
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rui Medeiros | -- | 2017 | 2022-08-25 16:06:19 | | | |
| 2 | Vivi Li | Meta information modification | 2017 | 2022-08-26 04:52:33 | | |
Video Upload Options
Coronavirus disease (COVID-19) is an infectious disease that is caused by a highly contagious and severe acute respiratory syndrome—coronavirus 2 (SARS-CoV-2). This infection started to spread across the world in 2019 and rapidly turned into a global pandemic, causing an urgent necessity for treatment strategies development. The mRNA vaccines against SARS-CoV-2 can trigger an immune response, providing genetic information that allows the production of spike glycoproteins. MiRNAs play a crucial role in diverse key cellular processes, including antiviral defense. Several miRNAs are described as key factors in SARS-CoV-2 human infection through the regulation of ACE2 levels and by the inhibition of SARS-CoV-2 replication and spike expression. Consequently, these molecules have been considered as highly promising biomarkers. In numerous human malignancies, it has been recognized that miRNAs expression is dysregulated. Since miRNAs can target SARS-CoV-2-associated mRNAs, in cancer patients, the deregulation of these molecules can impair the immune response to the vaccines.
1. Introduction
2. Evidence Synthesis
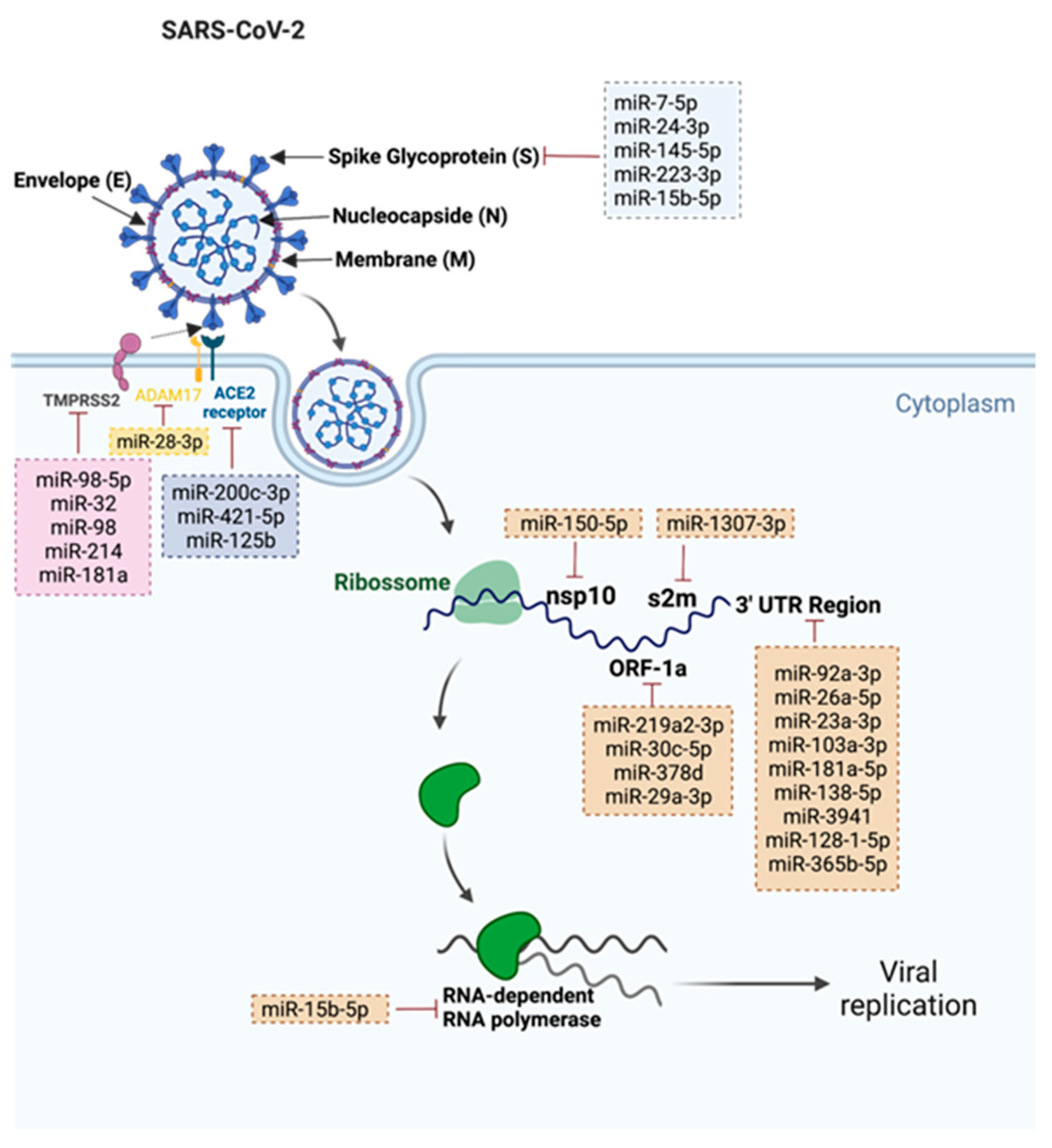
2.1. miRNAs That Target SARS-CoV-2
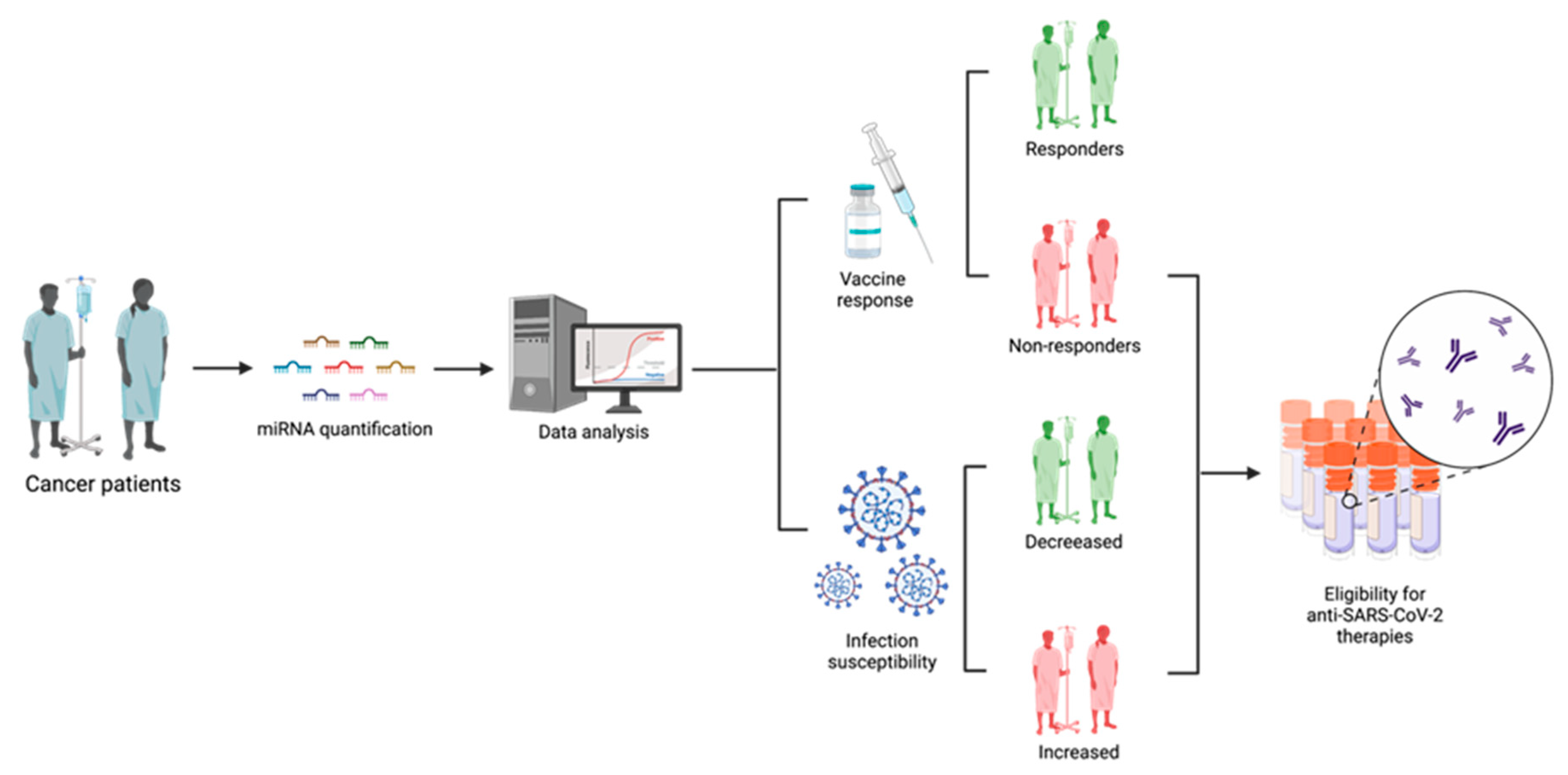
3. MiRNAs Applications in Cancer Patients’ Management Regarding SARS-CoV-2
References
- Abedi, F.; Rezaee, R.; Hayes, A.W.; Nasiripour, S.; Karimi, G. MicroRNAs and SARS-CoV-2 life cycle, pathogenesis, and mutations: Biomarkers or therapeutic agents? Cell Cycle 2021, 20, 143–153.
- Visacri, M.B.; Nicoletti, A.S.; Pincinato, E.C.; Loren, P.; Saavedra, N.; Saavedra, K.; Salazar, L.A.; Moriel, P. Role of miRNAs as biomarkers of COVID-19: A scoping review of the status and future directions for research in this field. Biomark Med. 2021, 15, 1785–1795.
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579.
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793.
- Keppel, G.; Cole, A.M.; Ramsbottom, M.; Nagpal, S.; Hornecker, J.; Thomson, C.; Nguyen, V.; Baldwin, L.M. Early Response of Primary Care Practices to COVID-19 Pandemic. J. Prim. Care Community Health 2022, 13, 21501319221085374.
- Shahbazi, F.; Solgi, M.; Khazaei, S. Predisposing risk factors for COVID-19 infection: A case-control study. Casp. J. Intern. Med. 2020, 11 (Suppl. S1), 495–500.
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407.
- Shahbazi, F.; Khazaei, S. Socio-economic inequality in global incidence and mortality rates from coronavirus disease 2019: An ecological study. New Microbes New Infect 2020, 38, 100762.
- Cordero-Franco, H.F.; De La Garza-Salinas, L.H.; Gomez-Garcia, S.; Moreno-Cuevas, J.E.; Vargas-Villarreal, J.; González-Salazar, F. Risk Factors for SARS-CoV-2 Infection, Pneumonia, Intubation, and Death in Northeast Mexico. Front. Public Health 2021, 9, 645739.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337.
- Desai, A.; Gupta, R.; Advani, S.; Ouellette, L.; Kuderer, N.M.; Lyman, G.H.; Li, A. Mortality in hospitalized patients with cancer and coronavirus disease 2019: A systematic review and meta-analysis of cohort studies. Cancer 2021, 127, 1459–1468.
- Ramasamy, C.; Mishra, A.K.; John, K.J.; Lal, A. Clinical considerations for critically ill COVID-19 cancer patients: A systematic review. World J. Clin. Cases 2021, 9, 8441–8452.
- Rajan, S.; Akhtar, N.; Sharma, S.; Chakrabarti, D.; Kumar, V. COVID-19 vaccination for cancer patients: Evidence, priority, and practice. Vaccine 2021, 39, 5075–5077.
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23.
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423.
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020, 19, 100682.
- Narayanan, K.; Ramirez, S.I.; Lokugamage, K.G.; Makino, S. Coronavirus nonstructural protein 1: Common and distinct functions in the regulation of host and viral gene expression. Virus Res. 2015, 202, 89–100.
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868.
- Petrovszki, D.; Walter, F.R.; Vigh, J.P.; Kocsis, A.; Valkai, S.; Deli, M.A.; Dér, A. Penetration of the SARS-CoV-2 Spike Protein across the Blood-Brain Barrier, as Revealed by a Combination of a Human Cell Culture Model System and Optical Biosensing. Biomedicines 2022, 10, 188.
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454.
- Adhikari, P.; Jawad, B.; Podgornik, R.; Ching, W.Y. Mutations of Omicron Variant at the Interface of the Receptor Domain Motif and Human Angiotensin-Converting Enzyme-2. Int. J. Mol. Sci. 2022, 23, 2870.
- Jawad, B.; Adhikari, P.; Cheng, K.; Podgornik, R.; Ching, W.-Y. Computational Design of Miniproteins as SARS-CoV-2 Therapeutic Inhibitors. Int. J. Mol. Sci. 2022, 23, 838.
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577.
- Touati, R.; Elngar, A.A. Intelligent system based comparative analysis study of SARS-CoV-2 spike protein and antigenic proteins in different types of vaccines. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 34.
- Fendler, A.; de Vries, E.G.E.; GeurtsvanKessel, C.H.; Haanen, J.B.; Wörmann, B.; Turajlic, S.; von Lilienfeld-Toal, M. COVID-19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401.
- Slimano, F.; Baudouin, A.; Zerbit, J.; Toulemonde-Deldicque, A.; Thomas-Schoemann, A.; Chevrier, R.; Daouphars, M.; Madelaine, I.; Pourroy, B.; Tournamille, J.F.; et al. Cancer, immune suppression and Coronavirus Disease-19 (COVID-19): Need to manage drug safety (French Society for Oncology Pharmacy guidelines). Cancer Treat. Rev. 2020, 88, 102063.
- Addeo, A.; Friedlaender, A. Cancer and COVID-19: Unmasking their ties. Cancer Treat. Rev. 2020, 88, 102041.
- Machado, M.T.; Navega, S.; Dias, F.; de Sousa, M.J.; Teixeira, A.L.; Medeiros, R. microRNAs for peripheral blood fraction identification: Origin, pathways and forensic relevance. Life Sci. 2015, 143, 98–104.
- Dias, F.; Teixeira, A.L.; Ferreira, M.; Adem, B.; Bastos, N.; Vieira, J.; Fernandes, M.; Sequeira, M.I.; Maurício, J.; Lobo, F.; et al. Plasmatic miR-210, miR-221 and miR-1233 profile: Potential liquid biopsies candidates for renal cell carcinoma. Oncotarget 2017, 8, 103315–103326.
- Cho, W.C. MicroRNAs: Potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int. J. Biochem. Cell Biol. 2010, 42, 1273–1281.
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561.
- Marchi, R.; Sugita, B.; Centa, A.; Fonseca, A.S.; Bortoletto, S.; Fiorentin, K.; Ferreira, S.; Cavalli, L.R. The role of microRNAs in modulating SARS-CoV-2 infection in human cells: A systematic review. Infect Genet Evol. 2021, 91, 104832.
- Hummel, R.; Hussey, D.J.; Haier, J. MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur. J. Cancer 2010, 46, 298–311.
- Lu, J.; Clark, A.G. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012, 22, 1243–1254.
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016, 1, 15004.
- Park, J.H.; Choi, Y.; Lim, C.W.; Park, J.M.; Yu, S.H.; Kim, Y.; Han, H.J.; Kim, C.H.; Song, Y.S.; Kim, C.; et al. Potential Therapeutic Effect of Micrornas in Extracellular Vesicles from Mesenchymal Stem Cells against SARS-CoV-2. Cells 2021, 10, 2393.
- Barreda-Manso, M.A.; Nieto-Díaz, M.; Soto, A.; Muñoz-Galdeano, T.; Reigada, D.; Maza, R.M. In Silico and In Vitro Analyses Validate Human MicroRNAs Targeting the SARS-CoV-2 3′-UTR. Int. J. Mol. Sci. 2021, 22, 6094.
- Imperatore, J.A.; Cunningham, C.L.; Pellegrene, K.A.; Brinson, R.G.; Marino, J.P.; Evanseck, J.D.; Mihailescu, M.R. Highly conserved s2m element of SARS-CoV-2 dimerizes via a kissing complex and interacts with host miRNA-1307-3p. Nucleic Acids Res. 2022, 50, 1017–1032.
- Akula, S.M.; Bolin, P.; Cook, P.P. Cellular miR-150-5p may have a crucial role to play in the biology of SARS-CoV-2 infection by regulating nsp10 gene. RNA Biol. 2022, 19, 1–11.
- Wang, Y.; Zhu, X.; Jiang, X.M.; Guo, J.; Fu, Z.; Zhou, Z.; Yang, P.; Guo, H.; Guo, X.; Liang, G.; et al. Decreased inhibition of exosomal miRNAs on SARS-CoV-2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduct. Target Ther. 2021, 6, 300.
- Siniscalchi, C.; Di Palo, A.; Russo, A.; Potenza, N. Human MicroRNAs Interacting With SARS-CoV-2 RNA Sequences: Computational Analysis and Experimental Target Validation. Front. Genet 2021, 12, 678994.
- Sato, A.; Ogino, Y.; Tanuma, S.I.; Uchiumi, F. Human microRNA hsa-miR-15b-5p targets the RNA template component of the RNA-dependent RNA polymerase structure in severe acute respiratory syndrome coronavirus 2. Nucleosides Nucleotides Nucleic Acids 2021, 40, 790–797.
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197.
- Galvão-Lima, L.J.; Morais, A.H.F.; Valentim, R.A.M.; Barreto, E. miRNAs as biomarkers for early cancer detection and their application in the development of new diagnostic tools. Biomed. Eng. Online 2021, 20, 21.
- Yang, B.; Xiong, W.Y.; Hou, H.J.; Xu, Q.; Cai, X.L.; Zeng, T.X.; Ha, X.Q. Exosomal miRNAs as Biomarkers of Cancer: A Meta-Analysis. Clin. Lab. 2019, 65, 31115208.
- Walter, E.; Dellago, H.; Grillari, J.; Dimai, H.P.; Hackl, M. Cost-utility analysis of fracture risk assessment using microRNAs compared with standard tools and no monitoring in the Austrian female population. Bone 2018, 108, 44–54.
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. Ejifcc 2019, 30, 114–127.
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449.




