Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Filipa Mascarenhas-Melo | -- | 2696 | 2022-08-24 11:23:51 | | | |
| 2 | Camila Xu | -125 word(s) | 2571 | 2022-08-25 02:13:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Coimbra, S.C.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Peixoto, D.; Pereira-Silva, M.; Mathur, A.; Pawar, K.D.; Raza, F.; Mazzola, P.G.; Mascarenhas-Melo, F.; et al. Safety Assessment of Nanomaterials in Cosmetics. Encyclopedia. Available online: https://encyclopedia.pub/entry/26434 (accessed on 03 March 2026).
Coimbra SC, Sousa-Oliveira I, Ferreira-Faria I, Peixoto D, Pereira-Silva M, Mathur A, et al. Safety Assessment of Nanomaterials in Cosmetics. Encyclopedia. Available at: https://encyclopedia.pub/entry/26434. Accessed March 03, 2026.
Coimbra, Sara Cabanas, Inês Sousa-Oliveira, Inês Ferreira-Faria, Diana Peixoto, Miguel Pereira-Silva, Ankita Mathur, Kiran D. Pawar, Faisal Raza, Priscila Gava Mazzola, Filipa Mascarenhas-Melo, et al. "Safety Assessment of Nanomaterials in Cosmetics" Encyclopedia, https://encyclopedia.pub/entry/26434 (accessed March 03, 2026).
Coimbra, S.C., Sousa-Oliveira, I., Ferreira-Faria, I., Peixoto, D., Pereira-Silva, M., Mathur, A., Pawar, K.D., Raza, F., Mazzola, P.G., Mascarenhas-Melo, F., Veiga, F., & Paiva-Santos, A.C. (2022, August 24). Safety Assessment of Nanomaterials in Cosmetics. In Encyclopedia. https://encyclopedia.pub/entry/26434
Coimbra, Sara Cabanas, et al. "Safety Assessment of Nanomaterials in Cosmetics." Encyclopedia. Web. 24 August, 2022.
Copy Citation
Nanomaterials use in cosmetics is markedly enhancing, so their exposure and toxicity are important parameters to consider for their risk assessment.
nanomaterial
safety assessment test
toxicology
genotoxicity
hair dye cosmetic
1. Introduction
Topical delivery of active cosmetic ingredients (ACIs) can be efficiently made by nanoparticles (NPs), including the nanoencapsulated hair dyes, which are able to penetrate hair follicles to a much greater extent [1]. Due to their small size, NPs can be successful carriers of hair and skin ACIs. By searching for articles published in the field of nanotechnology it directs to a huge number of publications already done, attesting to the importance of this topic and its excellent upcoming prospects. Nevertheless, the number of papers considering its toxicological assessment, and depicting hair dyes, is much smaller. Further elucidation is needed about the possible impact that nanoscience and its specific nanomaterials (NMs) can have on living systems. Studies that could mimic the in vivo conditions should be carried out to clarify possible toxicological outcomes of a certain cosmetic formulation containing nanostructures. Several studies can be considered and analyzed regarding the wide range of possible interactions of the nanostructures with living systems, considering the broader range of NPs developed and conceived for different applications [2][3][4][5].
New approaches to the NMs use in cosmetic products enhanced safety requirements for a cosmetic product safety report preparation. Though the total replacement of animal testing with non-animal methods recurring to cell cultures and tissues is the final ideal. The 3R strategy is already an advancement and notable progress in the refinement, reduction, and replacement concepts related to laboratory animal testing. The “safety evaluation” of an ACI can be addressed by the process of risk assessment and its principles based on four main parts. The first part is hazard identification, in which the toxicological profile of the ACI of interest is undertaken via several tests (in vivo, in vitro), clinical and epidemiological studies, as well as case reports, that provide useful results and data, dose-response assessment, and some parameters can be measured, such as no observed adverse effect level (NOAEL) and no observed effect level (NOEL), in order to study the exposure—toxic response binomial, exposure assessment and, finally, risk characterization, where a margin of safety (MoS) can be calculated. Another important and inescapable point is the exposure assessment and the calculation of important parameters, such as the systemic exposure dosage (SED)—dermal exposure. Moreover, oral and inhalation exposures if needed, and the toxicological assessment, with similar parameters, when compared with the more conventional cosmetics formulations, have particular specifications considering nano-aspects as the absence of validation of conventional testing methods of toxicological endpoints for NM’s purpose and the possibility of existing surface interactions motivated by the possible high surface energy. Therefore, an accurate characterization of the NM is needed, along with its probable interaction with the surrounding environment. Toxicological measurement’s metric can be different once researchers are dealing with nanosized structures, the absorption, distribution, metabolism, and excretion (ADME) evaluation and bioavailability (BA)/toxicokinetic may assume even special importance, once their small properties and peculiar physicochemical characteristics that may increase their ability to cross membranes and attain different regions of the body that otherwise would remain loosely accessible, which can constitute both an advantage and a disadvantage [6][7]. Agglomeration/aggregation behavior should also be thoroughly examined, and dispersibility and solubility should be tested too. The risk of an NM is evaluated on the risk assessment and is calculated by dividing NO(A)EL (or LO(A)EL) by SED, yielding the margin of safety (MoS) of the NM on a final cosmetic product [8][9].
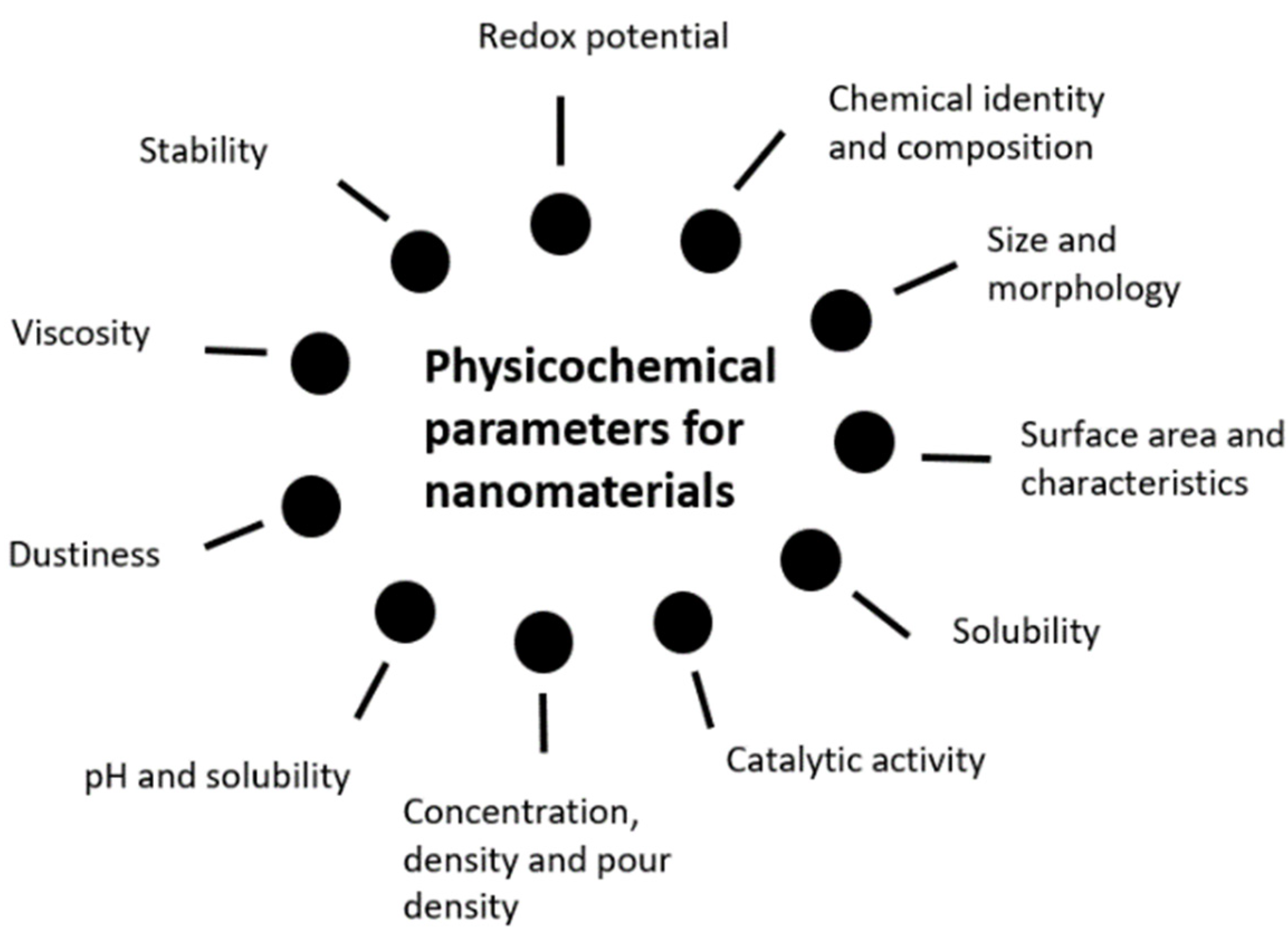 Figure 1. Physicochemical characterization for nanomaterials (NMs).
Figure 1. Physicochemical characterization for nanomaterials (NMs).
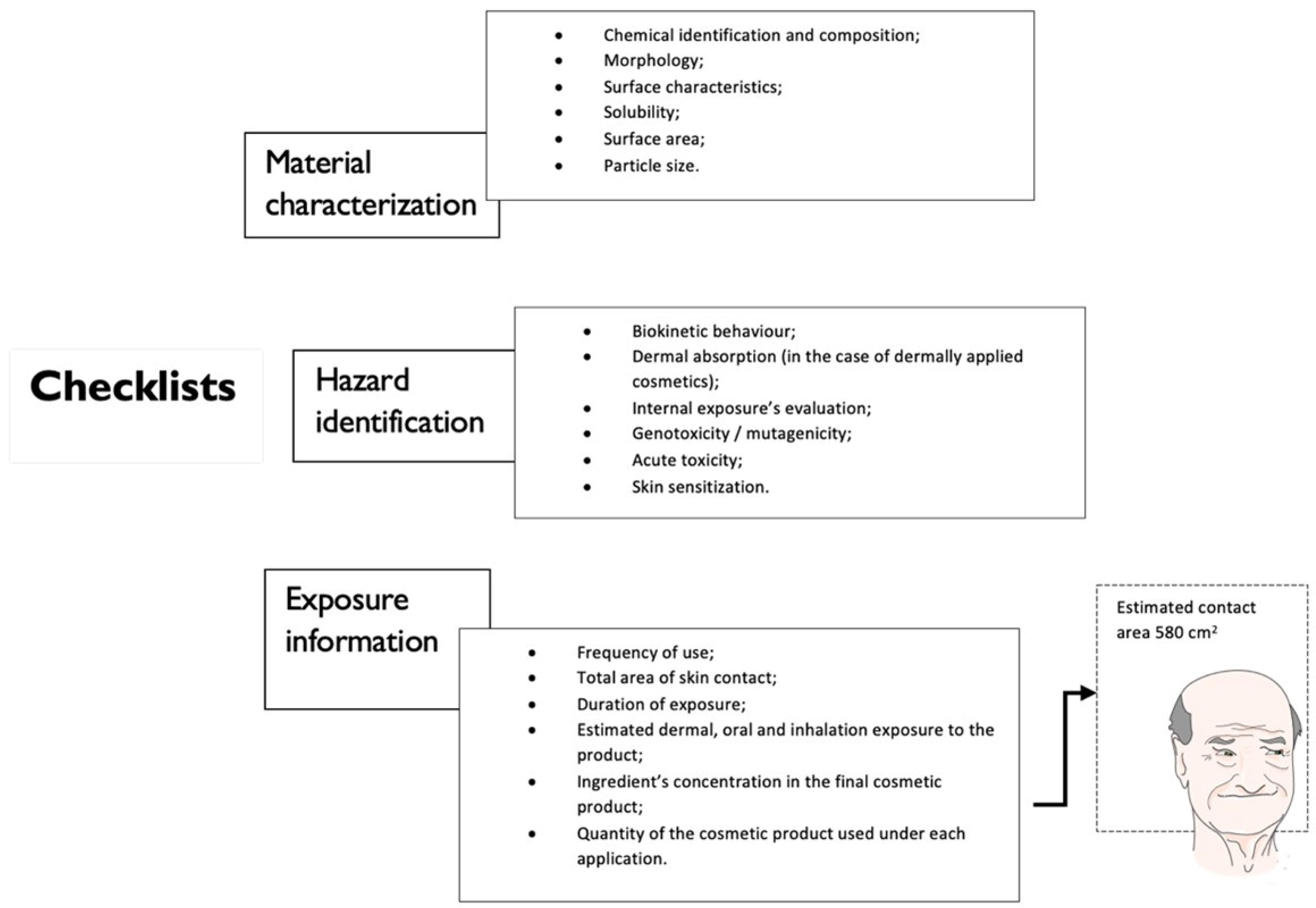 Figure 2. Schematic summary of SCCS’s checklists for applicants submitting dossiers on ACIs. SCCS stands for: Scientific Committee on Consumer Safety. ACIs stands for: active cosmetic ingredients.
Figure 2. Schematic summary of SCCS’s checklists for applicants submitting dossiers on ACIs. SCCS stands for: Scientific Committee on Consumer Safety. ACIs stands for: active cosmetic ingredients.
2. Hair Formulations and Their Impact on Living Systems
The emerging formulations and marketing of hair dyes brought some concerns regarding the possibility of certain ACIs contained in these formulations causing deleterious health outcomes.
Of note is the case of the permanent dyes 2-nitro-p-phenylenediamine and 4-amino-2-nytrophenol, among others, that were appointed as responsible for inducing carcinogenic activity on tested laboratory animals. They manifested the capability of being able to permeate the dermal barrier on humans, thereafter, culminating in the removal of these molecules from the definitive formulation of hair colorants by the Food and Drug Administration (FDA) and their substitution by other chemically similar molecules with safer properties [10]. p-phenylenediamine (PPD) containing cosmetic formulations have raised special concerns once it is considered to be a strong sensitizer and could initiate an allergic response, particularly by some specific oxidation products, such as p-benzoquinone diamine (BQDI), a product resulting from an oxidative transformation process of the initial PPD [11]. The complexity of the mixtures of different ACIs, with special regard not only to the more “problematic” aromatic amines but also to the huge quantity of other ACIs, leads to the necessity of further studies to fill the lack of information in this field of research. A recent study showed that the inflammatory mediator IL-1 alpha had its release augmented, suggesting skin irritation, after application of a mixture consisting of resorcinol, a coupler, PPD, an intermediate, and hydrogen peroxide, all of them commonly present on hair dye formulations. Moreover, it was verified a decrease in cell viability as well as an increase in skin cells apoptosis, epidermal damage, and impairment of the normal skin functions and properties, such as its pivotal natural barrier [12]. Studies in a dendritic cell model showed that the skin sensitizer PPD decreased membrane expression of certain receptors, more precisely chemokine receptors CCR6 and CXCR4. This way is able to impact the immune system, and dendritic cell migration and plays a key role in allergic contact dermatitis [13]. PPD allergy can also be acquired by cross-sensitization, in which other exposures for example latex products, local anesthetics such as benzocaine and procaine, other drugs such as sulfones and sulphonamides or p-aminobenzoic acid (PABA) used for example in sunscreens, are responsible for the installment of allergic symptomatology [14].
Toxicology assessment is one of the most relevant processes for the determination of deleterious health outcomes that can occur with hair dyes. Several studies are needed in order to conduct a toxicology assessment, as the exposure of the ACI to the skin could promote a possible percutaneous absorption and guide a systemic delivery and distribution of the ACI of interest, permeability, and exposition to the ACI, the period of time in which it will be contacted with the surface of the skin because the longer the time of contact, the greater could be the effects and the implications that could be triggered by the chemical, and all the formula ACIs, such as permanent dyeing ACIs long-term exposure [15]. Therefore, it is important to establish and test the ACIs by doing a safety assessment, due to their potential to interact and modify the activity and equilibrium of living systems, with some tests such as acute and sub-chronic systemic toxicity, local effects, sensitizing potential, mutagenicity, tumorigenicity, and teratogenicity. It is necessary to establish possible routes of entry and some relevant ACI’s characteristics, as a screening phase, that could provide important and useful data for the following studies.
3. Physicochemical Characteristics of NPs and Their Safety Testing
As far as the safety of NMs in cosmetic products is concerned, the first note that should be taken resides in the nanoscale issue. In fact, the NM’s incredibly small size can have an impact on numerous properties, when compared to its micro or normal size correspondent. A nanoscale material may not only have the physicochemical properties different, but also the biological ones, thus potentially affecting several domains such as quality, safety, effects, and activities. This way, it is comprehensible the possible lack of applicability and adequacy of the traditional testing assays normally used to test an ACI’s safety [16].
NP’s safety assessment is of utmost importance and despite the need for more studies and guidance to establish a definitive and robust safety assessment, the first major topic should be the assessment of its characteristics. Morphology and solubility can play a key role in immune cells’ uptake, as in the case of nanofibers, and dermal permeability respectively [17][18]. Both FDA and the Scientific Committee on Consumer Safety (SCCS) have provided useful information on the specific requirements and relevant testing methodologies for safety evaluation. Physicochemical characterization is perhaps the first to consider, allowing a thorough description of several useful information on the NMs, including its structural formula, purity degree, presence or absence of impurities, particle size measurement, aggregation, or agglomeration phenomena. Surface chemistry studies, such as zeta potential assessment and morphology parameters elucidation as shape and surface area are relevant, as well as solubility and stability. Once the chemical and physical properties have been properly evaluated, toxicological assessment is extremely important, concerning the routes of exposure, possible uptake and absorption, using a variety of in vitro or in vivo tests that best fit each case. Thereafter, FDA recommends at least, acute, repeated dose, and subcronic toxicity testing, skin and eye irritation testing, as well as genotoxicity or mutagenicity, skin sensitization, and dermal photoirritation testing [16]. Similarly, the SCCS, in the “Guidance on the safety assessment of NMs in cosmetics”, emphasizes the importance of characterization to identify and entail its particular properties and to detail its toxicological profile and safety issues, building upon previously studied physicochemical peculiarities. Physicochemical characterization can be summarized, according to the previously mentioned guidance, in the following Figure 1 representation [8].
 Figure 1. Physicochemical characterization for nanomaterials (NMs).
Figure 1. Physicochemical characterization for nanomaterials (NMs).Taking on the SCCS’s checklists for applicants submitting dossiers on ACIs to be evaluated by the SCCS, a final section is dedicated to addressing NMs principal assessments. Divided into three main “checklist” sections, offering a guide for the information previously mentioned and described characteristics, parameters, and general information regarding the NMs for cosmetic use, including in a cosmetic formulation, as seen summarily in Figure 2—checklist for NM’s characterization, hazard assessment as toxicological data and the checklist for information on exposure, exposure assessment that is adapted from the document, noting the importance of addressing a description regarding the raw material (the produced pristine NPs); the NPs in the finished cosmetic formulation and the NPs present under toxicological investigations and exposure assessment [19].
 Figure 2. Schematic summary of SCCS’s checklists for applicants submitting dossiers on ACIs. SCCS stands for: Scientific Committee on Consumer Safety. ACIs stands for: active cosmetic ingredients.
Figure 2. Schematic summary of SCCS’s checklists for applicants submitting dossiers on ACIs. SCCS stands for: Scientific Committee on Consumer Safety. ACIs stands for: active cosmetic ingredients.General studies for the safety of NMs, for example, a random search in vitro micronucleus assay [20], comet assay, and other tests are widely used to assess the toxicological profile of the NMs. Especially genotoxicity and cytotoxicity assays, several studies regarding zinc nanoparticles (ZnO), cosmetics, drugs, sensors [21][22][23], AgNPs [24], and gold nanoparticles (AuNP) [25], remark on the importance of the particle size and zeta potential on the overall toxicological contribution. Carbon NMs, including nanotubes and graphene [26][27] are also in deep focus, as well as silica nanoparticles [28], dendrimers [29], among others.
3.1. Exposure Assessment
NMs are widely used in products, such as cosmetics, so exposure and toxicity are important parameters to consider for their risk assessment [30]. It is required by the EU chemical legislation, acknowledged as Regulation on Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH), to do a consumer exposure assessment when there is a substance with a high hazard risk [31][32].
Nowadays, there is still no agreement regarding the methods to expect the consumer exposure assessment as mentioned above [33]. Predicting the consumer exposure assessment is difficult once it is required to know the nature, amount, exposure routes, and the intended use of the products, which makes it hard to monitor after the product is sold. For example, the application of hair dyes requires the use of gloves, but there is no way to monitor this after selling the product. This last example lead s to the dermal exposure which is the amount of ACIs that contact the exposed dermal surface [32]. The consumer’s exposure is expectable to be higher with NPs in a free form. There have been developed modeling tools to estimate the potential exposure [34]. Exposure assessments can be predicted using the NanoRisk framework, which was supported by two organizations, and consists of six steps, which are considered the physicochemical characterization of the NP, the defined life cycle, the assessment risks, and the assessment of risk management [35].
3.2. Safety Testing
Different studies can be encountered with regards to safety testing of metal-oxide NMs [36], carbon NMs [37][38], and gold NMs [39]. Delayed-type hypersensitivity is one of the reasons why ACIs are retired from the market. Testing methods to predict delayed-type hypersensivity were performed in a recent study, where several methods and strategies for testing engineered NMs were described and proposed, such as the human cell line activation test (hCLAT) and the myeloid U937 skin sensitization test (MUSST or U-SENS). Regarding the hCLAT and MUSST tests, the in vitro expression of surface markers CD54 and CD86 in THP-1 and U937 cell lines, respectively, was evaluated. The in vitro tests consist in calculating the expression of the surface marker by flow cytometry. The fixed minimum stimulation index was 3, so values above 3 are considered as having a positive stimulation. Generally, the MUSST test is more sensitive than the hCLAT [40].
The main definition of NM stated by the Cosmetics Regulation No. 1223/2009 considers it as a material that satisfies some requirements, such as its insolubility or bio persistence, made intentionally and “with one or more external dimensions, or an internal structure, on the scale of 1 to 100 nm” [41]. An NP is defined according to ISO/TS 80004:2015 as a nano-object “with all external dimensions in the nanoscale, where the lengths of the longest and the shortest axes of the nano-object do not differ significantly”. Nanofibers and nanoplates can be separated from this definition, once they have only two and one external dimension in the nanoscale, respectively [42]. There are several nanocarriers for nanocosmetic delivery, allowing the successful improvement of not only the cosmetic formulation’s properties at a completely different level but also toward a set of key-concepts such as toxicity, permeability, controlled-release, and efficacy. Therefore, the relevance of both the variety of the vehicles on display and the clear advantages of the more conventional normal sized forms, confers an attractiveness to the field of cosmetics, allowing the fusion of nanotechnology and cosmetology into one unique and enthralling concept.
Regarding the physicochemical characteristics of NMs, as described above, and the lack of safety testing evidence about these nanosized particles, it is important to perform more studies that also consider their long-term impact on safety. The following Table 1 presents a compilation with examples of models and testing performed on NPs for safety testing.
Table 1. Compilation of models and tests performed on NPs for safety testing.
| NPs | Study | Model | Test | Ref. |
|---|---|---|---|---|
| Iron Oxide and Ionic Iron NPs | Genotoxicity (in vivo) |
Earthworm Coelomocytes |
Comet Assay Micronucleus test |
[43] |
| Cerium Dioxide NPs | Genotoxicity (in vitro) |
Human peripheral blood lymphocytes |
Comet assay Micronucleus test Gamma H2AX |
[44] |
| Titanium Dioxide NPs | Genotoxicity | Human bronchial epithelial BEAS-2B cells | Mini-gel comet assay and micronucleus test |
[45] |
| AgNPs | Toxicity | Caenorhabditis elegans |
Population-based observations Gene expression analysis |
[46] |
| Multiwalled Carbon Nanotubes | ||||
| Dendrimers | ||||
| Micro-nano zinc oxide NPs | Cytotoxicity Genotoxicity Phototoxicity |
Human skin keratinocyte cells | Comet assay (geno) UV radiation (photo) NRU assay MTT assay Intracellular ROS determination JC-1 staining |
[47] |
| AgNPs | Toxicity | Zebrafish embryos |
NA | [48] |
| AuNPs | Genotoxicity (in vitro/in vivo) |
Drosophylla (in vivo) |
Comet assay SMART assay |
[49] |
| Fullerene | Genotoxicity (in vivo) |
Rat lung cells | Comet assay | [50] |
| Graphene | Toxicology | Caenorhabditis elegans | Transcriptomics Network-based pathway analysis | [51] |
| Indium-based quantum dot NPs | Biodistribution (in vivo) Toxicology |
Rat model | NA | [52] |
| Chitosan NPs | Toxicology (embryonic) |
Zebrafish | NA | [53] |
AgNP—silver nanoparticle; AuNP—gold nanoparticle; MTT—3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide; NA—not applicable; NP—nanoparticle; NRU—neutral red uptake; QSAR—quantitative structure—activity relationship; ROS—reactive oxygen species; SMART—single molecule analysis of resection tracks; UV—ultraviolet.
References
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.M.; Wepf, R.; et al. Nanoparticles—an efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164.
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 2018, 14, 1–12.
- Vazquez-Munoz, R.; Borrego, B.; Juarez-Moreno, K.; Garcia-Garcia, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20.
- Wang, Z.; Zhang, T.; Huang, F.; Wang, Z. The reproductive and developmental toxicity of nanoparticles: A bibliometric analysis. Toxicol. Ind. Health 2018, 34, 169–177.
- Andreo-Filho, N.; Bim, A.V.K.; Kaneko, T.M.; Kitice, N.A.; Haridass, I.N.; Abd, E.; Santos Lopes, P.; Thakur, S.S.; Parekh, H.S.; Roberts, M.S.; et al. Development and Evaluation of Lipid Nanoparticles Containing Natural Botanical Oil for Sun Protection: Characterization and in vitro and in vivo Human Skin Permeation and Toxicity. Ski. Pharmacol. Physiol. 2018, 31, 1–9.
- Schipper, O. Nanoparticle agglomeration restricts uptake into living cells. Environ. Sci. Technol. 2005, 39, 473A.
- Nystrom, A.M.; Fadeel, B. Safety assessment of nanomaterials: Implications for nanomedicine. J. Control. Release 2012, 161, 403–408.
- Scientific Committee on Consumer Safety. Guidance on the Safety Assessment of Nanomaterials in Cosmetics; European Commission: Brussels, Belgium, 2012.
- Yoshida, T.; Yoshioka, Y.; Tsutsumi, Y. Safety assessment of nanomaterials for development of nano-cosmetics. Yakugaku Zasshi n 2012, 132, 1231–1236.
- Saitta, P.; Cook, C.E.; Messina, J.L.; Brancaccio, R.; Wu, B.C.; Grekin, S.K.; Holland, J. Is There a True Concern Regarding the Use of Hair Dye and Malignancy Development? A Review of the Epidemiological Evidence Relating Personal Hair Dye Use to the Risk of Malignancy. Clin. Aesthet. Dermatol. 2013, 6, 8.
- Meyer, A.; Fischer, K. Oxidative transformation processes and products of para-phenylenediamine (PPD) and para-toluenediamine (PTD)—A review. Environ. Sci. Eur. 2015, 27, 11.
- Zanoni, T.B.; Pedrosa, T.N.; Catarino, C.M.; Spiekstra, S.W.; de Oliveira, D.P.; Den Hartog, G.; Bast, A.; Hagemann, G.; Gibbs, S.; de Moraes Barros, S.B.; et al. Allergens of permanent hair dyes induces epidermal damage, skin barrier loss and IL-1 alpha increase in epidermal in vitro model. Food Chem. Toxicol. 2018, 112, 265–272.
- Cruz, M.T.; Goncalo, M.; Paiva, A.; Morgado, J.M.; Figueiredo, A.; Duarte, C.B.; Lopes, M.C. Contact sensitizers downregulate the expression of the chemokine receptors CCR6 and CXCR4 in a skin dendritic cell line. Arch. Dermatol. Res. 2005, 297, 43–47.
- U.S. Food & Drug Administration. Hair Dyes. Available online: https://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm143066.htm (accessed on 20 March 2018).
- Bünger, H. Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2003.
- U.S. Department of Health and Human Services; FDA; Center for Food Safety and Applied Nutrition. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2014.
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910.
- Poland, C.A.; Duffin, R.; Donaldson, K. High Aspect Ratio Nanoparticles and the Fibre Pathogenicity Paradigm. In Nanotoxicity: From In Vivo and In Vitro Models to Health Risks; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 61–79.
- Scientific Committee on Consumer Safety SCCS. Checklists for Applicants Submitting Dossiers on Cosmetic Ingredients to be Evaluated by the SCCS; European Commission: Brussels, Belgium, 2017.
- Li, Y.; Doak, S.H.; Yan, J.; Chen, D.H.; Zhou, M.; Mittelstaedt, R.A.; Chen, Y.; Li, C.; Chen, T. Factors affecting the in vitro micronucleus assay for evaluation of nanomaterials. Mutagenesis 2017, 32, 151–159.
- Scherzad, A.; Meyer, T.; Kleinsasser, N.; Hackenberg, S. Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity Short Running Title: Genotoxicity of ZnO NPs. Materials 2017, 10, 1427.
- Senapati, V.A.; Kumar, A.; Gupta, G.S.; Pandey, A.K.; Dhawan, A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: A mechanistic approach. Food Chem. Toxicol. 2015, 85, 61–70.
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Milczarek, J.; Toma, M.; Korycinska, A.; Szemraj, J.; Sliwinski, T. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol. Mech. Methods 2015, 25, 176–183.
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239.
- George, J.M.; Magogotya, M.; Vetten, M.A.; Buys, A.V.; Gulumian, M. From the Cover: An Investigation of the Genotoxicity and Interference of Gold Nanoparticles in Commonly Used In Vitro Mutagenicity and Genotoxicity Assays. Toxicol. Sci. 2017, 156, 149–166.
- Asghar, W.; Shafiee, H.; Velasco, V.; Sah, V.R.; Guo, S.; El Assal, R.; Inci, F.; Rajagopalan, A.; Jahangir, M.; Anchan, R.M.; et al. Toxicology Study of Single-walled Carbon Nanotubes and Reduced Graphene Oxide in Human Sperm. Sci. Rep. 2016, 6, 30270.
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144.
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010.
- Naha, P.C.; Mukherjee, S.P.; Byrne, H.J. Toxicology of Engineered Nanoparticles: Focus on Poly(amidoamine) Dendrimers. Int. J. Environ. Res. Public Health 2018, 15, 338.
- Zhao, P.; Zhang, Y. The Overview of Methods of Nanoparticle Exposure Assessment. Methods Mol. Biol. 2019, 1894, 353–367.
- Chawla, J.; Singh, D.; Sundaram, B.; Kumar, A. Identifying Challenges in Assessing Risks of Exposures of Silver Nanoparticles. Expo. Health 2017, 10, 61–75.
- Mackevica, A.; Foss Hansen, S. Release of nanomaterials from solid nanocomposites and consumer exposure assessment—A forward-looking review. Nanotoxicology 2016, 10, 641–653.
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979.
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513.
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376.
- Landsiedel, R.; Ma-Hock, L.; Kroll, A.; Hahn, D.; Schnekenburger, J.; Wiench, K.; Wohlleben, W. Testing metal-oxide nanomaterials for human safety. Adv. Mater. 2010, 22, 2601–2627.
- Yamashita, T.; Yamashita, K.; Nabeshi, H.; Yoshikawa, T.; Yoshioka, Y.; Tsunoda, S.I.; Tsutsumi, Y. Carbon Nanomaterials: Efficacy and Safety for Nanomedicine. Materials 2012, 5, 350–363.
- Coccini, T.; Manzo, L.; Roda, E. Safety evaluation of engineered nanomaterials for health risk assessment: An experimental tiered testing approach using pristine and functionalized carbon nanotubes. ISRN Toxicol. 2013, 2013, 825427.
- Cao, M.; Li, J.; Tang, J.; Chen, C.; Zhao, Y. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses, Characterization, and Dermal Safety Assessment. Small 2016, 12, 5488–5496.
- Potter, T.M.; Neun, B.W.; Dobrovolskaia, M.A. In Vitro and In Vivo Methods for Analysis of Nanoparticle Potential to Induce Delayed-Type Hypersensitivity Reactions. Methods Mol. Biol. 2018, 1682, 197–210.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223&from=PT (accessed on 20 February 2018).
- ISO. ISO/TS 80004-2:2015. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-2:ed-1:v1:en (accessed on 16 April 2018).
- Cigerci, I.H.; Ali, M.M.; Kaygisiz, S.Y.; Kaya, B.; Liman, R. Genotoxic Assessment of Different Sizes of Iron Oxide Nanoparticles and Ionic Iron in Earthworm (Eisenia hortensis) Coelomocytes by Comet Assay and Micronucleus Test. Bull. Environ. Contam. Toxicol. 2018, 101, 105–109.
- Konen-Adiguzel, S.; Ergene, S. In vitro evaluation of the genotoxicity of CeO2 nanoparticles in human peripheral blood lymphocytes using cytokinesis-block micronucleus test, comet assay, and gamma H2AX. Toxicol. Ind. Health 2018, 34, 293–300.
- Di Bucchianico, S.; Cappellini, F.; Le Bihanic, F.; Zhang, Y.; Dreij, K.; Karlsson, H.L. Genotoxicity of TiO2 nanoparticles assessed by mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 2017, 32, 127–137.
- Walczynska, M.; Jakubowski, W.; Wasiak, T.; Kadziola, K.; Bartoszek, N.; Kotarba, S.; Siatkowska, M.; Komorowski, P.; Walkowiak, B. Toxicity of silver nanoparticles, multiwalled carbon nanotubes, and dendrimers assessed with multicellular organism Caenorhabditis elegans. Toxicol. Mech. Methods 2018, 28, 432–439.
- Genc, H.; Barutca, B.; Koparal, A.T.; Ozogut, U.; Sahin, Y.; Suvaci, E. Biocompatibility of designed MicNo-ZnO particles: Cytotoxicity, genotoxicity and phototoxicity in human skin keratinocyte cells. Toxicol. Vitr. 2018, 47, 238–248.
- Lee, W.S.; Kim, E.; Cho, H.J.; Kang, T.; Kim, B.; Kim, M.Y.; Kim, Y.S.; Song, N.W.; Lee, J.S.; Jeong, J. The Relationship between Dissolution Behavior and the Toxicity of Silver Nanoparticles on Zebrafish Embryos in Different Ionic Environments. Nanomaterials 2018, 8, 652.
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. In vitro and in vivo genotoxicity assessment of gold nanoparticles of different sizes by comet and SMART assays. Food Chem. Toxicol. 2018, 120, 81–88.
- Ema, M.; Tanaka, J.; Kobayashi, N.; Naya, M.; Endoh, S.; Maru, J.; Hosoi, M.; Nagai, M.; Nakajima, M.; Hayashi, M.; et al. Genotoxicity evaluation of fullerene C60 nanoparticles in a comet assay using lung cells of intratracheally instilled rats. Regul. Toxicol. Pharmacol. 2012, 62, 419–424.
- Chatterjee, N.; Kim, Y.; Yang, J.; Roca, C.P.; Joo, S.W.; Choi, J. A systems toxicology approach reveals the Wnt-MAPK crosstalk pathway mediated reproductive failure in Caenorhabditis elegans exposed to graphene oxide (GO) but not to reduced graphene oxide (rGO). Nanotoxicology 2017, 11, 76–86.
- Yaghini, E.; Turner, H.; Pilling, A.; Naasani, I.; MacRobert, A. In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomedicine 2018, 14, 2644–2655.
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr. Polym. 2016, 141, 204–210.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
25 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





