
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michiaki Nagai | -- | 3046 | 2022-08-25 14:25:43 | | | |
| 2 | Catherine Yang | -4 word(s) | 3042 | 2022-08-26 03:31:28 | | |
Video Upload Options
Diabetes mellitus is a common disease affecting more than 537 million adults worldwide. The microvascular complications that occur during the course of the disease are widespread and affect a variety of organ systems in the body. Diabetic retinopathy is one of the most common long-term complications, which include, amongst others, endothelial dysfunction, and thus, alterations in the blood-retinal barrier (BRB). This particularly restrictive physiological barrier is important for maintaining the neuroretina as a privileged site in the body by controlling the inflow and outflow of fluid, nutrients, metabolic end products, ions, and proteins. In addition, people with diabetic retinopathy (DR) have been shown to be at increased risk for systemic vascular complications, including subclinical and clinical stroke, coronary heart disease, heart failure, and nephropathy.
1. Targeting Inflammatory Mechanisms in Diabetes Mellitus
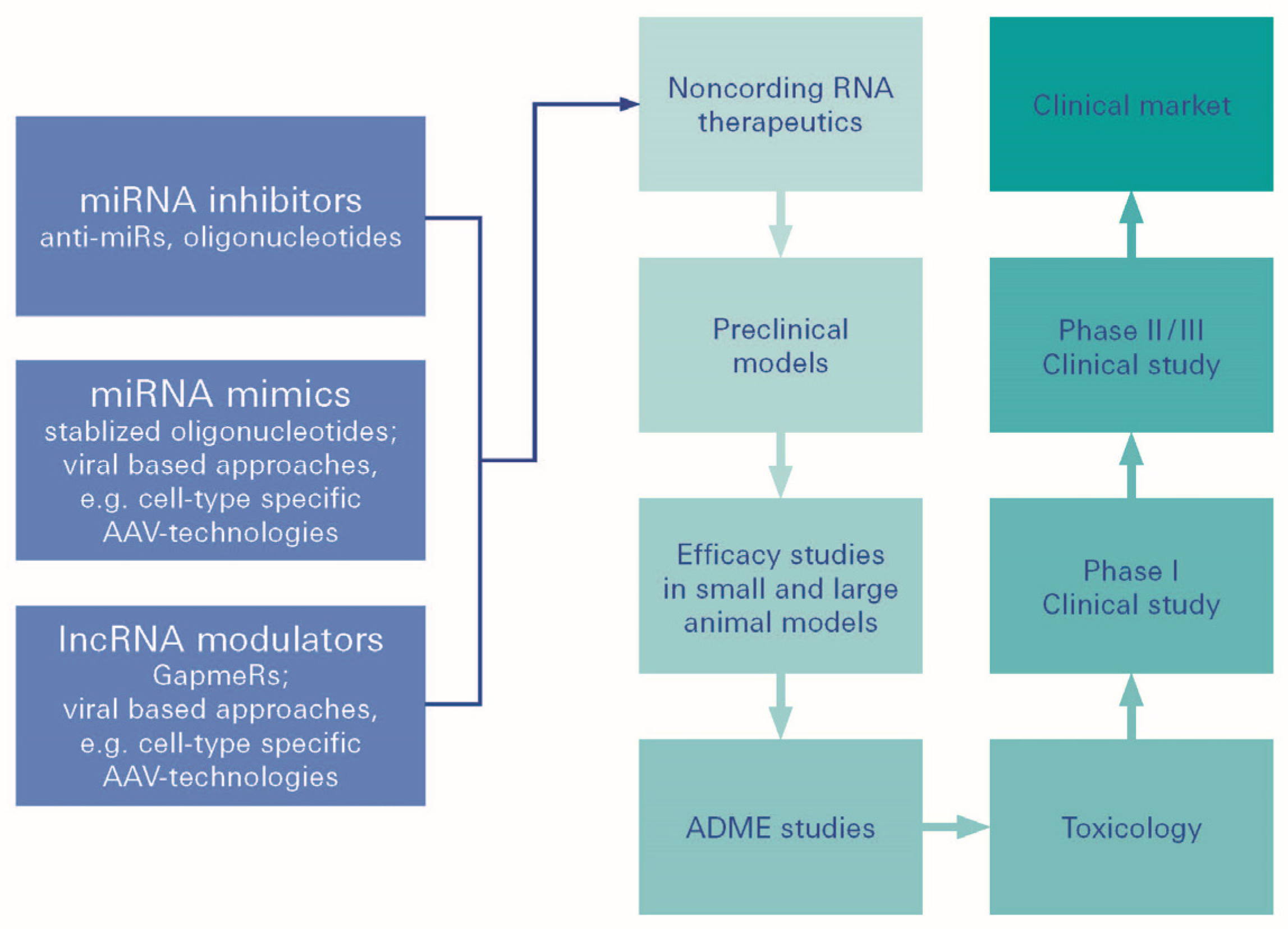
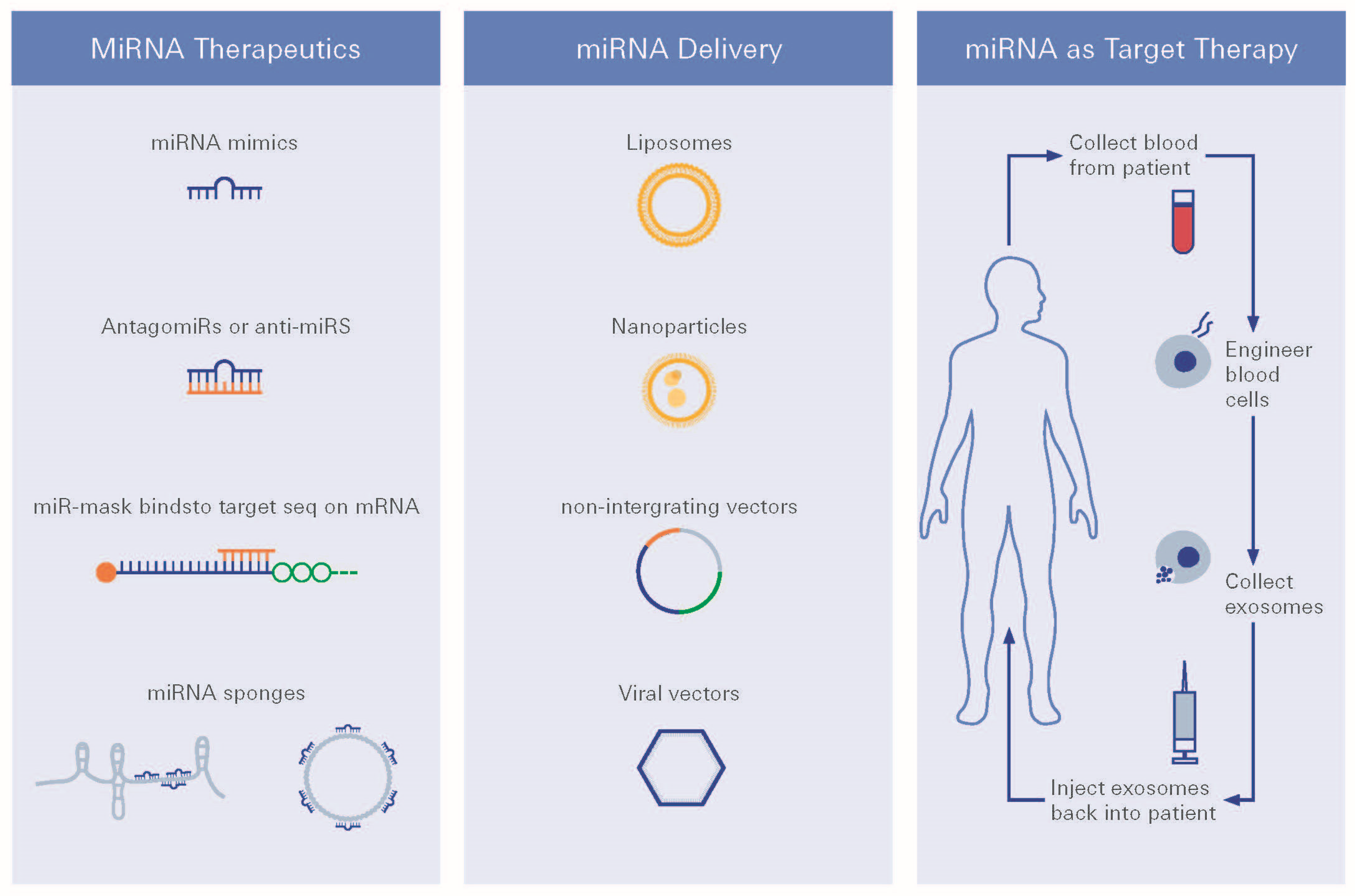
2. Identification of a Common MicroRNA Signature in Damage in Diabetic Retinopathy, Diabetic Nephropathy and Heart Failure
2.1. Neurotrophin Signaling and Neurotrophin-Related Treatment Opportunities in Diabetic Retinopathy
2.2. Targeted Delivery of Neurotrophic Factors to the Retina
References
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91.
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59.
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805.
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82.
- Ghosh, A.; Liu, T.; Khoury, M.J.; Valdez, R. Family history of diabetes and prevalence of the metabolic syndrome in U.S. adults without diabetes: 6-year results from the National Health and Nutrition Examination Survey (1999–2004). Public Health Genom. 2010, 13, 353–359.
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617.
- Himasa, F.I.; Singhal, M.; Ojha, A.; Kumar, B. Prospective for diagnosis and treatment of diabetic retinopathy. Curr. Pharm. Des. 2021, 28, 560–569.
- Mesquida, M.; Drawnel, F.; Fauser, S. The role of inflammation in diabetic eye disease. Semin. Immunopathol. 2019, 41, 427–445.
- Abcouwer, S.F.; Antonetti, D.A. A role for systemic inflammation in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2384.
- Lu, L.; Zou, G.; Chen, L.; Lu, Q.; Wu, M.; Li, C. Elevated NLRP3 Inflammasome Levels Correlate With Vitamin D in the Vitreous of Proliferative Diabetic Retinopathy. Front. Med. 2021, 8, 736316.
- Shimizu, I.; Yoshida, Y.; Katsuno, T.; Minamino, T. Adipose tissue inflammation in diabetes and heart failure. Microbes Infect. 2013, 15, 11–17.
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154.
- Fernandez-Hernando, C.; Ramirez, C.M.; Goedeke, L.; Suarez, Y. MicroRNAs in metabolic disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185.
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060.
- van de Vrie, M.; Heymans, S.; Schroen, B. MicroRNA involvement in immune activation during heart failure. Cardiovasc. Drugs Ther. 2011, 25, 161–170.
- Smit-McBride, Z.; Morse, L.S. MicroRNA and diabetic retinopathy-biomarkers and novel therapeutics. Ann. Transl. Med. 2021, 9, 1280.
- Gong, Q.; Su, G. Roles of miRNAs and long noncoding RNAs in the progression of diabetic retinopathy. Biosci. Rep. 2017, 37, BSR20171157.
- Flannick, J.; Johansson, S.; Njolstad, P.R. Common and rare forms of diabetes mellitus: Towards a continuum of diabetes subtypes. Nat. Rev. Endocrinol. 2016, 12, 394–406.
- Giugliano, D.; Maiorino, M.I.; Longo, M.; Bellastella, G.; Chiodini, P.; Esposito, K. Type 2 diabetes and risk of heart failure: A systematic review and meta-analysis from cardiovascular outcome trials. Endocrine 2019, 65, 15–24.
- Ruiz, M.A.; Chakrabarti, S. MicroRNAs: The underlying mediators of pathogenetic processes in vascular complications of diabetes. Can. J. Diabetes 2013, 37, 339–344.
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400.
- Leptidis, S.; el Azzouzi, H.; Lok, S.I.; de Weger, R.; Olieslagers, S.; Kisters, N.; Silva, G.J.; Heymans, S.; Cuppen, E.; Berezikov, E.; et al. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS ONE 2013, 8, e57800.
- Martin, M.M.; Buckenberger, J.A.; Jiang, J.; Malana, G.E.; Nuovo, G.J.; Chotani, M.; Feldman, D.S.; Schmittgen, T.D.; Elton, T.S. The human angiotensin II type 1 receptor+1166 A/C polymorphism attenuates MicroRNA-155 binding. J. Biol. Chem. 2007, 282, 24262–24269.
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of bic/microRNA-155 for normal immune function. Science 2007, 316, 608–611.
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486.
- Haasch, D.; Chen, Y.W.; Reilly, R.M.; Chiou, X.G.; Koterski, S.; Smith, M.L.; Kroeger, P.; McWeeny, K.; Halbert, D.N.; Mollison, K.W.; et al. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol. 2002, 217, 78–86.
- Zheng, D.; Ma, J.; Yu, Y.; Li, M.; Ni, R.; Wang, G.; Chen, R.; Li, J.; Fan, G.C.; Lacefield, J.C.; et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015, 58, 1949–1958.
- Baseler, W.A.; Thapa, D.; Jagannathan, R.; Dabkowski, E.R.; Croston, T.L.; Hollander, J.M. miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. Am. J. Physiol. Cell Physiol. 2012, 303, C1244–C1251.
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. TMicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288.
- Horie, T.; Ono, K.; Nishi, H.; Iwanaga, Y.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Takanabe, R.; Hasegawa, K.; Kita, T.; et al. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009, 389, 315–320.
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787.
- He, K.; Chen, Z.; Zhao, J.; He, Y.; Deng, R.; Fan, X.; Wang, J.; Zhou, X. The role of microRNA-155 in glomerular endothelial cell injury induced by high glucose. Mol. Biol. Rep. 2022, 49, 2915–2924.
- Mikhail, M.; Vachon, P.H.; D’Orleans-Juste, P.; Jacques, D.; Bkaily, G. Role of endothelin-1 and its receptors, ETA and ETB, in the survival of human vascular endothelial cells. Can. J. Physiol. Pharmacol. 2017, 95, 1298–1305.
- Gao, J.; Liang, Z.; Zhao, F.; Liu, X.; Ma, N. Triptolide inhibits oxidative stress and inflammation via the microRNA-155-5p/brain-derived neurotrophic factor to reduce podocyte injury in mice with diabetic nephropathy. Bioengineered 2022, 13, 12275–12288.
- Chen, Y.Q.; Wang, X.X.; Yao, X.M.; Zhang, D.L.; Yang, X.F.; Tian, S.F.; Wang, N.S. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am. J. Nephrol. 2011, 34, 549–559.
- YChen, Q.; Wang, X.X.; Yao, X.M.; Zhang, D.L.; Yang, X.F.; Tian, S.F.; Wang, N.S. Abated microRNA-195 expression protected mesangial cells from apoptosis in early diabetic renal injury in mice. J. Nephrol. 2012, 25, 566–576.
- Wu, J.; Liu, J.; Ding, Y.; Zhu, M.; Lu, K.; Zhou, J.; Xie, X.; Xu, Y.; Shen, X.; Chen, Y.; et al. MiR-455-3p suppresses renal fibrosis through repression of ROCK2 expression in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2018, 503, 977–983.
- Wang, J.; Yang, S.; Li, W.; Zhao, M.; Li, K. Circ_0000491 Promotes Apoptosis, Inflammation, Oxidative Stress, and Fibrosis in High Glucose-Induced Mesangial Cells by Regulating miR-455-3p/Hmgb1 Axis. Nephron 2022, 146, 72–83.
- Polina, E.R.; Oliveira, F.M.; Sbruzzi, R.C.; Crispim, D.; Canani, L.H.; Santos, K.G. Gene polymorphism and plasma levels of miR-155 in diabetic retinopathy. Endocr. Connect. 2019, 8, 1591–1599.
- He, J.; Zhang, R.; Wang, S.; Xie, L.; Yu, C.; Xu, T.; Li, Y.; Yan, T. Expression of microRNA-155-5p in patients with refractory diabetic macular edema and its regulatory mechanism. Exp. Ther. Med. 2021, 22, 975.
- Mortuza, R.; Feng, B.; Chakrabarti, S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 2014, 57, 1037–1046.
- Shan, L.; Zhang, H.; Han, Y.; Kuang, R. Expression and mechanism of microRNA 195 in diabetic retinopathy. Endocr. J. 2022, 69, 529–537.
- Chen, P.; Miao, Y.; Yan, P.; Wang, X.J.; Jiang, C.; Lei, Y. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J. Cell. Physiol. 2019, 234, 21915–21924.
- Surina, S.; Fontanella, R.A.; Scisciola, L.; Marfella, R.; Paolisso, G.; Barbieri, M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 767064.
- Ajuyah, P.; Hill, M.; Ahadi, A.; Lu, J.; Hutvagner, G.; Tran, N. MicroRNA (miRNA)-to-miRNA Regulation of Programmed Cell Death 4 (PDCD4). Mol. Cell. Biol. 2019, 39, e00086–e00119.
- Ali, T.K.; Al-Gayyar, M.M.H.; Matragoon, S.; Pillai, B.A.; Abdelsaid, M.A.; Nussbaum, J.J.; El-Remessy, A.B. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia 2011, 54, 657–668.
- Al-Gayyar, M.M.; Matragoon, S.; Pillai, B.A.; Ali, T.K.; Abdelsaid, M.A.; El-Remessy, A.B. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes . Diabetologia 2011, 54, 669–680.
- Barcelona, P.F.; Sitaras, N.; Galan, A.; Esquiva, G.; Jmaeff, S.; Jian, Y.; Sarunic, M.V.; Cuenca, N.; Sapieha, P.; Saragovi, H.U. p75NTR and Its Ligand ProNGF Activate Paracrine Mechanisms Etiological to the Vascular, Inflammatory, and Neurodegenerative Pathologies of Diabetic Retinopathy. J. Neurosci. 2016, 36, 8826–8841.
- Hammes, H.-P.; Federoff, H.J.; Brownlee, M. Nerve Growth Factor Prevents Both Neuroretinal Programmed Cell Death and Capillary Pathology in Experimental Diabetes. Mol. Med. 1995, 1, 527–534.
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A Nerve Growth-Stimulating Factor Isolated from Sarcom as 37 and 180. Proc. Natl. Acad. Sci. USA 1954, 40, 1014–1018.
- Jones, K.R.; Reichardt, L.F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc. Natl. Acad. Sci. USA 1990, 87, 8060–8064.
- Ip, N.Y.; Ibanez, C.F.; Nye, S.H.; McClain, J.; Jones, P.F.; Gies, D.R.; Belluscio, L.; le Beau, M.M.; Espinosa, R., 3rd; Squinto, S.P. Mammalian neurotrophin-4: Structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 3060–3064.
- Hofer, M.M.; Barde, Y.A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature 1988, 331, 261–262.
- Caporali, A.; Emanueli, C. Cardiovascular actions of neurotrophins. Physiol. Rev. 2009, 89, 279–308.
- Johnson, D.; Lanahan, A.; Buck, C.R.; Sehgal, A.; Morgan, C.; Mercer, E.; Bothwell, M.; Chao, M. Expression and structure of the human NGF receptor. Cell 1986, 47, 545–554.
- Kaplan, D.R.; Hempstead, B.L.; Martin-Zanca, D.; Chao, M.V.; Parada, L.F. The trk proto-oncogene product: A signal transducing receptor for nerve growth factor. Science 1991, 252, 554–558.
- Klein, R.; Jing, S.Q.; Nanduri, V.; O’Rourke, E.; Barbacid, M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991, 65, 189–197.
- Gibon, J.; Barker, P.A. Neurotrophins and Proneurotrophins: Focus on Synaptic Activity and Plasticity in the Brain. Neuroscientist 2017, 23, 587–604.
- Cheung, N.; Wang, J.J.; Rogers, S.L.; Brancati, F.; Klein, R.; Sharrett, A.R.; Wong, T.Y. Diabetic retinopathy and risk of heart failure. J. Am. Coll. Cardiol. 2008, 51, 1573–1578.
- Bai, Y.; Dergham, P.; Nedev, H.; Xu, J.; Galan, A.; Rivera, J.C.; ZhiHua, S.; Mehta, H.M.; Woo, S.B.; Sarunic, M.V.; et al. Chronic and acute models of retinal neurodegeneration TrkA activity are neuroprotective whereas p75NTR activity is neurotoxic through a paracrine mechanism. J. Biol. Chem. 2010, 285, 39392–39400.
- Mysona, B.A.; Al-Gayyar, M.M.H.; Matragoon, S.; Abdelsaid, M.A.; El-Azab, M.F.; Saragovi, H.U.; El-Remessy, A.B. Modulation of p75NTR prevents diabetes- and proNGF-induced retinal inflammation and blood–retina barrier breakdown in mice and rats. Diabetologia 2013, 56, 2329–2339.
- Colafrancesco, V.; Coassin, M.; Rossi, S.; Aloe, L. Effect of eye NGF administration on two animal models of retinal ganglion cells degeneration. Ann. Dell’Istituto Super. Sanita 2011, 47, 284–289.
- Mysona, B.A.; Shanab, A.Y.; Elshaer, S.L.; El-Remessy, A.B. Nerve growth factor in diabetic retinopathy: Beyond neurons. Expert Rev. Ophthalmol. 2014, 9, 99–107.
- El-Asrar, A.M.A.; Mohammad, G.; de Hertogh, G.; Nawaz, M.I.; van den Eynde, K.; Siddiquei, M.M.; Struyf, S.; Opdenakker, G.; Geboes, K. Neurotrophins and neurotrophin receptors in proliferative diabetic retinopathy. PLoS ONE 2013, 8, e65472.
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594–5603.
- Tadlipinar Uzel, A.G.; UĞurlu, N.; Toklu, Y.; Çİçek, M.; Boral, B.; Sener, B.; CaGil, N. Relationship between Stages of Diabetic Retinopathy and Levels of Brain-Derived Neurotrophic Factor in Aqueous Humor and Serum. Retina 2020, 40, 121–125.
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol. 2013, 33, 359–367.
- Cristofaro, B.; Stone, O.A.; Caporali, A.; Dawbarn, D.; Ieronimakis, N.; Reyes, M.; Madeddu, P.; Bates, D.O.; Emanueli, C. Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1143–1150.
- Nakamura, K.; Tan, F.; Li, Z.; Thiele, C.J. NGF activation of TrkA induces vascular endothelial growth factor expression via induction of hypoxia-inducible factor-1alpha. Mol. Cell Neurosci. 2011, 46, 498–506.
- Nico, B.; Mangieri, D.; Benagiano, V.; Crivellato, E.; Ribatti, D. Nerve growth factor as an angiogenic factor. Microvasc. Res. 2008, 75, 135–141.
- Nakamura, K.; Martin, K.C.; Jackson, J.K.; Beppu, K.; Woo, C.W.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006, 66, 4249–4255.
- Halade, G.V.; Ma, Y.; Ramirez, T.A.; Zhang, J.; Dai, Q.; Hensler, J.G.; Lopez, E.F.; Ghasemi, O.; Jin, Y.F.; Lindsey, M.L. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1830–H1842.
- Schlecht, A.; Vallon, M.; Wagner, N.; Ergun, S.; Braunger, B.M. TGFbeta-Neurotrophin Interactions in Heart, Retina, and Brain. Biomolecules 2021, 11, 1360.
- Jee, D.; Lee, W.K. Inhibitory effect of intravitreal injection of bevacizumab on nerve growth factor. Curr. Eye Res. 2012, 37, 408–415.
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell. Mol. Life Sci. CMLS 2018, 75, 1255–1267.
- Loftsson, T.; Hreinsdottir, D.; Stefansson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635.
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203.
- Yang, J.Y.; Lu, B.; Feng, Q.; Alfaro, J.S.; Chen, P.H.; Loscalzo, J.; Wei, W.B.; Zhang, Y.Y.; Lu, S.J.; Wang, S. Retinal Protection by Sustained Nanoparticle Delivery of Oncostatin M and Ciliary Neurotrophic Factor Into Rodent Models of Retinal Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 6.
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm.: Off. J. Arb. Fur Pharm. Verfahr. E.V 2011, 78, 10–18.
- McGill, T.J.; Prusky, G.T.; Douglas, R.M.; Yasumura, D.; Matthes, M.T.; Nune, G.; Donohue-Rolfe, K.; Yang, H.; Niculescu, D.; Hauswirth, W.W.; et al. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5756–5766.
- Garcia-Ayuso, D.; di Pierdomenico, J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen. Res. 2018, 13, 1885–1886.
- di Pierdomenico, J.; Scholz, R.; Valiente-Soriano, F.J.; Sanchez-Migallon, M.C.; Vidal-Sanz, M.; Langmann, T.; Agudo-Barriuso, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P. Neuroprotective Effects of FGF2 and Minocycline in Two Animal Models of Inherited Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4392–4403.
- Bok, D.; Yasumura, D.; Matthes, M.T.; Ruiz, A.; Duncan, J.L.; Chappelow, A.V.; Zolutukhin, S.; Hauswirth, W.; LaVail, M.M. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp. Eye Res. 2002, 74, 719–735.
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901.
- Wang, D.; Luo, M.; Huang, B.; Gao, W.; Jiang, Y.; Li, Q.; Nan, K.; Lin, S. Localized co-delivery of CNTF and FK506 using a thermosensitive hydrogel for retina ganglion cells protection after traumatic optic nerve injury. Drug Deliv. 2020, 27, 556–564.
- Shityakov, S.; Broscheit, J.; Forster, C. alpha-Cyclodextrin dimer complexes of dopamine and levodopa derivatives to assess drug delivery to the central nervous system: ADME and molecular docking studies. Int. J. Nanomed. 2012, 7, 3211–3219.
- Sauer, R.S.; Rittner, H.L.; Roewer, N.; Sohajda, T.; Shityakov, S.; Brack, A.; Broscheit, J.A. A Novel Approach for the Control of Inflammatory Pain: Prostaglandin E2 Complexation by Randomly Methylated beta-Cyclodextrins. Anesth. Analg. 2017, 124, 675–685.
- Shityakov, S.; Forster, C. Pharmacokinetic delivery and metabolizing rate of nicardipine incorporated in hydrophilic and hydrophobic cyclodextrins using two-compartment mathematical model. Sci. World J. 2013, 2013, 131358.
- Rajagopal, R.; Zhang, S.; Wei, X.; Doggett, T.; Adak, S.; Enright, J.; Shah, V.; Ling, G.; Chen, S.; Yoshino, J.; et al. Retinal de novo lipogenesis coordinates neurotrophic signaling to maintain vision. JCI Insight 2018, 3, e97076.




