1. Targeting Inflammatory Mechanisms in Diabetes Mellitus
Various studies delivered compelling evidence for the possible link between inflammation and diabetes in animal models, revealing the key role of TNF-alpha in this pathology [
120,
121]. Some biomarkers and mediators, such as C-reactive protein, IL-6, fibrinogen, plasminogen activator inhibitor-1, and sialic acid, were determined in elevated concentrations based on epidemiological data, connecting diabetes and inflammation [
122]. However, the etiology of T1D is mainly an autoimmune inflammation of insulin-producing pancreatic beta cells, while T2D is a metabolic disorder [
123,
124]. Therefore, given cytokines’ (IL-1beta) and TNF-alpha involvement in the process of beta-cell pathology, they might be plausible therapeutic targets for T1D treatment [
121]. On the other hand, due to the B-cells’ immunity contribution to the adipose tissue inflammation as a result of the T2D metabolic imbalance, some B-cell knockout mice models and anti-CD20 therapies were proposed to improve metabolic and inflammatory phenotypes [
125]. DR progresses through different pathophysiological pathways, namely in the form of oxidative stress, inflammation, and stimulation of the growth factor in the eye’s vasculature [
126]. Increasing evidence has been given that inflammatory mechanisms are chiefly important in the pathogenesis of DR [
86,
127]. In detail, the upregulation of cytokines such as IL-6, IL-1β, IL-8, TNF-α, ICAM-1, vascular cell adhesion molecule V-CAM, selected integrins, and other proinflammatory cytokines and cell adhesion receptors have been demonstrated to lead to persistent low-grade inflammation. It is hypothesised that these actively play a part in the development of DR-associated damage to the iBRB, inducing breakdown, leading to subsequent macular edema formation, and promoting retinal neovascularization [
127].
Moreover, VEGF and angiopoietins have shown themselves to be critical in the pathogenesis of diabetic eye disease. Worth noting is a novel biomarker specific for the development of proliferative DR, elevated NLRP3 inflammasome levels in the vitreous have been shown to correlate positively with the onset and progress of this variant of the disease [
128].
Regarding adipose tissue inflammation and heart failure, several clinical studies have shown an association between systemic insulin resistance, inflammation and heart failure. For example, glucose metabolism is impaired in patients without type 2 diabetes who have idiopathic dilated cardiomyopathy [
129].
Diabetic nephropathy has, so far, not been traditionally considered an inflammatory disease. However, recent studies have shown that kidney inflammation is crucial in promoting the development and progression of diabetic nephropathy [
130].
Following this notion, currently, emerging therapeuties targeting inflammation are under evaluation, including the blockade of angiopoietin 2 and other inflammatory targets such as interleukins IL-6, IL-1β, plasma kallikrein, and integrins [
126].
2. Identification of a Common MicroRNA Signature in Damage in Diabetic Retinopathy, Diabetic Nephropathy and Heart Failure
Diabetes mellitus is a metabolic disease, with miRNAs playing a crucial role in regulating metabolism [
131].
miRNAs are highly conserved, endogenous, small, non-coding RNAs with a length of ~22 nucleotides, that regulate gene expression by binding to partially complementary sequences of mRNA [
132]. In cardiac insufficiency, chronic immune activation and aberrant miRNA expression are consistently evident [
133,
134].
On the other hand, blood, serum, and plasma are accessible data sources for physiological and pathological status. Recently, it was shown that miRNAs in a more stable form circulate in the blood. This would suggest that circulating miRNAs are used as biomarkers for cardiovascular diseases such as DR and diabetes-associated heart failure. In addition, it has been shown that restoring normal levels of altered miRNAs is a promising approach to prevent diabetes-related changes. The assessment of blood-borne DR microRNA profiles might, therefore, present clinicians a unique opportunity to detect the onset of diabetic microvascular damage at an early stage, thus allowing for the performance of a blood-borne marker-based progression analysis.
New approaches to treat DR are being developed via precision medicine that aims to target novel epigenetic changes associated with the development of DR: amongst these approaches, targeting specific miRNA is a very promising avenue for managing DR [
134,
135]. The restoration of altered miRNA expression to normal levels has recently been shown as a promising approach to prevent diabetes-associated changes [
2,
7,
20].
On one hand, it is known now that several miRNAs have been associated with the severity of DR, and future studies could even pinpoint whether circulatory miRNAs could serve as novel biomarkers to detect or predict DR [
134]. On the other hand, current functional screenings have identified miRNA as multi-cellular regulators of heart failure [
136]; among the differentially regulated entities, miR-155 plays an important role in the inflammation in the heart in diabetes-associated heart failure; these regulate processes such as hypertrophy, fibrosis (cardiac fibroid collagen content), and inflammation [
137]. miR-155, therefore, plays an important role in the immune system in mammals as well as in HF, and is abundantly expressed in T cells, B cells, and monocytes [
138,
139,
140,
141]. An STZ-induced diabetic heart expresses higher levels of miR-195, and silencing miR-195 reduces DCM [
142]. MiR-141 is also increased in the diabetic heart and influences mitochondrial function and ATP production [
143]. Palmitate-stimulated neonatal rat cardiomyocytes (NRCs) and diet-induced obese (DIO) mouse heart also showed increased expression of miR-451, which lowers LKB1/AMPK signal transduction [
144]. The expression of miR-133a decreases the Glut4 expression with the consequence of a decrease of the insulin-mediated glucose uptake in NRCs [
145]. Remarkably, the diabetic heart is also characterized by a clear downregulation of recently discovered cardioprotective miRNA [
146]. The development of further methods and research to establish a greater understanding of miRNA alterations in T2DM related to HF might become an important future focus area. Specifically, to assess more profoundly the association of these changes with respect to HfpEF or HfrEF, there will be greater importance placed on the ability to relate DR to HF subtype. This approach could facilitate the development of concepts for early detection and progression analysis of heart failure in diabetic patients. The relative expression of heart failure-related miRNA in cases of DR has, however, not been investigated so far.
miR-155 negatively regulates the expression of target gene E26 transformation-specific Sequence 1 (ETS-1) and its downstream factors VCAM-1, MCP-1, and cleaved caspase-3, thus mediating the inflammatory response and apoptosis of human renal glomerular endothelial cells [
147]. The miR-155 expression was increased in renal tissues of DN patients, and mainly expressed in glomerular vascular endothelial cells, mesangial cells and renal tubule interstitium [
148]. The results of luciferase reporter gene showed that ETS-1 may be a potential target gene of miR-155. Further detection of serum miRNAs in diabetic patients showed abnormal expression of miR-155 in diabetic patients compared with healthy controls, and the expression of miR-155 was significantly different in microproteinuria and macroproteinuria groups, and was positively correlated with eGFR in diabetic nephropathy patients and negatively correlated with urinary protein excretion rate. Triptolide upregulated BDNF by inhibiting miR-155-5p, thus inhibiting oxidative stress and inflammatory damage and alleviating podocyte injury in diabetic nephropathy mice [
149]. miRNA-195 promotes apoptosis of podocytes under high-glucose conditions via enhanced caspase cascades for BCL2 insufficiency [
150]. The abated miRNA-195 expression protected mesangial cells from apoptosis, suggesting that the antiapoptosis in a miRNA-regulated manner may play an important role in the early stages of diabetic nephropathy [
151] (Chen et al., 2012). miR-455-3p suppresses renal fibrosis through repression of ROCK2 expression in diabetic nephropathy [
152]. In the DN mouse model, Circ_0000491 knockdown inhibited high glucose-induced apoptosis, inflammation, oxidative stress, and fibrosis in SV40-MES13 cells by regulating miR-455-3p/Hmgb1 axis [
153].
In T2DM retinopathy, miR-155 plays an important role in the pathogenesis of T2DM retinopathy by regulating the Treg cells with TGF-β. rs767649 polymorphism in the pre-MIR155 gene is associated with DR in T2DM, and miR-155 plasma levels might be associated with T2DM [
154]. miR-155-5p expression was significantly upregulated in human retinal microvascular endothelial cells induced by high glucose. After inhibiting the expression of miR-155-5p, cell proliferation, angiogenesis, and VEGF protein levels were significantly downregulated, whereas miR-155-5p mimics had the opposite effect. miR-155-5p is closely associated with diabetic macular edema and is a potential target for refractory diabetic macular edema treatment [
155]. miR-195 regulates SIRT1-mediated tissue damage in DR [
156]. miR-195 knockdown led to the downregulation of the mRNA and protein expression levels of BAX and the upregulation of the mRNA and protein expression levels of SIRT1 and BCL-2 as well as improvement in cell growth and a decrease of the apoptosis rate [
157]. miR-195 is overexpressed in DR, and its targeted relationship with SIRT1 inhibits the growth of cells in the retina and accelerates apoptosis [
157]. miR-455-5p ameliorates high glucose-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells [
158].
Another promising biomarker to diagnose various cardiomyopathies, including heart failure and diabetic cardiomyopathy, might be miR-21 [
159]. This 22-nucleotide long microRNA has a paradoxical effect on cardiomyocytes against stress-overloaded conditions. It activates fibroblast to trigger the fibrosis process, stimulating the proliferation of the heart cells at the same time. Besides, the high level of miR-21 expression might be linked to the suppression of programmed cell death protein 4 in head and neck squamous cell carcinoma [
160].
One prospective long-term goal could, therefore, be the development of miRNA therapeutics for the prevention or treatment of diabetes-associated, inflammation-based heart failure: by targeting specific miRNAs, which govern whole pathways implicated in the pathogenesis of both DR and diabetes associated heart failure, it could be possible to develop novel diagnostic and therapeutic avenues to tackle the life-threatening condition of diabetes-associated HF.
Such therapeutic strategies could be developed utilizing miRNA therapeutics (
Figure 5 and
Figure 6). Establishing greater evidence and understanding has, moreover, even demonstrated that noncoding RNAs (ncRNAs) may also be expressed in various ways. DR expression may play a vital role in the development of DR. Amongst the non-coding RNAs, ncRNAs, besides miRNAs, and also the long ncRNAs (lncRNAs), have recently been described for their regulatory functions [
135].
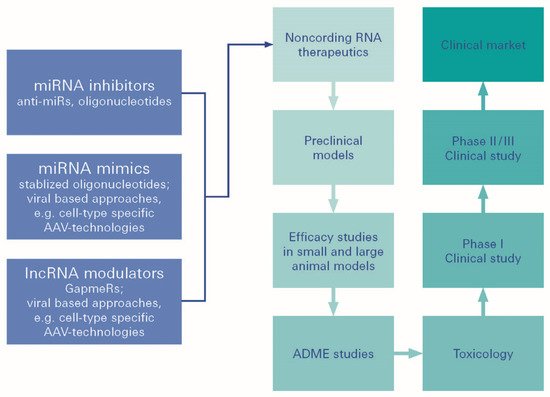
Figure 5. MiRNA therapeutics. MiRNA (miRNA)-based therapeutics can be divided into miRNA mimics and miRNA inhibitors, the so-called antimiRs. miRNA mimics are synthetic double-stranded small RNA molecules that match the corresponding miRNA sequence, and therefore, aim to functionally replenish lacking, dysfunctional, or lost miRNA expression in diseases.
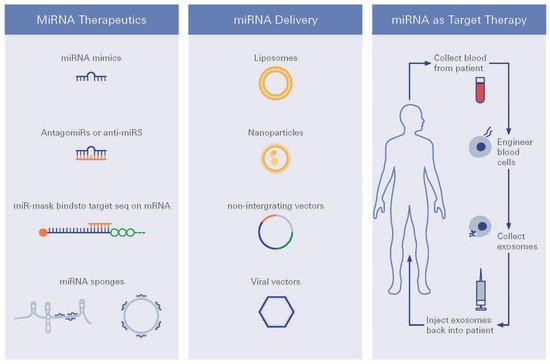
Figure 6. miRNA therapeutic strategies. Different strategies for miRNA targeted therapy, i.e., choice of delivery vehicles and route of delivery are being presented.
For future treatment regimens, several miRNA delivery strategies have been developed for use in practice, such as viral vectors, plasmid, piggybacks expression vectors, nanoparticles, exosomes, and liposomes [
134].
However, although plenty of miRNA-based therapeutics have been investigated in preclinical studies, only a few of these have been selected for further clinical development. Challenges concern difficulties in proper target selection, varying stability in body fluids, and insufficient target specificity. It also relates to off-target effects, which remain to be resolved in the future, to optimize the delivery and efficiency of miRNA-based therapeutics. As miRNAs have been shown to be important in basic potential, there could be greater expectations from miRNA-based constructs.
2.1. Neurotrophin Signaling and Neurotrophin-Related Treatment Opportunities in Diabetic Retinopathy
Also of significance, several components of the neurotrophin family are dysregulated upon diabetic challenges [
161,
162,
163,
164]. The mammalian neurotrophin family of growth factors comprises four ligands: nerve growth factor (NGF) [
165], neurotrophin-3 (NT-3) [
166], neurotrophin-4/5 (NT-4/5) [
167], and brain-derived growth factor (BDNF) [
168]. Initially, neurotrophins are expressed as precursor proteins (pro-neurotrophins) and proteolytically cleaved to their mature forms. This is to signal through tropomyosin receptor kinases (Trks) A-C and neurotrophin receptor p75 (p75
NTR), even though biological activity has also been attributed to pro-neurotrophins [
33,
169,
170,
171,
172].
While each neurotrophin has altered binding affinities to these receptors, it is well-established knowledge that the activation of Trk receptors primarily mediates cell survival, whereas p75
NTR-mediated signaling promotes, with some exceptions, degeneration of neuronal and non-neuronal cells [
94,
129,
134].
In streptozotocin (STZ)-induced diabetic rats, p75
NTR and proNGF are upregulated at an early stage in glial cells and pericytes [
161,
162,
163,
164], concomitant with an impaired TrkA receptor phosphorylation that shifts the TrkA/p75
NTR balance towards activation of the pro-apoptotic p75
NTR receptor in retinal ganglion cells. It eventually results in neuronal cell death [
162]. Accordingly, in human ocular samples (aqueous humor, vitreous, and retinae) from diabetic patients and in STZ-induced diabetic retinae, an accumulation of proNGF and reduced NGF levels were observed [
161]. Upregulation of p75
NTR and proNGF was furthermore associated with an increase of tumor necrosis factor alpha (TNFα) and alpha-2-macroglobulin (α
2M) in the retina, pointing again towards a proinflammatory and neurotoxic environment [
163]. Intriguingly, therapeutic treatment of diabetic rats with vitreal injections of a p75
NTR small-molecule antagonist (THX-B) or an anti-(pro)NGF blocking mAb (NGF30 mAb [
173]) significantly reduced retinal TNFα and α
2M levels and prevented death of retinal ganglion cells [
163]. Accordingly, STZ-induced diabetic p75
NTR knockout mice showed an attenuation of the diabetes-induced increases in proNGF, nuclear factor κB (NFκB), phospho-NFκB, and TNF-α in the retina, concomitant with a reduced decrease of retinal NGF expression and mitigated retinal ganglion cell loss [
174]. STZ-induced diabetic p75
NTR knockout mice were further protected against diabetes-induced BRB breakdown [
174]. Similarly, STZ-induced diabetic rats treated with NGF eye drops showed a not significant trend toward protection of retinal ganglion cells from diabetes-induced degeneration [
175]. Taken together, this data indicated that the proNGF–p75
NTR axis contributes to retinal inflammation and vascular dysfunction in STZ-induced diabetic retinae [
174].
However, although current knowledge of neurotrophin signaling in diabetic retinopathy predominantly centers around the proNGF/NGF axis, other neurotrophins, such as BDNF, NT3, and NT4/5 are also dysregulated in diabetic retinae [
176,
177,
178]. There are studies reporting significantly increased neurotrophin levels (BDNF, NGF, NT3, and NT4/5) in the vitreous of diabetic patients and in animals with experimentally induced proliferative diabetic retinopathy [
177,
178], but others demonstrate significantly decreased BDNF and NGF levels in serum and aqueous humor of diabetic patients [
179,
180]. These reports, contradictory at first sight, may well be attributed to the fact that investigation of diabetic retinal tissue most likely originated from diabetic patients in the late stages of the disease. Meanwhile, other samples (vitreous/aqueous humor) may have been collected during surgery at earlier stages of the disease.
Studies of the molecular interactions of BDNF and NGF in the diabetic retina have shown that both molecules exhibit pro-angiogenic effects and contribute to the formation of neovascularization in proliferative diabetic retinopathy [
176]. This is either directly, by binding to Trk receptors on endothelial cells, or indirectly, by promoting vascular endothelial growth factor (VEGF) expression in other cells [
177,
181,
182,
183,
184,
185,
186]. Thus, inhibition of VEGF by intravitreal injection of bevacizumab, a humanized anti-VEGF monoclonal antibody commonly used to inhibit neovascularization in patients with proliferative diabetic retinopathy, results in marked reduction of retinal NGF levels [
187].
Notably, comparable to patients with cardiovascular disease, decreased serum BDNF and NGF levels can be detected before the onset of clinically manifested diabetic retinopathy, and are, therefore, discussed as biomarkers and critical indicators for the development of diabetic retinopathy [
186].
2.2. Targeted Delivery of Neurotrophic Factors to the Retina
A novel approach for DR treatment might be via API delivery to the retina using various drug delivery vectors, including various organic and inorganic nanoparticles (NPs), proteins, adenoassociated virus vectors (AVV), hydrogels, and even cellar implants to prevent retinal damage [
188,
189,
190]. The use of NT-conjugated magnetic nanoparticles (MNTs) has been shown to be a strategy with potential to prevent the loss of retinal ganglion cells induced by oxidative stress damage [
188]. Indeed, these MNTs tend to progressively migrate from the vitreous chamber to the retina within 24 h after eye microinjection, maintaining the NGF, GDNF, and FGF-2 factors in situ [
188]. On the other hand, stable and biocompatible NPs incorporating CNTF and OSM were recently formulated and applied for the treatment of retinitis pigmentosa. The results of this study have shown significant photoreceptor preservation in an in vitro mouse model, offering long-term efficacy [
191,
192]. Direct intravitreal injection of CNTF, PEDF, and FGF2 can also be performed [
193] to access photoreceptor survival and macro/microglial reactivity in two rat models of inherited retinal degeneration [
194]. As an outcome, these neurotrophic factors were able to improve the outer segment morphology of photoreceptors and maintain their quantity [
195].
Similarly, the subretinal delivery of AVV-CNTF was also measured in adult Long–Evans rats to improve electroretinogram (ERG) amplitudes caused by the retinal degeneration [
193]. Despite some promising results, a few dose-related side effects were detected, such as a change in rod photoreceptor nucleus phenotype and a paradoxical decrease of ERG amplitudes, most likely due to the changes in gene expression [
196].
Furthermore, Bush and colleagues showed that some neuroprotective factors can be successfully delivered to the retina by cells transfected with the human CNTF and sequestered within capsules suitable for intraocular implantation [
197]. This Phase I clinical trial indicated that CNTF using encapsulated cell implants is safe for the human retina, even with severely compromised photoreceptors.
On the other hand, the usage of thermosensitive hydrogel enriched by CNTF for retinal ganglion cells (RGC) protection was proposed by Lin and co-authors. In this study, a pre-hydrogel liquid containing chitosan, hydrophobic macrolide immunosuppressant, CNTF, and the gelling agent, was directly smeared on the injured site, exhibiting in vivo RGCs protective action against the adverse effects caused by traumatic optic nerve injury [
198].
Other alternative strategies can be proposed to regulate NT levels in the retina indirectly by implementing amphiphilic cyclodextrins (CDs). These molecules have already been shown in different studies to be promising drug delivery vectors comprising native and modified CDs [
199,
200,
201]. In one study, neurotrophic signaling was restored by the CD-mediated exogenous cholesterol delivery using methyl-β cyclodextrin via de novo lipogenesis in the NT-dependent cell survival [
202]. It could also be achieved by stabilizing retinal membrane configuration and lipid composition to prevent cell death in many forms of retinopathy [
202]. Despite the limitation of CDs regarding their ability to engulf big molecules such as proteins, they might be suitable for formulation and delivery to the retina in the form of small neuroprotective peptides, such as neuropeptide Y (
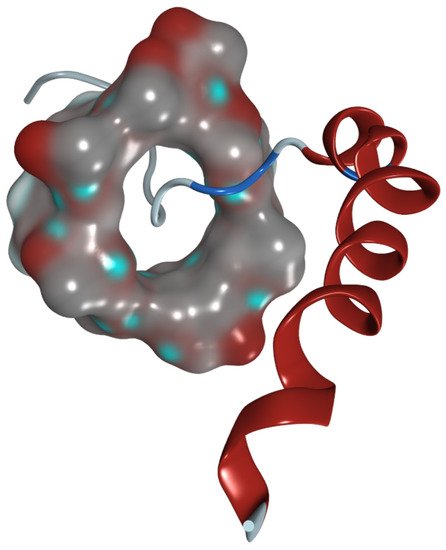
Figure 7).
Figure 7. Hypothesized 3D model of methyl-β-cyclodextrin with neuropeptide Y. The HP-β-CD molecule is shown as a molecular surface. The peptide is presented as a ribbon model.
This entry is adapted from the peer-reviewed paper 10.3390/biom12081113