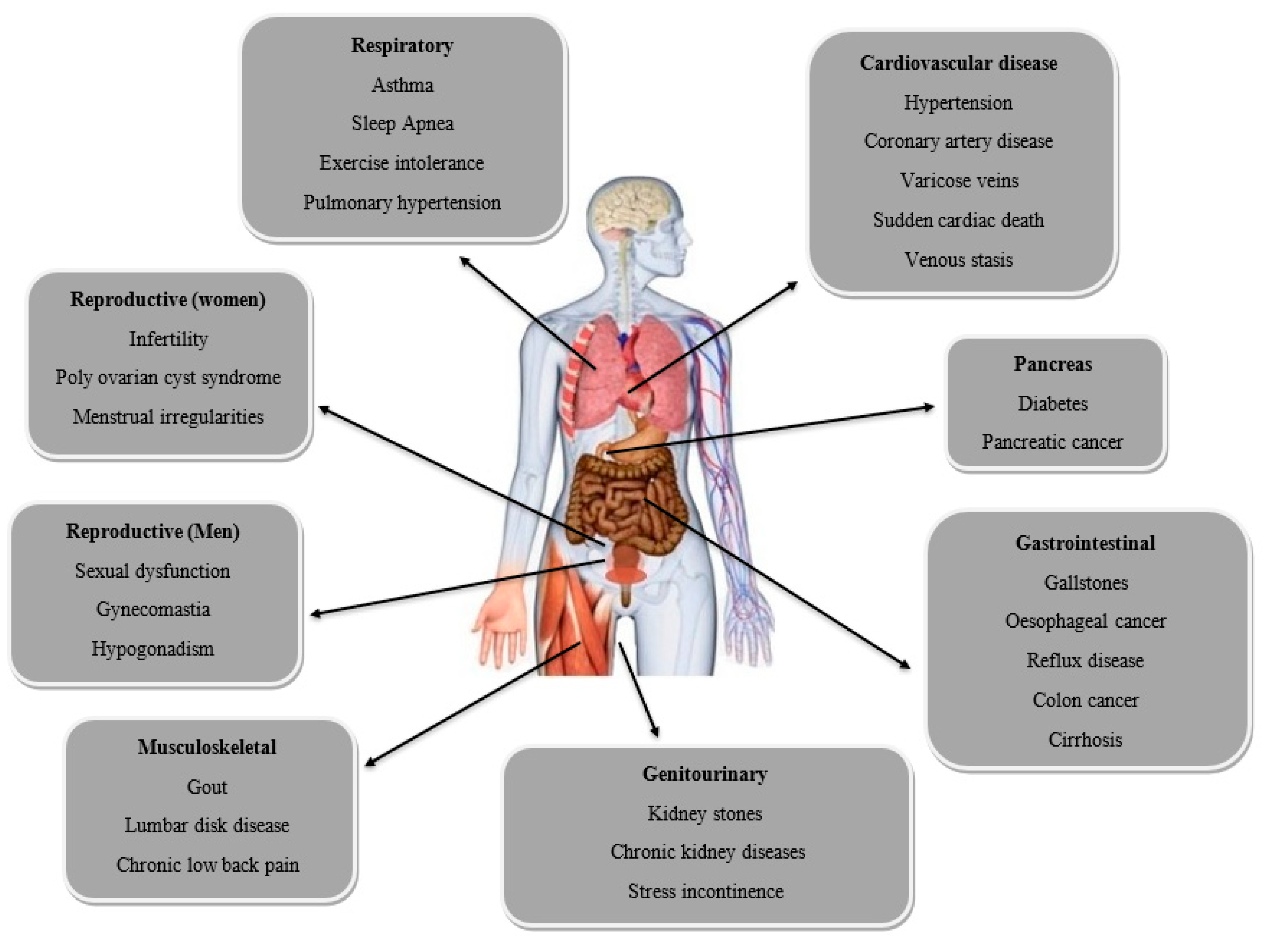
2. Hormones Related to Obesity
2.1. Leptin
2.2. Ghrelin
2.3. Insulin
2.4. Adiponectin
3. Synthetic Drugs
3.1. Orlistat
3.2. Lorcaserin
3.3. Phentermine/Extended-Release Topiramate (Qnexa)
3.4. Bupropion/Naltrexone (Contrave)
3.5. Liraglutide
4. Herbs That Control Obesity
4.1. Nigella sativa
4.2. Hibiscus sabdariffa
4.3. Ilex paraguariensis
4.4. Rosmarinus officinalis
References
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Still, C.D. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362.
- World Health Organization. Noncommunicable Diseases Country Profiles 2022; World Health Organization: Geneva, Switzerland, 2022.
- Forse, R.A.; Betancourt-Garcia, M.M.; Kissee, M.C. Epidemiology and Discrimination in Obesity. In The ASMBS Textbook of Bariatric Surgery; Springer: Cham, Switzerland, 2020; pp. 3–14.
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135, ix.
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S.
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256.
- Sidhu, S.; Parikh, T.; Burman, K.D. Endocrine Changes in Obesity. In Endotext; Feingold, K.R., Ed.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017.
- Zhang, Y.; Yu, L.; Cai, W.; Fan, S.; Feng, L.; Ji, G.; Huang, C. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci. Rep. 2014, 4, 7375.
- Miranda, J.; Vázquez-Polo, M.; Pérez-Junkera, G.; Fernández-Gil, M.D.P.; Bustamante, M.Á.; Navarro, V.; Martínez, O. FODMAP intake in Spanish population: Open approach for risk assessment. Int. J. Environ. Res. Public Health 2020, 17, 5882.
- Pollex, R.L.; Hanley, A.J.; Zinman, B.; Harris, S.B.; Khan, H.M.; Hegele, R.A. Metabolic syndrome in aboriginal Canadians: Prevalence and genetic associations. Atherosclerosis 2006, 184, 121–129.
- Kumar, M.; Guleria, S.; Chawla, P.; Khan, A.; Modi, V.K.; Kumar, N.; Kaushik, R. Anti-obesity efficacy of the selected high altitude Himalayan herbs: In vitro studies. J. Food Sci. Technol. 2020, 57, 3081–3090.
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555.
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978.
- Mishra, D.; Naorem, K.; Saraswathy, K.N. Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism and Cardiometabolic Risk Factors: A Study Among Bhil Tribal Population from Two Environmental Settings. Biochem. Genet. 2018, 56, 295–314.
- Kandpal, V.; Sachdeva, M.P.; Saraswathy, K.N. An assessment study of CVD-related risk factors in a tribal population of India. BMC Public Health 2016, 16, 434.
- Cameron, A.J.; Zimmet, P.Z.; Dunstan, D.W.; Dalton, M.; Shaw, J.E.; Welborn, T.A.; Jolley, D. Overweight and obesity in Australia: The 1999–2000 Australian diabetes, obesity, and lifestyle study (AusDiab). Med. J. Aust. 2003, 178, 427–432.
- Ruxton, C.H.S. Nutritional implications of obesity and dieting. Nutr. Bull. 2011, 36, 199–211.
- Mendez, M.A.; Popkin, B.M.; Buckland, G.; Schroder, H.; Amiano, P.; Barricarte, A.; González, C.A. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am. J. Epidemiol. 2011, 173, 448–458.
- Paradis, A.M.; Godin, G.; Pérusse, L.; Vohl, M.C. Associations between dietary patterns and obesity phenotypes. Int. J. Obes. 2009, 33, 1419–1426.
- Paz-Filho, G.; Mastronardi, C.A.; Licinio, J. Leptin treatment: Facts and expectations. Metabolism 2015, 64, 146–156.
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575.
- Fogteloo, A.J.; Pijl, H.; Frölich, M.; McCamish, M.; Meinders, A.E. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr. Metab. 2003, 16, 109–114.
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437.
- Cordido, F.; Penalva, A.; Dieguez, C.; Casanueva, F.F. Massive growth hormone (GH) discharge in obese subjects after the combined administration of GH-releasing hormone and GHRP-6: Evidence for a marked somatotroph secretory capability in obesity. J. Clin. Endocrinol. Metab. 1993, 76, 819–823.
- Alvarez-Castro, P.; Isidro, M.L.; Garcia-Buela, J.; Leal-Cerro, A.; Broglio, F.; Tassone, F. Marked GH secretion after ghrelin alone or combined with GH-releasing hormone (GHRH) in obese patients. Clin. Endocrinol. 2004, 61, 250–255.
- Kojima, M.; Hosoda, H.; Matsuo, H.; Kangawa, K. Ghrelin: Discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol. Metab. 2001, 12, 118–122.
- Tschop, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709.
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673.
- Ukkola, O. Ghrelin and insulin metabolism. Eur. J. Clin. Investig. 2003, 33, 183–185.
- Shuto, Y.; Shibasaki, T.; Otagiri, A.; Kuriyama, H.; Ohata, H.; Tamura, H. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J. Clin. Investig. 2002, 109, 1429–1436.
- Cummings, D.E.; Frayo, R.S.; Marmonier, C.; Aubert, R.; Chapelot, D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E297–E304.
- Abate, N. Insulin resistance and obesity. The role of fat distribution pattern. Diabetes Care 1996, 19, 292–294.
- Bjorntorp, P. Metabolic abnormalities in visceral obesity. Ann. Med. 1992, 24, 3–5.
- Flier, J.S. Insulin receptors and insulin resistance. Annu. Rev. Med. 1983, 34, 145–160.
- Kahn, C.R. Role of insulin receptors in insulin-resistant states. Metabolism 1980, 29, 455–466.
- Moller, D.E.; Flier, J.S. Insulin resistance-mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948.
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543.
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751.
- Guerre-Millo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18.
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099.
- Frohmader, K.S.; Pitchers, K.K.; Balfour, M.E.; Coolen, L.M. Mixing pleasures: Review of the effects of drugs on sex behavior in humans and animal models. Horm. Behav. 2010, 58, 149–162.
- Oh, S.; Kim, K.S.; Chung, Y.S.; Shong, M.; Park, S.B. Anti-obesity agents: A focused review on the structural classification of therapeutic entities. Curr. Top Med. Chem. 2009, 9, 466–481.
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25.
- United States Food and Drug Administration. Lorcaserin Briefing Information: Endocrinologic and Metabolic Drugs Advisory Committee. 2012. Available online: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm225628.htm (accessed on 28 October 2015).
- Weissman, N.J.; Sanchez, M.; Koch, G.G.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ. Cardiovasc. Imaging 2013, 6, 560–567.
- Fidler, M.C.; Sanchez, M.; Raether, B.; Weissman, N.J.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J. Clin. Endocrinol. Metab. 2011, 96, 3067–3077.
- Scozzafava, A.; Supuran, C.T.; Carta, F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert. Opin. Ther. Pat. 2013, 23, 725–735.
- Gadde, K.M.; Allison, D.B.; Ryan, D.H.; Peterson, C.A.; Troupin, B.; Schwiers, M.L.; Day, W.W. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1341–1352.
- Unites States Food and Drug Administration. Contrave (Naltrexone 4 mg, 8 mg/Bupropion HCL 90 mg Extended Release Tablet) Briefing Document, NDA 200063. 2010. Available online: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommitee/ucm235671.pdf (accessed on 28 October 2015).
- Wadden, T.A.; Foreyt, J.P.; Foster, G.D. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity 2011, 19, 110–120.
- European Medicines Agency. Victoza Summary of Product Characteristics. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf (accessed on 28 October 2015).
- Turton, M.D.; O’Shea, D.; Gunn, I. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72.
- Van Can, J.; Sloth, B.; Jensen, C.B. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793.
- United States Food and Drug Administration. FDA Approves Weight-Management Drug Saxenda. 2014. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm (accessed on 28 October 2015).
- Bahmani, M.; Eftekhari, Z.; Saki, K.; Fazeli-Moghadam, E.; Jelodari, M.; Rafieian-Kopaei, M. Obesity phytotherapy: Review of native herbs used in traditional medicine for obesity. J. Evid.-Based Complement. Altern. Med. 2016, 21, 228–234.
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641.
- Dessie, A.B.; Abate, T.M.; Adane, B.T.; Tesfa, T.; Getu, S. Estimation of technical efficiency of black cumin (Nigella sativa L.) farming in northwest Ethiopia: A stochastic frontier approach. J. Econ. Struct. 2020, 9, 18.
- Goreja, W.G. Black Seed: Nature’s Miracle Remedy; Karger Publishers: Basel, Switzerland, 2003.
- Cheikh-Rouhou, S.; Besbes, S.; Lognay, G.; Blecker, C.; Deroanne, C.; Attia, H. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J. Food Compos. Anal. 2008, 21, 162–168.
- Mehta, B.K.; Verma, M.; Gupta, M. Novel lipid constituents identified in seeds of Nigella sativa (Linn). J. Braz. Chem. Soc. 2008, 19, 458–462.
- Nickavar, B.; Mojab, F.; Javidnia, K.; Amoli, M.A.R. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Nat. C 2003, 58, 629–631.
- Morikawa, T.; Xu, F.; Ninomiya, K.; Matsuda, H.; Yoshikawa, M. Nigellamines A3, A4, A5, and C, new dolabellane-type diterpene alkaloids, with lipid metabolism-promoting activities from the Egyptian medicinal food black cumin. Chem. Pharm. Bull. 2004, 52, 494–497.
- Datau, E.A.; Surachmanto, E.E.; Pandelaki, K.; Langi, J.A. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med. Indones. 2010, 42, 130–134.
- Adebayo-Tayo, B.C.; Samuel, U.A. Microbial Quality and Proximate Composition of Dried Hibiscus sabdariffa Calyxes in Uyo, Eastern Nigeria. Malays. J. Microbiol. 2009, 5, 13–18.
- Hirunpanich, V.; Utaipat, A.; Morales, N.P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 2006, 103, 252–260.
- Sheba, A.L.; Ilakkia, A. Anti-obesity effect of hibiscus Sabdariffa L.—A review. Int. J. Pharma Bio Sci. 2016, 7, 13.
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210.
- Janson, B.; Prasomthong, J.; Malakul, W.; Boonsong, T.; Tunsophon, S. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed. Pharmacother. 2021, 138, 111438.
- Mosimann, A.L.P.; Wilhelm-Filho, D.; Da Silva, E.L. Aqueous extract of Ilex paraguariensis attenuates the progression of atherosclerosis in cholesterol-fed rabbits. Biofactors 2006, 26, 59–70.
- Kim, S.Y.; Oh, M.R.; Kim, M.G.; Chae, H.J.; Chae, S.W. Anti-obesity effects of Yerba Mate (Ilex paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015, 15, 338.
- Hussein, G.M.; Matsuda, H.; Nakamura, S.; Akiyama, T.; Tamura, K.; Yoshikawa, M. Protective and ameliorative effects of maté (Ilex paraguariensis) on metabolic syndrome in TSOD mice. Phytomedicine 2011, 19, 88–97.
- Kang, Y.R.; Lee, H.Y.; Kim, J.H.; Moon, D.I.; Seo, M.Y.; Park, S.H.; Cho, S.W. Anti-obesity and anti-diabetic effects of Yerba Mate (Ilex paraguariensis) in C57BL/6J mice fed a high-fat diet. Lab. Anim. Res. 2012, 28, 23–29.
- Al-Mukhtar, E.; Selman, S.; Sahib, Z.; Naji, H. Antidepressant-Like Effect of Rosmarinus officinalis Extract in Male Mice. Med. J. Babylon 2013, 10, 803–808.
- Hussain, A.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and anti-bacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078.
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521.
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; de Lima Portella, R.; da Silva, M.H.; Athayde, M.L. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55.
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3796–3806.
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plant Res. 2010, 4, 2235–2242.
- Shatla, I.M.; Abdel-Hamid, A.M.; Metwally, M. The Effect of Rosmarinus officinalis L. Extract on High Fat Diet-Induced Obesity in Adult Male Albino Rats. Al-Azhar Med. J. 2017, 46, 749–764.





