Nanomaterials use in cosmetics is markedly enhancing, so their exposure and toxicity are
important parameters to consider for their risk assessment. This review article provides an overview
of the active cosmetic ingredients used for cosmetic application, including dermal cosmetics and
also hair dye cosmetics, as well as their safety assessment, enriched with a compilation of the safety
assessment tests available to evaluate the different types of toxicity. In fact, despite the increase
in research and the number of papers published in the field of nanotechnology, the related safety
assessment is still insufficient. To elucidate the possible effects that nanosized particles can have
on living systems, more studies reproducing similar conditions to what happens in vivo should be
conducted, particularly considering the complex interactions of the biological systems and active
cosmetic ingredients to achieve newer, safer, and more efficient nanomaterials. Toward this end,
ecological issues and the toxicological pattern should also be a study target.
- nanomaterial
- safety assessment test
- toxicology
- genotoxicity
- hair dye cosmetic
1. Introduction
2. Hair Formulations and Their Impact on Living Systems
The emerging formulations and marketing of hair dyes brought some concerns regarding the possibility of certain ACIs contained in these formulations causing deleterious health outcomes. Of note is the case of the permanent dyes 2-nitro-p-phenylenediamine and 4-amino-2-nytrophenol, among others, that were appointed as responsible for inducing carcinogenic activity on tested laboratory animals. They manifested the capability of being able to permeate the dermal barrier on humans, thereafter, culminating in the removal of these molecules from the definitive formulation of hair colorants by the Food and Drug Administration (FDA) and their substitution by other chemically similar molecules with safer properties [10][11]. p-phenylenediamine (PPD) containing cosmetic formulations have raised special concerns once it is considered to be a strong sensitizer and could initiate an allergic response, particularly by some specific oxidation products, such as p-benzoquinone diamine (BQDI), a product resulting from an oxidative transformation process of the initial PPD [11][12]. The complexity of the mixtures of different ACIs, with special regard not only to the more “problematic” aromatic amines but also to the huge quantity of other ACIs, leads to the necessity of further studies to fill the lack of information in this field of research. A recent study showed that the inflammatory mediator IL-1 alpha had its release augmented, suggesting skin irritation, after application of a mixture consisting of resorcinol, a coupler, PPD, an intermediate, and hydrogen peroxide, all of them commonly present on hair dye formulations. Moreover, it was verified a decrease in cell viability as well as an increase in skin cells apoptosis, epidermal damage, and impairment of the normal skin functions and properties, such as its pivotal natural barrier [12][13]. Studies in a dendritic cell model showed that the skin sensitizer PPD decreased membrane expression of certain receptors, more precisely chemokine receptors CCR6 and CXCR4. This way is able to impact the immune system, and dendritic cell migration and plays a key role in allergic contact dermatitis [13][14]. PPD allergy can also be acquired by cross-sensitization, in which other exposures for example latex products, local anesthetics such as benzocaine and procaine, other drugs such as sulfones and sulphonamides or p-aminobenzoic acid (PABA) used for example in sunscreens, are responsible for the installment of allergic symptomatology [14][15]. Toxicology assessment is one of the most relevant processes for the determination of deleterious health outcomes that can occur with hair dyes. Several studies are needed in order to conduct a toxicology assessment, as the exposure of the ACI to the skin could promote a possible percutaneous absorption and guide a systemic delivery and distribution of the ACI of interest, permeability, and exposition to the ACI, the period of time in which it will be contacted with the surface of the skin because the longer the time of contact, the greater could be the effects and the implications that could be triggered by the chemical, and all the formula ACIs, such as permanent dyeing ACIs long-term exposure [15][16]. Therefore, it is important to establish and test the ACIs by doing a safety assessment, due to their potential to interact and modify the activity and equilibrium of living systems, with some tests such as acute and sub-chronic systemic toxicity, local effects, sensitizing potential, mutagenicity, tumorigenicity, and teratogenicity. It is necessary to establish possible routes of entry and some relevant ACI’s characteristics, as a screening phase, that could provide important and useful data for the following studies.3. Physicochemical Characteristics of NPs and Their Safety Testing
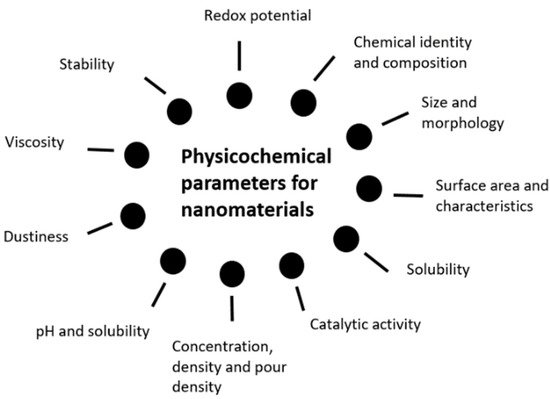
As far as the safety of NMs in cosmetic products is concerned, the first note that should be taken resides in the nanoscale issue. In fact, the NM’s incredibly small size can have an impact on numerous properties, when compared to its micro or normal size correspondent. A nanoscale material may not only have the physicochemical properties different, but also the biological ones, thus potentially affecting several domains such as quality, safety, effects, and activities. This way, it is comprehensible the possible lack of applicability and adequacy of the traditional testing assays normally used to test an ACI’s safety [16][17]. NP’s safety assessment is of utmost importance and despite the need for more studies and guidance to establish a definitive and robust safety assessment, the first major topic should be the assessment of its characteristics. Morphology and solubility can play a key role in immune cells’ uptake, as in the case of nanofibers, and dermal permeability respectively [17][18][18,19]. Both FDA and the Scientific Committee on Consumer Safety (SCCS) have provided useful information on the specific requirements and relevant testing methodologies for safety evaluation. Physicochemical characterization is perhaps the first to consider, allowing a thorough description of several useful information on the NMs, including its structural formula, purity degree, presence or absence of impurities, particle size measurement, aggregation, or agglomeration phenomena. Surface chemistry studies, such as zeta potential assessment and morphology parameters elucidation as shape and surface area are relevant, as well as solubility and stability. Once the chemical and physical properties have been properly evaluated, toxicological assessment is extremely important, concerning the routes of exposure, possible uptake and absorption, using a variety of in vitro or in vivo tests that best fit each case. Thereafter, FDA recommends at least, acute, repeated dose, and subcronic toxicity testing, skin and eye irritation testing, as well as genotoxicity or mutagenicity, skin sensitization, and dermal photoirritation testing [16][17]. Similarly, the SCCS, in the “Guidance on the safety assessment of NMs in cosmetics”, emphasizes the importance of characterization to identify and entail its particular properties and to detail its toxicological profile and safety issues, building upon previously studied physicochemical peculiarities. Physicochemical characterization can be summarized, according to the previously mentioned guidance, in the following Figure 1 representation [8].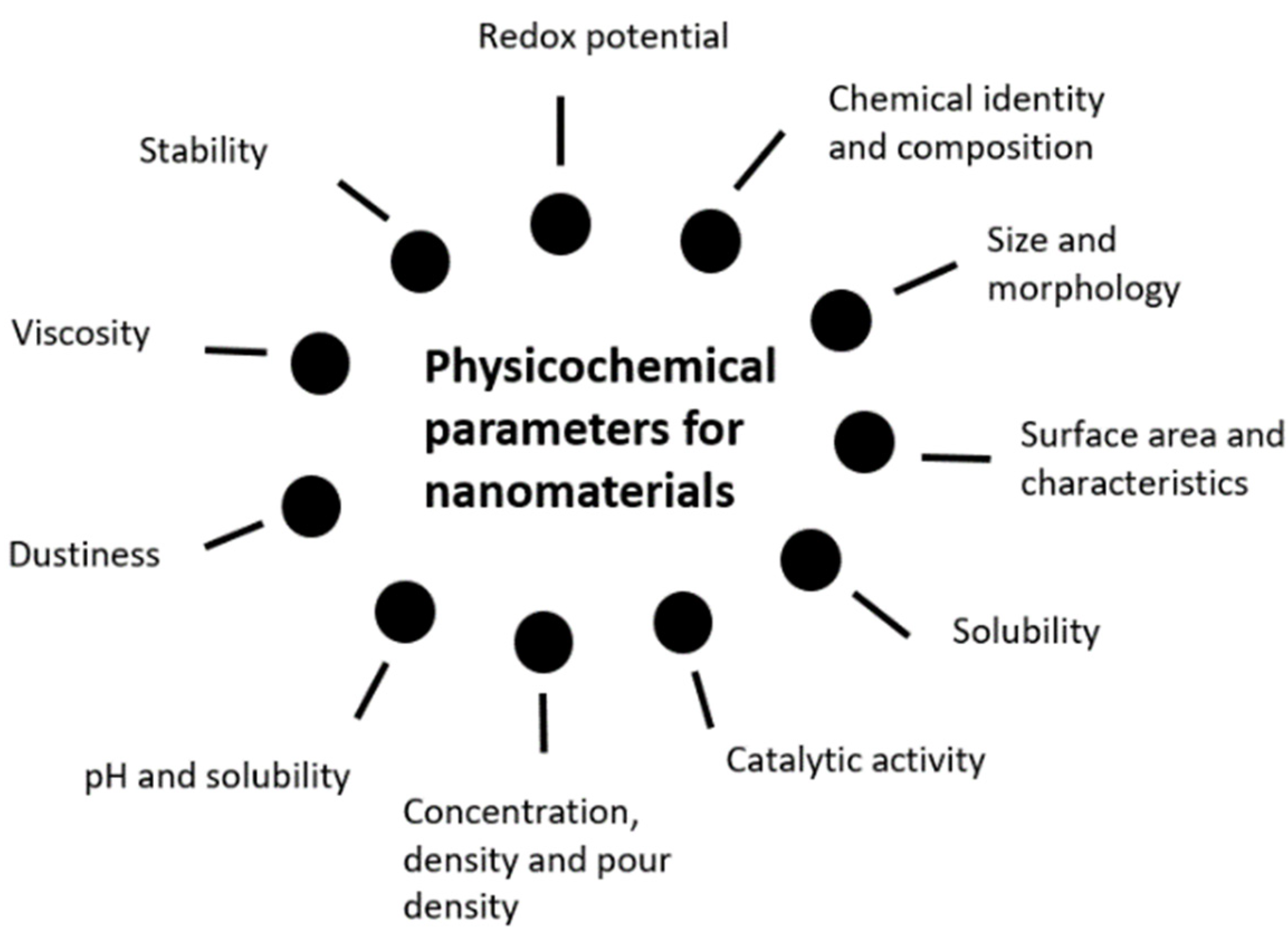
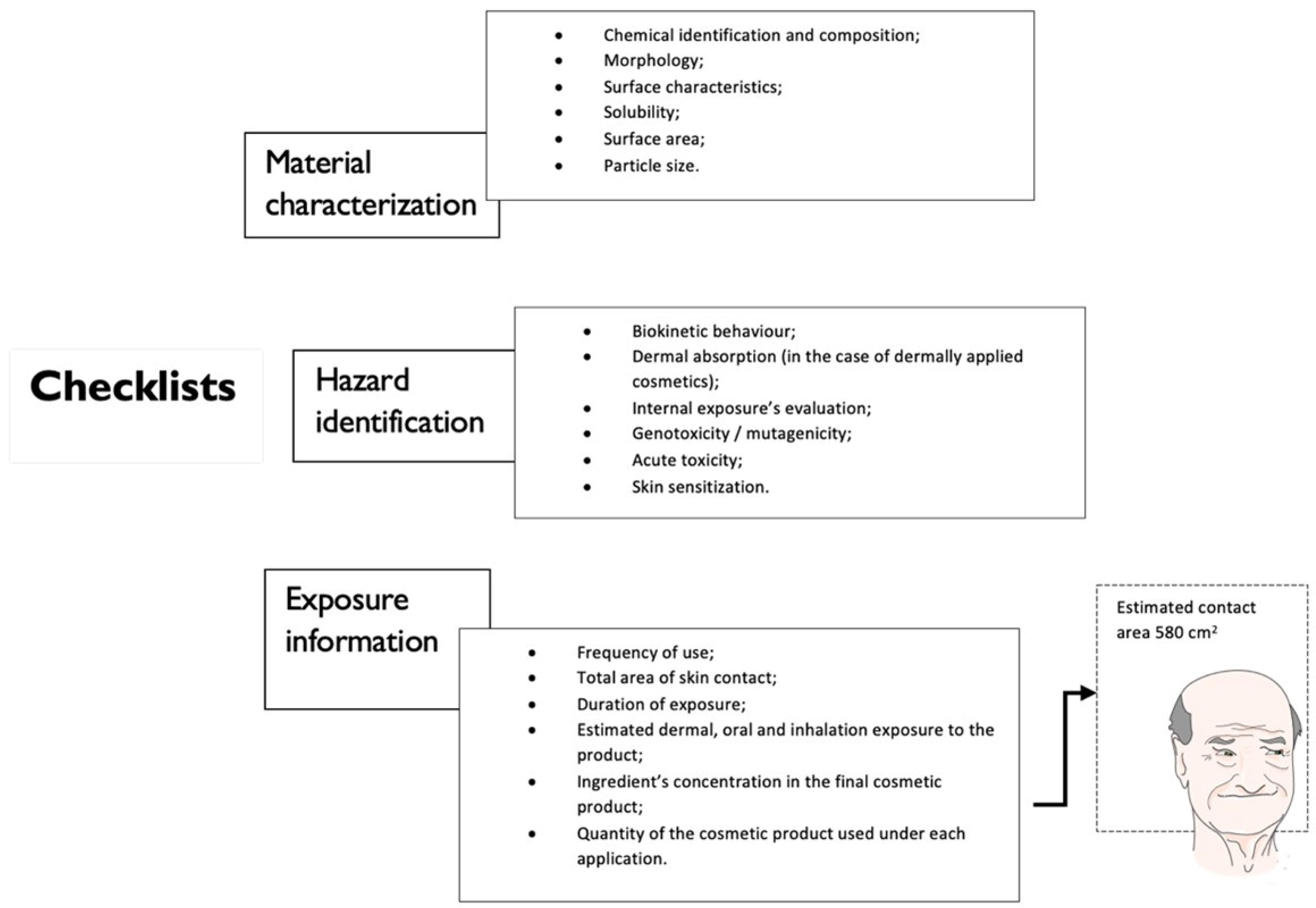
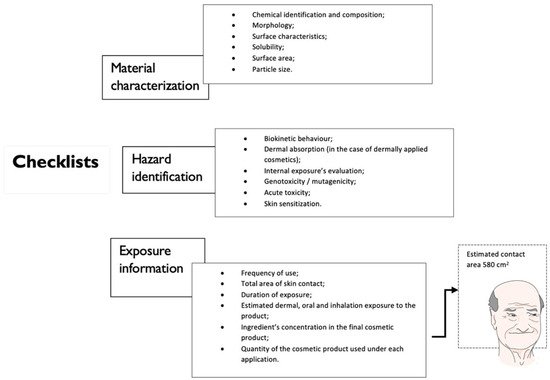
3.1. Exposure Assessment
NMs are widely used in products, such as cosmetics, so exposure and toxicity are important parameters to consider for their risk assessment [30][31]. It is required by the EU chemical legislation, acknowledged as Regulation on Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH), to do a consumer exposure assessment when there is a substance with a high hazard risk [31][32][32,33]. Nowadays, there is still no agreement regarding the methods to expect the consumer exposure assessment as mentioned above [33][34]. Predicting the consumer exposure assessment is difficult once it is required to know the nature, amount, exposure routes, and the intended use of the products, which makes it hard to monitor after the product is sold. For example, the application of hair dyes requires the use of gloves, but there is no way to monitor this after selling the product. This last example leads us to the dermal exposure which is the amount of ACIs that contact the exposed dermal surface [32][33]. The consumer’s exposure is expectable to be higher with NPs in a free form. There have been developed modeling tools to estimate the potential exposure [34][35]. Exposure assessments can be predicted using the NanoRisk framework, which was supported by two organizations, and consists of six steps, which are considered the physicochemical characterization of the NP, the defined life cycle, the assessment risks, and the assessment of risk management [35][36].3.2. Safety Testing
Different studies can be encountered with regards to safety testing of metal-oxide NMs [36][37], carbon NMs [37][38][38,39], and gold NMs [39][40]. Delayed-type hypersensitivity is one of the reasons why ACIs are retired from the market. Testing methods to predict delayed-type hypersensivity were performed in a recent study, where several methods and strategies for testing engineered NMs were described and proposed, such as the human cell line activation test (hCLAT) and the myeloid U937 skin sensitization test (MUSST or U-SENS). Regarding the hCLAT and MUSST tests, the in vitro expression of surface markers CD54 and CD86 in THP-1 and U937 cell lines, respectively, was evaluated. The in vitro tests consist in calculating the expression of the surface marker by flow cytometry. The fixed minimum stimulation index was 3, so values above 3 are considered as having a positive stimulation. Generally, the MUSST test is more sensitive than the hCLAT [40][41]. The main definition of NM stated by the Cosmetics Regulation No. 1223/2009 considers it as a material that satisfies some requirements, such as its insolubility or bio persistence, made intentionally and “with one or more external dimensions, or an internal structure, on the scale of 1 to 100 nm” [41][42]. An NP is defined according to ISO/TS 80004:2015 as a nano-object “with all external dimensions in the nanoscale, where the lengths of the longest and the shortest axes of the nano-object do not differ significantly”. Nanofibers and nanoplates can be separated from this definition, once they have only two and one external dimension in the nanoscale, respectively [42][43]. There are several nanocarriers for nanocosmetic delivery, allowing the successful improvement of not only the cosmetic formulation’s properties at a completely different level but also toward a set of key-concepts such as toxicity, permeability, controlled-release, and efficacy. Therefore, the relevance of both the variety of the vehicles on display and the clear advantages of the more conventional normal sized forms, confers an attractiveness to the field of cosmetics, allowing the fusion of nanotechnology and cosmetology into one unique and enthralling concept. Regarding the physicochemical characteristics of NMs, as described above, and the lack of safety testing evidence about these nanosized particles, it is important to perform more studies that also consider their long-term impact on safety. The following Table 1 presents a compilation with examples of models and testing performed on NPs for safety testing.| NPs | Study | Model | Test | Ref. | |
|---|---|---|---|---|---|
| Iron Oxide and Ionic Iron NPs | Genotoxicity (in vivo) |
Earthworm Coelomocytes |
Comet Assay Micronucleus test |
[43] | [44] |
| Cerium Dioxide NPs | Genotoxicity (in vitro) |
Human peripheral blood lymphocytes |
Comet assay Micronucleus test Gamma H2AX |
[44] | [45] |
| Titanium Dioxide NPs | Genotoxicity | Human bronchial epithelial BEAS-2B cells | Mini-gel comet assay and micronucleus test |
[45] | [46] |
| AgNPs | Toxicity | Caenorhabditis elegans |
Population-based observations Gene expression analysis |
[46] | [47] |
| Multiwalled Carbon Nanotubes | |||||
| Dendrimers | |||||
| Micro-nano zinc oxide NPs | Cytotoxicity Genotoxicity Phototoxicity |
Human skin keratinocyte cells | Comet assay (geno) UV radiation (photo) NRU assay MTT assay Intracellular ROS determination JC-1 staining |
[47] | [48] |
| AgNPs | Toxicity | Zebrafish embryos |
NA | [48] | [49] |
| AuNPs | Genotoxicity (in vitro/in vivo) |
Drosophylla (in vivo) |
Comet assay SMART assay |
[49] | [50] |
| Fullerene | Genotoxicity (in vivo) |
Rat lung cells | Comet assay | [50] | [51] |
| Graphene | Toxicology | Caenorhabditis elegans | Transcriptomics Network-based pathway analysis | [51] | [52] |
| Indium-based quantum dot NPs | Biodistribution (in vivo) Toxicology |
Rat model | NA | [52] | [53] |
| Chitosan NPs | Toxicology (embryonic) |
Zebrafish | NA | [53] | [54] |
