Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jelle D'Helft | -- | 1909 | 2022-08-24 16:49:52 | | | |
| 2 | Vivi Li | Meta information modification | 1909 | 2022-08-25 03:25:45 | | | | |
| 3 | Vivi Li | + 4 word(s) | 1913 | 2022-08-26 08:31:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
D’helft, J.; Caccialanza, R.; Derbyshire, E.; Maes, M. Gamma-Linolenic Acid and Attention Deficit Hyperactivity Disorder. Encyclopedia. Available online: https://encyclopedia.pub/entry/26452 (accessed on 07 February 2026).
D’helft J, Caccialanza R, Derbyshire E, Maes M. Gamma-Linolenic Acid and Attention Deficit Hyperactivity Disorder. Encyclopedia. Available at: https://encyclopedia.pub/entry/26452. Accessed February 07, 2026.
D’helft, Jelle, Riccardo Caccialanza, Emma Derbyshire, Michael Maes. "Gamma-Linolenic Acid and Attention Deficit Hyperactivity Disorder" Encyclopedia, https://encyclopedia.pub/entry/26452 (accessed February 07, 2026).
D’helft, J., Caccialanza, R., Derbyshire, E., & Maes, M. (2022, August 24). Gamma-Linolenic Acid and Attention Deficit Hyperactivity Disorder. In Encyclopedia. https://encyclopedia.pub/entry/26452
D’helft, Jelle, et al. "Gamma-Linolenic Acid and Attention Deficit Hyperactivity Disorder." Encyclopedia. Web. 24 August, 2022.
Copy Citation
The use of polyunsaturated fatty acids in Attention-Deficit/Hyperactivity Disorder (ADHD) and developmental disorders has been gaining interest with preparations containing different dosages and combinations. Gamma-linolenic acid is an ω-6 fatty acid of emerging interest with potential roles as an adjuvant anti-inflammatory agent that could be used with ω-3 Polyunsaturated fatty acids (PUFAs) in the treatment of ADHD and associated symptoms.
attention deficit hyperactivity disorder (ADHD)
brain function
brain structure
cognition
developmental
difference
gamma-linolenic acid (GLA)
hyperactivity
neuro-immune
neuroinflammatory
ω-3 PUFAs
oxidative stress
wellbeing
1. Introduction
Polyunsaturated fatty acids (PUFAs) include two pathways of fatty acids—ω-3 and ω-6—which are both known to play major biological roles as structural and functional components of cell membranes and have a profound influence on the development of the central nervous system [1]. As shown in Figure 1, linoleic acid (LA) and alpha-linolenic acid (ALA) are precursors of ω-6 and ω-3 families, respectively and are regarded as essential fatty acids (EFAs) as they cannot be synthesized by the human body in amounts needed for health and wellbeing, and thus need to be supplied by diet [2][3]. These parent fatty acids yield arachidonic acid (ARA, ω-6), eicosapentaenoic acid (EPA, ω-3), and docosahexaenoic acid (DHA, ω-3) which regulate body homeostasis and act locally via bioactive signaling lipids called eicosanoids [3].
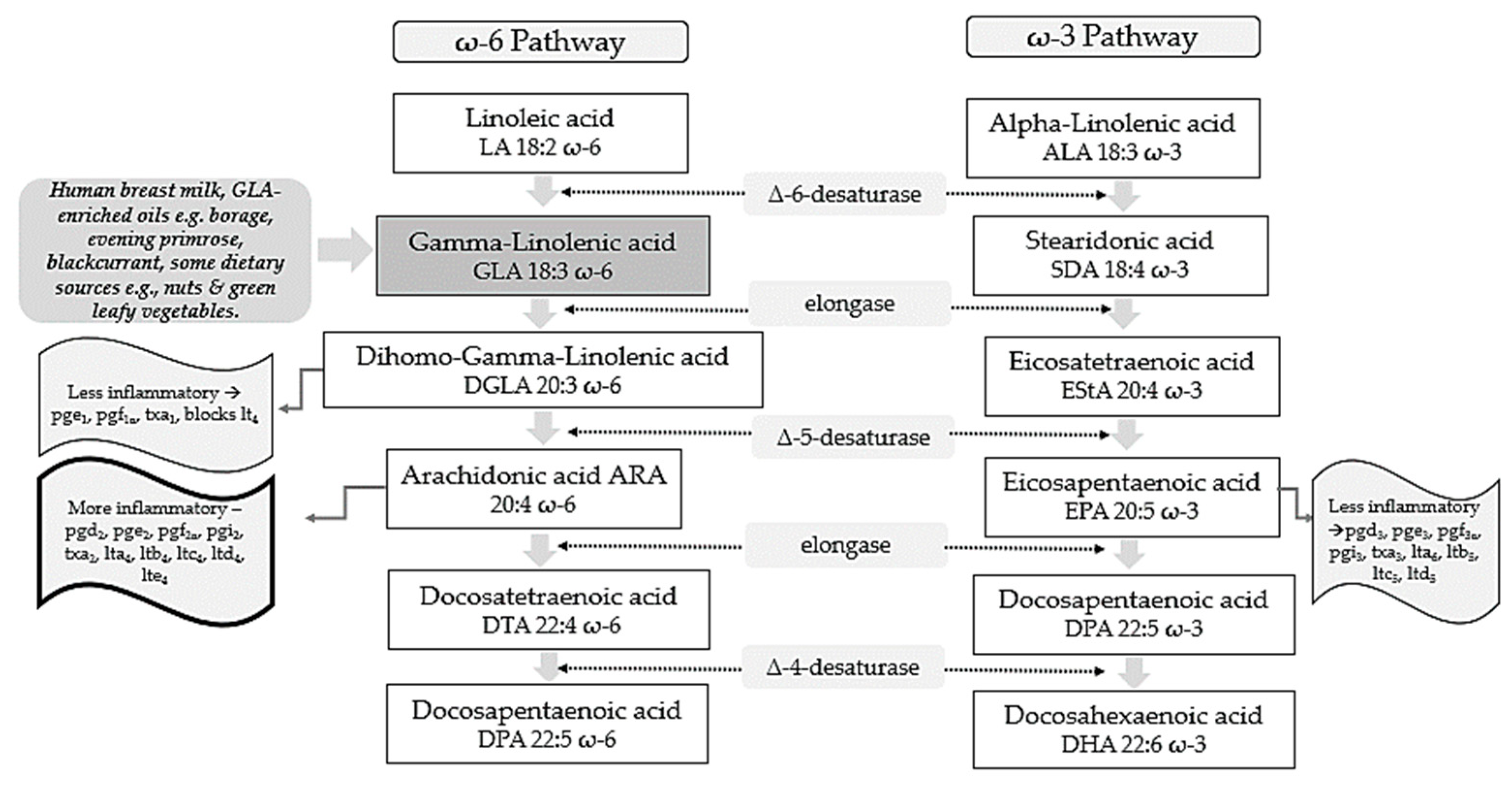
Figure 1. ω-6 and ω-3 family pathways. Key: pg, prostaglandin; pgi, prostacyclin; tx, thromboxane; lt, leukotriene.
Studies on evolutionary aspects of human diets indicate that major changes have taken place concerning types and profiles of dietary essential fatty acids obtained [4][5]. Genetically speaking, human beings in modern life live in a nutritional environment that differs from that which gave birth to their genetic pattern back in the evolutionary history of the genus Homo [5]. Whilst the dietary ratio of ω-6 to ω-3 fatty acids was once balanced and 1 to 1, in Western lifestyles this is now around 15–17 to 1 [4]. This is in stark contrast to recommendations from national agencies which advise that an ω-6 to ω-3 ratio of 4:1 is preferable [6]. It has been reported that lowering the ω-6 to ω-3 ratio to such thresholds could reduce enzymatic metabolic competition and facilitate the metabolism of more downstream products from ALA [7].
According to the Diagnostic and Statistical Manual of Mental Disorders V Edition (DSM-V), neurodevelopmental disorders (NDDs) are defined as a group of conditions with onset in the developmental period, inducing deficits that produce impairments of functioning [8][9]. NDDs comprise intellectual disability (ID), communication disorders, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), neurodevelopmental motor disorders, and specific learning disorders [8].
According to the DSM-V, ADHD diagnosis is based on some age-dependent symptoms of inattention and/or hyperactivity/impulsivity that interfere with functioning of development and should occur for at least 6 months [9]. ADHD is a neurodevelopmental disorder with onset in childhood, however, 50% of subjects continue to experience symptoms throughout adolescence and 30–60% in adulthood [10]. Overall pooled prevalence in the world is 7.2%; it is higher in males than in females, and it is most common in school-aged children [11]. Community prevalence of ADHD globally in children and young people has been reported to be between 2% and 7%, and an average of approximately 5% [12]. Other research reports a higher ADHD prevalence amongst boys, urban young people and those born to mothers with a history of psychiatric hospitalization [13].
2. GLA as an Antioxidant and Anti-Inflammatory Molecule
GLA is composed of 18 carbon atoms with three double bonds and belongs to the category of ω-6 PUFAs [14]. GLA is present in human breast milk, a significant source for infants, and can be obtained from certain botanical seed oils and ingested from dietary supplements [14][15]. Natural sources of GLA include the oils of borage (Borago officinalis L., 20–26% GLA), black currant (Ribes nigrum L., 15–18%), and evening primrose (Oenothera biennis L., 8–12%) [14]. Some foods provide GLA in trace amounts, such as nuts and green leafy vegetables [15]. Unfortunately, GLA-rich foods are consumed in low quantities by the average person with the typical dietary intake of GLA being negligible [16].
Metabolically, as shown in Figure 1, GLA is produced in the body by the delta 6-desaturase enzyme acting on the parent fatty acid LA. It is then rapidly elongated to dihomo-gamma-linolenic acid (DGLA) by the enzyme elongase, acetylated, and incorporated into cell membrane phospholipids without resultant changes in ARA [17][18][19]. This has been demonstrated in in vivo metabolic research where GLA supplementation resulted in DGLA accumulation in neutrophil glycerolipids rather than ARA [20]. It has been postulated that this increase in DGLA relative to ARA within inflammatory cells such as neutrophils could diminish ARA metabolite biosynthesis [20]. This presents a plausible mechanism in terms of how dietary GLA could exert its anti-inflammatory effects [20].
Upon cell activation, DGLA is then released as free fatty acids (FAs) by phospholipase A2 and converted to several anti-inflammatory metabolites through competition with ARA for the enzymes cyclooxygenase (COX) and lipoxygenase (LOX) [21]. COX products from DGLA include prostaglandins 1 (PGE1), which can exert vasodilatory and anti-inflammatory actions [22][23]. 15-hydroxyeicosatrienoic acid (15-HETrE) is also the 15-lipoxygenase product of DGLA which can inhibit proinflammatory eicosanoid biosynthesis and exert anti-inflammatory properties [19][24]. The Δ-6-desaturase activity appears to be altered by various factors. For example, desaturase mRNA levels may be influenced by the quantity and composition of dietary carbohydrates, protein, fats, and micronutrients including vitamin A, B12, folate, iron, zinc, and polyphenols [25]. Reduced enzyme activity is also observed in elderly people [16]. Other work suggests that Δ-6-desaturase activity could also be reduced in young children presenting signs of dry skin, desquamation, and thickening of the skin, as well as growth failure [16].
GLA and its metabolites have also been found to influence the expression of several genes, regulating levels of gene products including matrix proteins [15]. Such gene products are thought to have central roles in immune function and programmed cell death (apoptosis) [15]. Critical associations are also proposed between ADHD and the level of oxidative stress which can induce cell membrane damage, changes in inner structure and function of proteins, and DNA structural damage which eventually culminate in ADHD development [26].
3. GLA Synergy with EPA
ARA can also be synthesized from DGLA through Δ-5 desaturase, encoded by fatty acid desaturase 1 (FADS1) within the FADS gene cluster (Figure 1). ARA and its potent eicosanoid products (prostaglandins, thromboxanes, leukotrienes, and lipoxins) play an important role in immune responses and inflammation [27]. Therefore, dietary supplementation with GLA has the capability to both increase levels of DGLA and its several anti-inflammatory metabolites, as well as ARA and its pro-inflammatory metabolic products and this might represent a therapeutic concern [14].
To counterbalance these pathways and make full use of the anti-inflammatory mechanisms of the molecule, the ω-3 long-chain-PUFAs (LC-PUFAs) EPA and DHA are often co-administered with GLA [28]. Humans supplemented with GLA, and EPA have substantially elevated blood EPA levels, but not ARA levels, suggesting that this supplement combination inhibits the development of pro-inflammatory ARA metabolites [28].
Evidence shows that 0.25 g/d EPA + DHA can block GLA-induced elevations in plasma ARA levels, while supplementation with borage + fish oil combinations inhibit leukotriene generation [29][30] and attenuate the expression of pro-inflammatory cytokines genes [31]. Furthermore, studies suggest that botanical oil combinations of borage oil, enriched in GLA, and echium oil (from Echium plantagineum L.) abundant in ω-3 PUFAs (ALA and stearidonic acids; SDA) enhanced the conversion of dietary GLA to DGLA whilst inhibiting the further conversion of DGLA to ARA [32]. Such supplementation strategies maintained the anti-inflammatory capacity of GLA, while increasing EPA, without causing accumulation of ARA.
This has been observed to directly translate into a clinical benefit in various therapeutic areas. For example, when enteral diets were enriched with marine oils containing EPA, DHA, and GLA, cytokine production and neutrophil recruitment in the lung were reduced, resulting in fewer days on ventilation and Intensive Care Unit stay-in patients with acute lung injury or acute respiratory distress syndrome (ARDS) [33]. Other research has observed decreased morbidity and mortality of critically ill patients with severe ARDS [34] and improved quality of life in asthma patients [29]. Positive outcomes of a combined ω-6/ω-3 PUFAs therapy have been observed in patients with rheumatoid and psoriatic arthritis in a RCT, where treatment with ω-3 LC-PUFAs and GLA led to an increase of GLA and DGLA concentrations in plasma lipids, cholesteryl esters, and erythrocyte membranes, indicating a reduction in the production of ARA inflammatory eicosanoids [35].
4. GLA and ADHD Focus
Even though the pathogenetic pathways of NDDs are still not completely clear, growing scientific evidence points to oxidative stress and neuroinflammation as triggers for their genesis, and might be pivotal in their clinical pattern and evolution [36][37]. ω-6 and ω-3 PUFAs and their metabolites are involved in immune-inflammatory and brain structural mechanisms and therefore these molecules may play a role in neurological disorders in which disruption of these mechanisms represent a contributing pathogenetic factor [38][39].
4.1. Pathogenesis and Role of Neuroinflammation
The etiology of ADHD is multifactorial and still not fully understood. Evidence indicates that ADHD is heritable, however, no genes of major effect have been detected so far [40]. Pre- and peri-natal inflammatory factors that can affect the in-utero environment seem to be also involved in the etiology of the disease i.e., infections [41], smoking [42], obesity and poor diet [43], and pollutants to which the mother might be exposed [44][45]. Prenatal exposures to inflammation have also been associated with a volume reduction of cortical areas associated with ADHD [46]. Furthermore, a bilateral decrease in grey matter volume in the cingulate and parietal areas was observed in children with ADHD [46].
The hypothesis that inflammation is part of the pathway to ADHD and more in general to NDDs is consistent. Neuroinflammation influences brain development and subsequent risk of neurodevelopmental disorders through mechanisms such as glial activation [47], increased oxidative stress [48], aberrant neuronal development [49], reduced neurotrophic support [50], and altered neurotransmitter function [51]. Studies have identified associations between ADHD and regulatory genes involved in cell adhesion and inflammation, such as the gene for the interleukin-1 receptor antagonist (IL-1 RA) [52]. Moreover, immune disorders such as eczema [53], asthma, rheumatoid arthritis, type 1 diabetes, and hypothyroidism [54] are associated with greater rates of ADHD diagnosis.
Higher levels of antibodies against basal ganglia [55] and dopamine transporter [56] have been detected in subjects with ADHD. Furthermore, ADHD patients have reported increased cerebrospinal fluid levels of pro-inflammatory cytokine tumor necrosis factor β (TNF- β) and lower amounts of anti-inflammatory cytokine IL-4 [57]. This translates into an overall vulnerability of central nervous system (CNS) structures. Under normal conditions the blood–brain barrier (BBB) separates the peripheral immune system, preventing peripheral immune cells from entering the CNS. However, injuries, inflammatory diseases, and psychological stress may compromise the BBB, consequently, peripheral activated monocytes and T-lymphocytes may penetrate the brain, thereby inducing microglial activation, neuroinflammation, and neurotoxic responses [58].
A lack of dopamine in ADHD pathogenesis has been supported by the evidence that medications like methylphenidate and amphetamines, which increase the levels of dopamine in the synaptic cleft, can temporarily improve symptoms [59]. However, up to 30% of ADHD patients do not respond to this treatment, while only about 50% show signs of improvement [60]. Recent studies have provided evidence for the role of the monoamine serotonin (5-HT), synthesized from the essential amino acid tryptophan in a few areas of the brain such as the dorsal raphe nucleus, and which is an important regulator of behavioral inhibition [61]. Children with ADHD may have lower blood levels of 5-HT [62], which might contribute to the symptoms of ADHD [63]. Therefore, alternative pharmacological treatments for ADHD include selective-serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tri-cyclic-antidepressants (TCA) all of which target the 5-HT system [64].
Relevantly, some ω-3 PUFAs and some ω-6 PUFAs such as GLA are directly involved in the synthesis, release, and re-uptake of neurotransmitters [65], Subsequently, an ω-3 PUFAs deficiency or an imbalance between ω-6 and ω-3 PUFAs may lead to impaired neurological functioning and behavioral disturbances similar to those which characterize ADHD [65].
References
- Tesei, A.; Crippa, A.; Ceccarelli, S.B.; Mauri, M.; Molteni, M.; Agostoni, C.; Nobile, M. The potential relevance of docosahexaenoic acid and eicosapentaenoic acid to the etiopathogenesis of childhood neuropsychiatric disorders. Eur. Child Adolesc. Psychiatry 2017, 26, 1011–1030.
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Diet Suppl. 2009, 6, 143–161.
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267.
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379.
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet 2003, 92, 1–22.
- Holub, B. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. JAMC 2002, 166, 608–615.
- Ander, B.; Dupasquier, C.; Prociuk, M.; Pierce, G. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172.
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin. NeuroSci. 2020, 22, 65–72.
- DSM. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013.
- Ahmed, R.; Borst, J.M.; Yong, C.W.; Aslani, P. Do parents of children with attention-deficit/hyperactivity disorder (ADHD) receive adequate information about the disorder and its treatments? A qualitative investigation. Patient Prefer. Adherence 2014, 8, 661–670.
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001.
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186.
- Mohammadi, M.R.; Zarafshan, H.; Khaleghi, A.; Ahmadi, N.; Hooshyari, Z.; Mostafavi, S.A.; Ahmadi, A.; Alavi, S.S.; Shakiba, A.; Salmanian, M. Prevalence of ADHD and Its Comorbidities in a Population-Based Sample. J. Atten. Disord. 2021, 25, 1058–1067.
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86.
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534.
- Dobryniewski, J.; Szajda, S.D.; Waszkiewicz, N.; Zwierz, K. Biology of essential fatty acids (EFA). Przegl. Lek. 2007, 64, 91–99.
- Williams, C.M.; Burdge, G. Long-chain n-3 PUFA: Plant v. marine sources. Proc. Nutr. Soc. 2006, 65, 42–50.
- Khan, S.A.; Ali, A.; Khan, S.A.; Zahran, S.A.; Damanhouri, G.; Azhar, E.; Qadri, I. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediat. Inflamm. 2014, 2014, 502749.
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414.
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 1997, 127, 1435–1444.
- Chilton, L.; Surette, M.E.; Swan, D.D.; Fonteh, A.N.; Johnson, M.M.; Chilton, F.H. Metabolism of gammalinolenic acid in human neutrophils. J. Immunol. 1996, 156, 2941–2947.
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. Role of oxylipins generated from dietary PUFAs in the modulation of endothelial cell function. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102160.
- Fan, Y.Y.; Ramos, K.S.; Chapkin, R.S. Dietary gamma-linolenic acid modulates macrophage-vascular smooth muscle cell interactions. Evidence for a macrophage-derived soluble factor that downregulates DNA synthesis in smooth muscle cells. Arter. Thromb. Vasc. Biol. 1995, 15, 1397–1403.
- Heitmann, J.; Iversen, L.; Kragballe, K.; Ziboh, V.A. Incorporation of 15-hydroxyeicosatrienoic acid in specific phospholipids of cultured human keratinocytes and psoriatic plaques. Exp. Dermatol. 1995, 4, 74–78.
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on Delta-5/Delta-6 Desaturases and Elongases 2/5. Adv. Nutr. 2021, 12, 980–994.
- Moghadas, M.; Essa, M.M.; Ba-Omar, T.; Al-Shehi, A.; Qoronfleh, M.W.; Eltayeb, E.A.; Guillemin, G.J.; Manivasagam, T.; Justin-Thenmozhi, A.; Al-Bulushi, B.S.; et al. Antioxidant therapies in attention deficit hyperactivity disorder. Front. BioSci. 2019, 24, 313–333.
- Boyce, J.A. Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 2008, 8, 335–349.
- Barham, J.B.; Edens, M.B.; Fonteh, A.N.; Johnson, M.M.; Easter, L.; Chilton, F.H. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J. Nutr. 2000, 130, 1925–1931.
- Surette, M.E.; Stull, D.; Lindemann, J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr. Med. Res. Opin. 2008, 24, 559–567.
- Surette, M.E.; Koumenis, I.L.; Edens, M.B.; Tramposch, K.M.; Chilton, F.H. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 2003, 25, 948–971.
- Weaver, K.L.; Ivester, P.; Seeds, M.; Case, L.D.; Arm, J.P.; Chilton, F.H. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J. Biol. Chem. 2009, 284, 15400–15407.
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196.
- Pontes-Arruda, A.; Aragao, A.M.; Albuquerque, J.D. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006, 34, 2325–2333.
- Li, C.; Bo, L.; Liu, W.; Lu, X.; Jin, F. Enteral Immunomodulatory Diet (Omega-3 Fatty Acid, gamma-Linolenic Acid and Antioxidant Supplementation) for Acute Lung Injury and Acute Respiratory Distress Syndrome: An Updated Systematic Review and Meta-Analysis. Nutrients 2015, 7, 5572–5585.
- Dawczynski, C.; Hackermeier, U.; Viehweger, M.; Stange, R.; Springer, M.; Jahreis, G. Incorporation of n-3 PUFA and gamma-linolenic acid in blood lipids and red blood cell lipids together with their influence on disease activity in patients with chronic inflammatory arthritis--a randomized controlled human intervention trial. Lipids Health Dis. 2011, 10, 130.
- Buss, C. Maternal oxidative stress during pregnancy and offspring neurodevelopment. Brain Behav. Immun. 2021, 93, 6–7.
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579.
- Laye, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38.
- Caramia, G. The essential fatty acids omega-6 and omega-3: From their discovery to their use in therapy. Minerva. Pediatr. 2008, 60, 219–233.
- Brikell, I.; Kuja-Halkola, R.; Larsson, H. Heritability of attention-deficit hyperactivity disorder in adults. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 406–413.
- Werenberg Dreier, J.; Nybo Andersen, A.M.; Hvolby, A.; Garne, E.; Kragh Andersen, P.; Berg-Beckhoff, G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J. Child Psychol. Psychiatry 2016, 57, 540–548.
- Silva, D.; Colvin, L.; Hagemann, E.; Bower, C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014, 133, e14–e22.
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484.
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. What have we learnt about the causes of ADHD? J. Child Psychol. Psychiatry 2013, 54, 3–16.
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental pollutants and child health-A review of recent concerns. Int. J. Hyg. Environ. Health 2016, 219, 331–342.
- Castellanos, F.X.; Lee, P.P.; Sharp, W.; Jeffries, N.O.; Greenstein, D.K.; Clasen, L.S.; Blumenthal, J.D.; James, R.S.; Ebens, C.L.; Walter, J.M.; et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002, 288, 1740–1748.
- Reus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154.
- Hassan, W.; Noreen, H.; Castro-Gomes, V.; Mohammadzai, I.; da Rocha, J.B.; Landeira-Fernandez, J. Association of Oxidative Stress with Psychiatric Disorders. Curr. Pharm. Des. 2016, 22, 2960–2974.
- Belmadani, A.; Tran, P.B.; Ren, D.; Miller, R.J. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. NeuroSci. 2006, 26, 3182–3191.
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry 2008, 64, 527–532.
- Kronfol, Z.; Remick, D.G. Cytokines and the brain: Implications for clinical psychiatry. Am. J. Psychiatry 2000, 157, 683–694.
- Zayats, T.; Athanasiu, L.; Sonderby, I.; Djurovic, S.; Westlye, L.T.; Tamnes, C.K.; Fladby, T.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Genome-wide analysis of attention deficit hyperactivity disorder in Norway. PLoS ONE 2015, 10, e0122501.
- Lin, Y.T.; Chen, Y.C.; Gau, S.S.; Yeh, T.H.; Fan, H.Y.; Hwang, Y.Y.; Lee, Y.L. Associations between allergic diseases and attention deficit hyperactivity/oppositional defiant disorders in children. Pediatr. Res. 2016, 80, 480–485.
- Instanes, J.T.; Halmoy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsoyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459.
- Toto, M.; Margari, F.; Simone, M.; Craig, F.; Petruzzelli, M.G.; Tafuri, S.; Margari, L. Antibasal Ganglia Antibodies and Antistreptolysin O in Noncomorbid ADHD. J. Atten. Disord. 2015, 19, 965–970.
- Giana, G.; Romano, E.; Porfirio, M.C.; D’Ambrosio, R.; Giovinazzo, S.; Troianiello, M.; Barlocci, E.; Travaglini, D.; Granstrem, O.; Pascale, E.; et al. Detection of auto-antibodies to DAT in the serum: Interactions with DAT genotype and psycho-stimulant therapy for ADHD. J. Neuroimmunol. 2015, 278, 212–222.
- Mittleman, B.B.; Castellanos, F.X.; Jacobsen, L.K.; Rapoport, J.L.; Swedo, S.E.; Shearer, G.M. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J. Immunol. 1997, 159, 2994–2999.
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. NeuroSci. 2014, 8, 447.
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Ding, Y.S. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1410–1415.
- Arnold, L.E.; Hurt, E.; Lofthouse, N. Attention-deficit/hyperactivity disorder: Dietary and nutritional treatments. Child Adolesc. Psychiatr. Clin. N. Am. 2013, 22, 381–402.
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34.
- Spivak, B.; Vered, Y.; Yoran-Hegesh, R.; Averbuch, E.; Mester, R.; Graf, E.; Weizman, A. Circulatory levels of catecholamines, serotonin and lipids in attention deficit hyperactivity disorder. Acta Psychiatr. Scand. 1999, 99, 300–304.
- Quist, J.F.; Kennedy, J.L. Genetics of childhood disorders: XXIII. ADHD, Part 7: The serotonin system. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 253–256.
- Park, P.; Caballero, J.; Omidian, H. Use of serotonin norepinephrine reuptake inhibitors in the treatment of attention-deficit hyperactivity disorder in pediatrics. Ann. Pharmacother. 2014, 48, 86–92.
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 2010, 169, 149–164.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Entry Collection:
Neurodegeneration
Revisions:
3 times
(View History)
Update Date:
26 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




