Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marc Righini | -- | 2090 | 2022-08-26 10:32:34 | | | |
| 2 | Camila Xu | Meta information modification | 2090 | 2022-08-29 03:00:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Robert-Ebadi, H.; Moumneh, T.; Gal, G.L.; Righini, M. Diagnosis of Pulmonary Embolism during Pregnancy. Encyclopedia. Available online: https://encyclopedia.pub/entry/26534 (accessed on 07 February 2026).
Robert-Ebadi H, Moumneh T, Gal GL, Righini M. Diagnosis of Pulmonary Embolism during Pregnancy. Encyclopedia. Available at: https://encyclopedia.pub/entry/26534. Accessed February 07, 2026.
Robert-Ebadi, Helia, Thomas Moumneh, Grégoire Le Gal, Marc Righini. "Diagnosis of Pulmonary Embolism during Pregnancy" Encyclopedia, https://encyclopedia.pub/entry/26534 (accessed February 07, 2026).
Robert-Ebadi, H., Moumneh, T., Gal, G.L., & Righini, M. (2022, August 26). Diagnosis of Pulmonary Embolism during Pregnancy. In Encyclopedia. https://encyclopedia.pub/entry/26534
Robert-Ebadi, Helia, et al. "Diagnosis of Pulmonary Embolism during Pregnancy." Encyclopedia. Web. 26 August, 2022.
Copy Citation
Many of the symptoms and signs reported in almost 50% of women during physiological pregnancy, such as shortness of breath or lower limb oedema—especially as pregnancy advances through the third trimester—may suggest the possibility of a pulmonary embolism (PE) and/or deep vein thrombosis (DVT).
pulmonary embolism
diagnostic strategy
D-dimer
clinical probability
1. Introduction
The diagnosis of pulmonary embolism during pregnancy is difficult as clinical presentation may be misleading and few prospective data is available. Hence, researchers performed a narrative review reporting recent knowledge in this challenging field. Women of childbearing age are at low risk of developing venous thromboembolism (VTE). Pregnancy however represents a period at risk, with an overall incidence of VTE estimated at 1/1000 pregnancies [1][2]. The risk is highest during the third trimester and the 6 to 12 weeks following delivery [3]. Many of the symptoms and signs reported in almost 50% of women during physiological pregnancy, such as shortness of breath or lower limb oedema—especially as pregnancy advances through the third trimester—may suggest the possibility of a pulmonary embolism (PE) and/or deep vein thrombosis (DVT) [4][5]. Syncope and pre-syncope are also reported by pregnant women [6]. Which clinical picture represents a clinical suspicion of PE is therefore even more difficult to define in pregnant women than in the general population. Most often, an acute onset of progressive dyspnea and/or chest pain without any other obvious explanation raises a clinical suspicion of PE, especially if additional transient risk factors of VTE (e.g., reduced mobility) have been present during the previous weeks.
Epidemiological Data
Although VTE risk is increased 7 to 10-fold during pregnancy compared to age-matched controls, the absolute incidence remains low at around 1/1000 [7]. However, PE is responsible for around 10% of pregnancy-associated mortality. Nevertheless, the fear of missing a PE during pregnancy leads to a low threshold to suspect the disease. This is well reflected by the very low prevalence of confirmed disease among pregnant women with suspected PE in clinical trials, of around 2 to 7% [4][8][9], compared to a prevalence of 10 to 20% in diagnostic studies in the general population [10][11]. Interestingly, among pregnant women with suspected DVT, the prevalence of confirmed disease was also shown to be low, at 9 to 10% [5][12]. Another important point is that falsely diagnosing PE might be associated with bleeding complications that can be lethal for the mother and her baby. Furthermore, falsely diagnosing a PE in a young woman will have potential major implications regarding antithrombotic prophylaxis in case of further pregnancy or regarding future administration of contraceptive pills or hormone replacement therapy.
2. Diagnostic Management of Pregnant Women with Suspected Pulmonary Embolism
Until recently, all pregnant women with suspected PE historically underwent a thoracic imaging test [13]. The first “non-invasive” alternative to pulmonary angiography assessed in the 1990s was ventilation-perfusion lung scintigraphy (V/Q scan), in the hallmark PIOPED trial in a general population of patients with suspected PE [14]. Despite the absence of any scientific validation in pregnant women, perfusion only lung scintigraphy was adopted in clinical practice, based on the hypothesis that ventilation phases were highly likely to be normal in this young population with very low prevalence of cardio-pulmonary comorbidities. In a study published in 2006 assessing the appropriateness of diagnostic management of patients with suspected PE, pregnancy was by far the strongest predictor of inappropriate management, 69% of pregnant women with suspected PE being inappropriately managed [15].
2.1. Assessing the Pre-Test Clinical Probability of PE in Pregnant Women
Modern PE diagnostic algorithms are based on the assessment of pre-test clinical probability (PTP) as the first step in front of a patient with clinically suspected PE, and this crucial step is strongly supported by international guidelines [16][17]. The aim of these PTP prediction rules is to identify a group of patients with a low prevalence of PE in whom a negative D-dimer provides a high negative predictive value and safely excludes PE without imaging. Currently used PTP scores were derived and validated in PE diagnostic studies in which pregnancy represented an exclusion criterion [18]. The absence of scores validated in pregnant women is, as a matter of fact, one of the reasons refraining physicians from using D-dimer in this population.
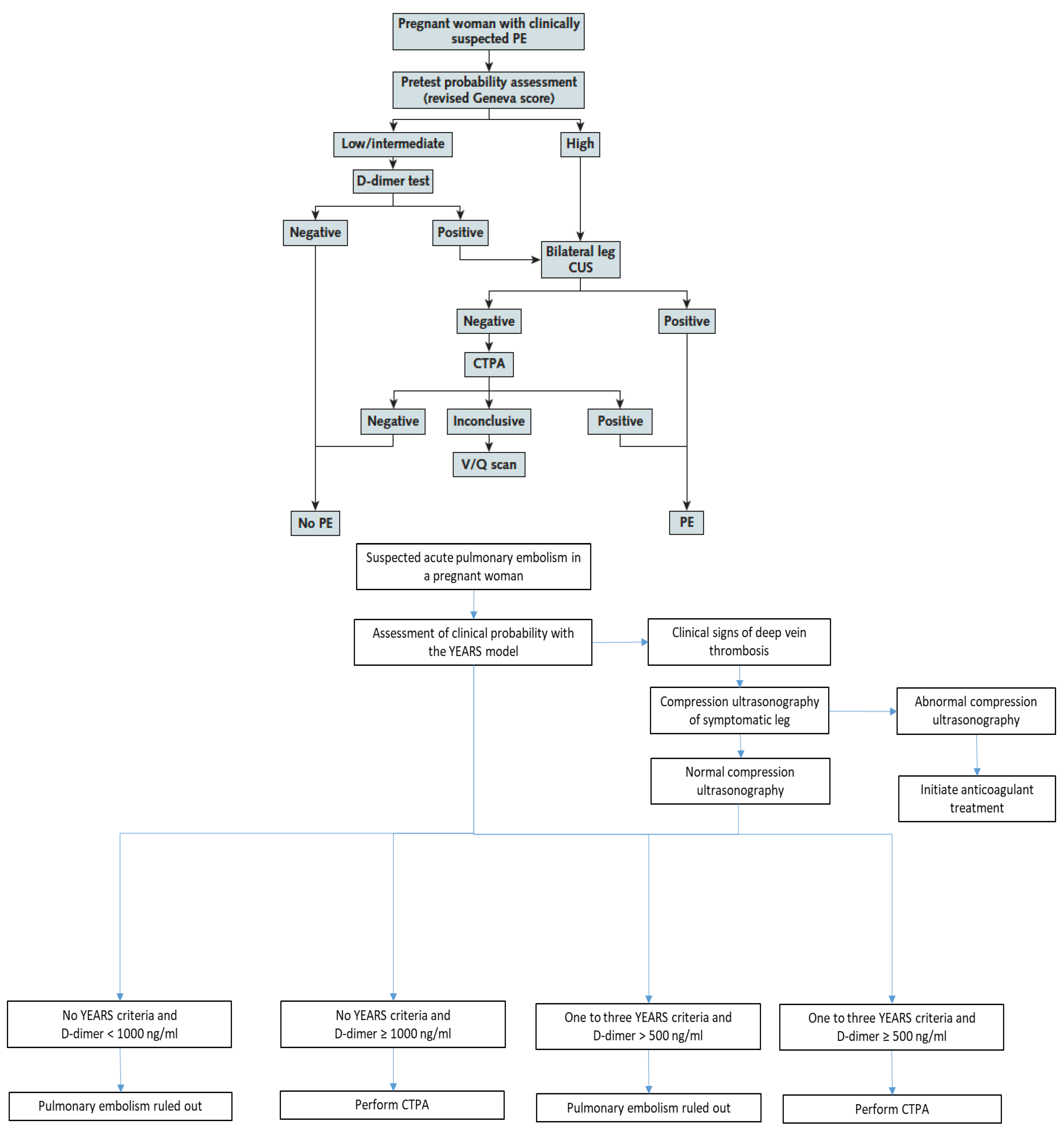
As stated above, the CT-PE pregnancy and ARTEMIS studies were the first studies to prospectively assess diagnostic strategies in pregnant women with suspected PE [8][9]. They applied two different PTP assessment models—the Geneva score and a pregnancy-adapted YEARS model—that had not been previously derived nor validated in pregnant women. Nevertheless, these two clinical decision rules (CDRs) both proved their usefulness in the integrated diagnostic algorithms used in these two studies (Figure 1) [8][9].
2.2. Safety and Usefulness of D-Dimer to Exclude PE during Pregnancy
Physicians tend to consider D-dimer tests as useless in pregnant women with suspected VTE. The lack of a PTP score validated in this population is one of the reasons why, as discussed above. However, the most frequent reason reported by clinicians is their belief that D-dimer does not represent a useful test in pregnant women due to the perception of D-dimer levels being always elevated during pregnancy. Moreover, even the safety of excluding VTE by a negative D-dimer test during pregnancy has been challenged by some authors [19].
The safe exclusion of PE by a negative D-dimer test associated with a non-high/unlikely PTP has been widely accepted outside pregnancy, based on large scale clinical trials demonstrating a very low 3-month VTE rate, and has been implemented in clinical practice for many years [10][11][20][21].
The CT-PE pregnancy and ARTEMIS studies both showed the safety of excluding PE during pregnancy by a negative D-dimer test in sequential diagnostic algorithms (Figure 1) with low failure rates. The number of women included in these studies remains limited compared to studies in the general population, and prospective trials are still needed to enrich these data. Nevertheless, integrating data from these two trials with previous data on D-dimer as a VTE exclusion test during pregnancy in a meta-analysis, the high negative predictive value of D-dimer was confirmed. The pooled sensitivity and negative predictive values were 99.5% (95% CI 95.0–100.0%) and 100% (95% CI 99.19–100.0%), respectively [22].
Regarding the diagnostic yield—also called efficiency—of a negative D-dimer test associated with a non-high PTP to safely rule out PE without any additional tests (CUS or chest imaging) was reported at 12% in the CT-PE pregnancy study. In other words, the number of pregnant women needed to test with D-dimer to exclude one PE was 8.3 [8].
The ARTEMIS study used a variable D-dimer cutoff depending on the presence or absence of YEARS criteria (Figure 1). Using a higher cutoff of 1000 ng/mL in women with no YEARS criteria, and a standard cutoff of 500 ng/mL in all other women, the efficiency of negative D-dimer to exclude PE without thoracic imaging was 39%. Of note, the non-invasive strategy tested in the ARTEMIS algorithm was not only based on PTP assessment and D-dimer measurement, but included the pre-exclusion of DVT by lower limb CUS in all women with clinical signs of DVT at baseline, representing a more complex selection of “low-risk” women in whom a higher D-dimer cutoff could be used to exclude PE (Figure 1) [9].
Pooling all the available evidence to assess efficiency of D-dimer to exclude VTE during pregnancy, the meta-analysis referred to in the last paragraph reported an overall efficiency of 34% (95% CI 15.9–55.23%) [22]. Although D-dimer levels are known to increase during physiological pregnancy as a result of a gradually increasing hypercoagulable state as pregnancy progresses towards delivery [7][23][24], they remain below the diagnostic cutoff for exclusion of VTE in a significant proportion of pregnant women, especially during the first half of pregnancy [23][24]. Giving a pregnant woman with a clinical suspicion of PE the chance to avoid a radiating thoracic imaging test should, in researchers' opinion, not be neglected, and in spite of the controversies in international guidelines [25], researchers believe that the use of D-dimer measurements in the decision algorithm should be strongly encouraged.
2.3. The Diagnostic Yield of Lower Limb Compression Ultrasound in Pregnant Women with Suspected PE
Bilateral lower limb compression ultrasound (CUS) was part of the diagnostic strategies of both CT-PE pregnancy and ARTEMIS studies but integrated in a different manner (Figure 1).
In the CT-PE pregnancy study, CUS was performed systematically in all women with a high PTP, or a low-intermediate PTP associated with positive D-dimer, as the first-line “imaging” modality. If a proximal DVT was confirmed, PE diagnosis was considered as confirmed without chest imaging. If no proximal DVT was present, CTPA was performed. Overall, CUS was performed in 88% of the overall population. Interestingly, proximal DVT was found in only 2% of these unselected women. Among women with clinical symptoms and signs of DVT, proximal DVT was found in 9% [8].
In the ARTEMIS study, CUS was part of the algorithm at baseline in all women with clinical signs of DVT, representing 9% of the whole cohort of pregnant women with suspected PE. When performed in this setting, CUS identified proximal DVT in 7% of women with leg symptoms. Of note, CUS was also performed in an additional 16% of the whole cohort in women without leg symptoms (reason not known) and showed DVT in only 1% of this group [9].
These interesting observations confirm that CUS seems to be mainly useful in pregnant women with suspected PE and lower limb symptoms suggestive of DVT (pain +/− edema). Nevertheless, even a diagnostic yield of only 1–2% when performing systematic CUS in pregnant women with suspected PE that could not be excluded by PTP and D-dimer before moving on to chest imaging is considered worthwhile by some, if the focus is set on minimizing radiating tests than on cost-effectiveness [18].
Despite this limited utility, some but not all, international guidelines currently recommend bilateral CUS in pregnant women with suspected PE in whom PE diagnosis could not be excluded by the combination of PTP and D-dimer, and in whom further testing is thus needed before proceeding to chest imaging [16].
2.4. Thoracic Imaging in Pregnant Women with Suspected PE
Although the use of PTP scores and D-dimer reduces the need for chest imaging, a significant proportion of pregnant women with suspected VTE still require imaging [22]. The two imaging modalities validated in the setting of PE diagnosis outside pregnancy are CTPA and V/Q lung scintigraphy. CTPA has now become the new gold standard in the diagnostic management of PE in clinical practice [26] and the recommended imaging test in most international guidelines [22]. The wide accessibility of CTPA has even led to concerns about over-testing and consequent radiation exposure, as well as over-diagnosis of small PE, some of which are of uncertain clinical significance. In pregnant women with suspected PE, radiation exposure both to the mother and the fetus are the first matter of concern [27], the second being the rate of inconclusive results.
A meta-analysis compared all available data on CTPA versus V/Q lung scintigraphy in pregnant women. No conclusion could be drawn regarding relative radiation risks between CTPA and V/Q lung scan due to the wide heterogeneity in radiation dose calculation methods and imaging protocol used across studies [27]. Nevertheless, this work highlighted the fact that all reported radiation doses for CTPA and V/Q lung scan, including older studies using protocols with no adaptation for pregnant women, were far below the accepted harmful threshold of 100 mGy [27]. The proportion of inconclusive tests varied very widely across the individual studies included in this meta-analysis both for CTPA (0–57%) and V/Q scan (1–40%). The pooled proportion of non-diagnostic results were similar between CTPA (12%, 95%CI 6–17%) and V/Q scan (14%, 95% CI 10–18%) [27]. Beyond the debate on the optimal imaging modality to use during pregnancy, experts and scientific societies all agree that the risks of an inappropriate diagnostic work-up, leading either to a missed diagnosis or to the introduction of therapeutic anticoagulation based only on clinical grounds, by far outweigh the risk of any of these two diagnostic modalities [13][28].
2.5. Scientific Societies’ Recommendations for PE Diagnosis during Pregnancy
The 2018 American society of Hematology (ASH) guidelines for PE diagnosis were strongly driven by the willingness to minimize radiation even in the general population, and thus advocate for V/Q scans for patients likely to have a diagnostic scan and in centers where V/Q scans are available with expertise to interpret the results in a timely manner [17].
The 2019 European Society of Cardiology (ESC) Guidelines provide specific recommendations for pregnant women [16]:
- -
-
formal diagnostic assessment with validated methods (Class I, level B)
- -
-
D-dimer measurement and clinical prediction rules to rule out PE (Class IIa, level B)
- -
-
venous CUS to avoid unnecessary irradiation (Class IIa, level B)
- -
-
and in terms of imaging test: perfusion scintigraphy or CTPA (with a low-radiation dose protocol); CTPA as the first-line option if chest X-ray is abnormal (Class IIa, level C) recommendation.
References
- Cantwell, R.; Clutton-Brock, T.; Cooper, G.; Dawson, A.; Drife, J.; Garrod, D.; Harper, A.; Hulbert, D.; Lucas, S.; McClure, J.; et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011, 118 (Suppl. 1), 1–203.
- Heit, J.A.; Kobbervig, C.E.; James, A.H.; Petterson, T.M.; Bailey, K.R.; Melton, L.J., 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann. Intern. Med. 2005, 143, 697–706.
- Kamel, H.; Navi, B.B.; Sriram, N.; Hovsepian, D.A.; Devereux, R.B.; Elkind, M.S. Risk of a thrombotic event after the 6-week postpartum period. N. Engl. J. Med. 2014, 370, 1307–1315.
- Chan, W.S.; Ray, J.G.; Murray, S.; Coady, G.E.; Coates, G.; Ginsberg, J.S. Suspected pulmonary embolism in pregnancy: Clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch. Intern. Med. 2002, 162, 1170–1175.
- Chan, W.S.; Lee, A.; Spencer, F.A.; Crowther, M.; Rodger, M.; Ramsay, T.; Ginsberg, J.S. Predicting deep venous thrombosis in pregnancy: Out in “LEFt” field? Ann. Intern. Med. 2009, 151, 85–92.
- Gibson, P.S.; Powrie, R.; Peipert, J. Prevalence of syncope and recurrent presyncope during pregnancy. Obstet. Gynecol. 2001, 97, S41–S42.
- Bourjeily, G.; Paidas, M.; Khalil, H.; Rosene-Montella, K.; Rodger, M. Pulmonary embolism in pregnancy. Lancet 2010, 375, 500–512.
- Righini, M.; Robert-Ebadi, H.; Elias, A.; Sanchez, O.; Le Moigne, E.; Schmidt, J.; Le Gall, C.; Cornuz, J.; Aujesky, D.; Roy, P.-M.; et al. Diagnosis of Pulmonary Embolism during Pregnancy: A Multicenter Prospective Management Outcome Study. Ann. Intern. Med. 2018, 169, 766–773.
- van der Pol, L.M.; Tromeur, C.; Bistervels, I.M.; Ni Ainle, F.; van Bemmel, T.; Bertoletti, L.; Couturaud, F.; van Dooren, Y.P.; Elias, A.; Faber, L.M.; et al. Pregnancy-Adapted YEARS Algorithm for Diagnosis of Suspected Pulmonary Embolism. N. Engl. J. Med. 2019, 380, 1139–1149.
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124.
- van der Hulle, T.; Cheung, W.Y.; Kooij, S.; Beenen, L.F.M.; van Bemmel, T.; van Es, J.; Faber, L.M.; Hazelaar, G.M.; Heringhaus, C.; Hofstee, H.; et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): A prospective, multicentre, cohort study. Lancet 2017, 390, 289–297.
- Le Gal, G.; Kercret, G.; Ben Yahmed, K.; Bressollette, L.; Robert-Ebadi, H.; Riberdy, L.; Louis, P.; Delluc, A.; Labalette, M.; Baba-Ahmed, M.; et al. Diagnostic value of single complete compression ultrasonography in pregnant and postpartum women with suspected deep vein thrombosis: Prospective study. BMJ 2012, 344, e2635.
- Leung, A.N.; Bull, T.M.; Jaeschke, R.; Lockwood, C.J.; Boiselle, P.M.; Hurwitz, L.M.; James, A.H.; McCullough, L.B.; Menda, Y.; Paidas, M.J.; et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: Evaluation of suspected pulmonary embolism in pregnancy. Am. J. Respir. Crit. Care Med. 2011, 184, 1200–1208.
- Investigators, P. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990, 263, 2753–2759.
- Roy, P.-M.; Meyer, G.; Carpentier, F.; Leveau, P.; The EMDEPU Study Group; Vielle, B.; Le Gall, C.; Verschuren, F.; Furber, A. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann. Intern. Med. 2006, 144, 157–164.
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart, J. 2020, 41, 543–603.
- Lim, W.; Le Gal, G.; Bates, S.M.; Righini, M.; Haramati, L.B.; Lang, E.; Kline, J.A.; Chasteen, S.; Snyder, M.; Patel, P.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018, 2, 3226–3256.
- Chan, W.S. Can pregnancy-adapted algorithms avoid diagnostic imaging for pulmonary embolism? Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 184–189.
- Goodacre, S.; Horspool, K.; Nelson-Piercy, C.; Knight, M.; Shephard, N.; Lecky, F.; Thomas, S.; Hunt, B.J.; Fuller, G.W. The DiPEP study: An observational study of the diagnostic accuracy of clinical assessment, D-dimer and chest x-ray for suspected pulmonary embolism in pregnancy and postpartum. BJOG 2019, 126, 383–392.
- Robert-Ebadi, H.; Robin, P.; Hugli, O.; Verschuren, F.; Trinh-Duc, A.; Roy, P.M.; Schmidt, J.; Fumeaux, T.; Meyer, G.; Hayoz, D.; et al. Impact of the Age-Adjusted D-Dimer Cutoff to Exclude Pulmonary Embolism: A Multinational Prospective Real-Life Study (the RELAX-PE Study). Circulation 2021, 143, 1828–1830.
- Carrier, M.; Righini, M.; Djurabi, R.K.; Huisman, M.V.; Perrier, A.; Wells, P.S.; Rodger, M.; Wuillemin, M.A.; Le Gal, G. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb. Haemost. 2009, 101, 886–892.
- Bellesini, M.; Robert-Ebadi, H.; Combescure, C.; Dedionigi, C.; Le Gal, G.; Righini, M. D-dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta-analysis. J. Thromb. Haemost. 2021, 19, 2454–2467.
- Chabloz, P.; Reber, G.; Boehlen, F.; Hohlfeld, P.; de Moerloose, P. TAFI antigen and D-dimer levels during normal pregnancy and at delivery. Br. J. Haematol. 2001, 115, 150–152.
- Murphy, N.; Broadhurst, D.I.; Khashan, A.S.; Gilligan, O.; Kenny, L.C.; O’Donoghue, K. Gestation-specific D-dimer reference ranges: A cross-sectional study. BJOG 2015, 122, 395–400.
- Wan, T.; Skeith, L.; Karovitch, A.; Rodger, M.; Le Gal, G. Guidance for the diagnosis of pulmonary embolism during pregnancy: Consensus and controversies. Thromb. Res. 2017, 157, 23–28.
- Robert-Ebadi, H.; Le Gal, G.; Righini, M. Evolving imaging techniques in diagnostic strategies of pulmonary embolism. Expert Rev. Cardiovasc. Ther. 2016, 14, 495–503.
- Tromeur, C.; van der Pol, L.M.; Le Roux, P.-Y.; Ende-Verhaar, Y.; Salaun, P.-Y.; Leroyer, C.; Couturaud, F.; Kroft, L.J.; Huisman, M.V.; Klok, F.A. Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: A systematic review and meta-analysis. Haematologica 2019, 104, 176–188.
- McLintock, C.; Brighton, T.; Chunilal, S.; Dekker, G.; McDonnell, N.; McRae, S.; Muller, P.; Tran, H.; Walters, B.N.; Young, L. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 14–22.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
29 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No