Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Mohammed | -- | 1346 | 2022-08-19 05:31:19 | | | |
| 2 | Sirius Huang | Meta information modification | 1346 | 2022-08-19 06:21:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alaerjani, W.M.A.; Mohammed, M.E.A. Enzymatic Reactions in Honey. Encyclopedia. Available online: https://encyclopedia.pub/entry/26305 (accessed on 28 February 2026).
Alaerjani WMA, Mohammed MEA. Enzymatic Reactions in Honey. Encyclopedia. Available at: https://encyclopedia.pub/entry/26305. Accessed February 28, 2026.
Alaerjani, Wed Mohammed Ali, Mohammed Elimam Ahamed Mohammed. "Enzymatic Reactions in Honey" Encyclopedia, https://encyclopedia.pub/entry/26305 (accessed February 28, 2026).
Alaerjani, W.M.A., & Mohammed, M.E.A. (2022, August 19). Enzymatic Reactions in Honey. In Encyclopedia. https://encyclopedia.pub/entry/26305
Alaerjani, Wed Mohammed Ali and Mohammed Elimam Ahamed Mohammed. "Enzymatic Reactions in Honey." Encyclopedia. Web. 19 August, 2022.
Copy Citation
Honey is known for its content of biomolecules, such as enzymes. The enzymes of honey originate from bees, plant nectars, secretions or excretions of plant-sucking insects, or microorganisms such as yeasts. Honey can be characterized by enzyme-catalyzed and non-enzymatic reactions. Notable examples of enzyme-catalyzed reactions are the production of hydrogen peroxide through glucose oxidase activity and the conversion of hydrogen peroxide to water and oxygen by catalase enzymes.
glucose oxidase
catalase
carbohydrates
amino acids
honey
1. Introduction
Honey is a sweet honeybee product which is mainly composed of sugars and water. Biomedical activities of honey are mostly due to its minor components, which include proteins, amino acids, organic acids, dicarbonyl molecules, hydrogen peroxide, phenolic acids, flavonoids, and enzymes [1][2][3]. Honey enzymes originate from three major sources: plant nectars and secretions, honeybees, and excretions of plant-sucking insects. Biochemical reactions can be divided to two types: enzyme-catalyzed and non-enzymatic reactions [4]. Enzyme-catalyzed reactions in honey are known to affect its quality and biological activities [5][6][7]. Enzymes present in honey include the diastase, invertase, glucose oxidase, catalase, glucosylceramidase, α-amylase, α-glucosidase, β-glucosidase, and proteases [6][8].
Enzymatic reactions in honey include the conversion of oligosaccharides and disaccharides (sucrose and maltose) to glucose and fructose by diastase and invertase enzyme activity. Glucose is converted to gluconic acid and hydrogen peroxide by glucose oxidase. Moreover, hydrogen peroxide is degraded to water and oxygen by catalase enzymes. Honey samples which exhibit high catalase activity are low in hydrogen peroxide [9]. Honey samples with high hydrogen peroxide concentrations are known to be useful for the treatment of wounds, and are characterized by a high activity of glucose oxidase and low activity of catalase [10]. Furthermore, honey and other honeybee products are characterized by enzyme-catalyzed reactions such as the proteases, glucosylceramidase and acid phosphatase [6][11].
2. Production and Degradation of Hydrogen Peroxide
Hydrogen peroxide is produced as a product of glucose oxidation by glucose oxidase and by the non-enzymatic autoxidation of polyphenols [12][13]. Glucose oxidase is secreted by honeybees; some studies have reported its production by plants, honey fungi, yeast, and bacteria [14][15][16][17]. Glucose oxidase catalyzes the conversion of glucose to gluconic acid and hydrogen peroxide, using molecular oxygen and vitamin B2 as cofactors (FAD) (Figure 1).
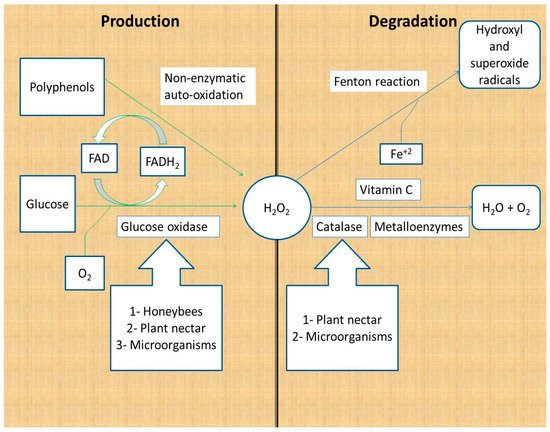
Figure 1. Production and degradation of hydrogen peroxide in honey. Hydrogen peroxide is produced from glucose by the action of glucose oxidase and non-enzymatically by polyphenols. Hydrogen peroxide is degraded to water and oxygen by enzymes and vitamin C, whereas it is degraded to hydroxyl and superoxide radicals through the Fenton reaction [12][13][14][15][16][17][18][19].
Honey hydrogen peroxide is degraded by enzymatic and non-enzymatic reactions. The major catabolic enzyme of hydrogen peroxide is Fe+3-dependent catalase, which catalyzes the conversion of two H2O2 molecules to two water molecules and one oxygen molecule. Plant nectar and the microorganisms in honey are the major sources of catalase in honey [12][18][19]. Other metal-containing enzymes (metalloenzymes) are capable of converting hydrogen peroxide to water following a different mechanism of action. Metalloenzymes include peroxidases and superoxide dismutase (Figure 1) [20].
Non-enzymatic reactions responsible for the degradation of hydrogen peroxide are the vitamin C and Fenton reactions. Vitamin C (hydro ascorbic acid) donates two protons and two electrons to hydrogen peroxide, reducing it to water and oxygen. It is reported that the addition of vitamin C to honey samples leads to decreased hydrogen peroxide levels, which reinforces the suggestion that vitamin C degrades hydrogen peroxide [12][21]. The second non-enzymatic reaction that depletes hydrogen peroxide from honey is the Fenton reaction. Fenton reactions involve the reaction of hydrogen peroxide with Fe+2 and/or polyphenols to produce hydroxide ions, oxygen, and hydroxyl and superoxide radicals. The hydroxyl and superoxide free radicals contribute to the antibacterial activity of honey because of their powerful damaging effect on the bacterial cells and DNA (Figure 1) [22][23].
Hydrogen peroxide generation in honey is influenced by its colloidal structure, which is due to the interactions between its macromolecules. Macromolecules in honey involved in the formation of the colloidal structure include oligo-sugars, proteins, and polyphenols. Colloidal particles of honey are composed of complexes of proteins, polyphenols, and melanoidins. The colloids of honey are compact and stable, with multiple layers. Colloidal honey samples are characterized by the generation of hydrogen peroxide, and antibiotic and antioxidant activities. Moreover, the colloidal structure of honey increases in dark and medium-color honeys, whereas it is not a character of the light color honeys [12][24].
Most of the antibacterial activity of honeys is due to the hydrogen peroxide concentration, high sugar concentration, and the low pH, due to the content of organic acids in honey, such as gluconic acid. Hydrogen-peroxide-producing honeys are used in wound and burn dressings, either alone or in combination with other medicines such as calcium alginate [12][24][25] (Table 1).
Table 1. Honey reactions and their biological activities.
| Reaction | Enzyme | Products | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| 1 | Production of hydrogen peroxide | Glucose oxidase | Hydrogen peroxide and gluconic acid | Antibacterial and wound and burn dressings | [12][24][25] |
| 2 | Production of short peptides | Proteases | Short peptides | Antimicrobial, antioxidant, antitumor and weight loss inducers | [26][27][28][29][30][31] |
| 3 | Degradation of amylose | Diastase | Glucose and maltose | Honey quality parameter that indicates storage conditions | [1] |
| 4 | Degradation of sucrose | Invertase | Glucose and fructose | Indicator for honey storage conditions | [32] |
| 5 | Degradation of organic phosphates | Acid phosphatase | Inorganic phosphate | Marker of honey floral origin and indicator of honey fermentation | [33][34][35] |
| 6 | Trans-glycosylation | Non-enzymatic | Disaccharides Oligosacharides |
Artificial sweeteners, increase bone mineral density in postmenopausal women (fructooligosaccharides), and classified as prebiotic molecules |
[36][37][38][39] |
| 7 | Production and degradation of dicarbonyls | Enzymatic (dihydroxyacetone phosphatase) and non-enzymatic | Dicarbonyls (glyoxal, methylglyoxal and 3-deoxyglucosone), methional and methylbutanal, AGEs and nucleoside AGEs and melanoidins | 1-Antibacterial, antitumor, antioxidant and contribution to the honey color, flavor and odorant. 2-High level of dicarbonyl molecules are reported to be with some toxicity to the humans such as tumerigenic, negative impact on blood vessels and induction of diabtes and uremia |
[40][41][42][43][44][45][46][47][48] |
| 8 | Production and degradation of HMF | Non-enzymatic | HMF, formic and levulinic acids, soluble HMF polymers and insoluble humins | Threatening honeybee life, human hepatorenal toxicity, induction of neoplastic changes, and irritation of mucous membranes HMF has positive impacts on human health, such as antioxidant, anti-carcinogen, anti-allergenic, and anti-hyperuricemic activities |
[49][50][51][52][53][54][55][56] |
| 9 | Maillard reaction | Non-enzymatic | Complexes of sugars and amino acids, amino aldoses and ketoses, dicarbonyls, enediols, 2-amino-2-deoxy-ald-oses and melanoidins | Antibacterial and antioxidant | [57][58][59][60][61] |
| 10 | Caramelization | Non-enzymatic | Deoxyosones, furan and pyran derivatives, HMF, hydroxydimethylfuranone (HDF) and hydroxyacetilfuran (HAF) | Contribution to the color, aroma, and flavor of honey and antioxidants | [62][63][64][65] |
3. Proteases
Proteases are responsible for the degradation of proteins to produce amino acids and short peptides according to the type of protease. According to their substrate preference, proteases are classified as endopeptidases and exopeptidases [66][67]. Unifloral honey samples are reported to contain serine protease enzymes, which contribute to their quality and biological activities [6]. A Croatian study reported the presence of three serine proteases in honey: trypsin, chymotrypsin, and elastase [33]. Trypsin cleaves the peptide bonds formed by the carboxyl groups of arginine and lysine [68]. The sites of chymotrypsin cleavage are peptide bonds formed by the carboxyl groups of aromatic amino acids (tyrosine, phenylalanine, and tryptophan) and leucine [69]. Elastase is associated with breakdown of the peptide bonds formed by the short aliphatic amino acids such as glycine, alanine, and valine [70]. The major products of honey proteases are short peptides that function as antioxidants, antitumor, and antimicrobials, and are used as weight loss inducers (Figure 2). Alaerjani et al. (2021) published an article which proved the presence of short peptides in honey samples from Saudi Arabia. They analyzed five honey samples using LC–MS and concluded that short peptides in honey samples are of floral origin and storage-dependent [26] (Table 1).
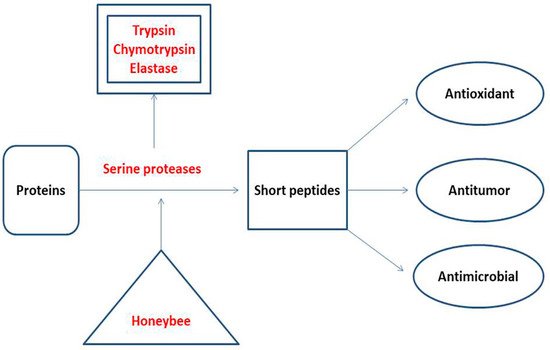
Amino acids and their sequences in short peptides are responsible for their activity. Short peptides that contain cysteine, methionine, tyrosine, lysine, histidine, and tryptophan are known to act as antioxidants [27][28][29]. Short peptides rich in hydrophobic amino acids, such as glysine, alanine, valine, leucine, and isoleucine, are active antimicrobial peptides because of their ability to disrupt the plasma membrane of the microbes [26][30]. Short and cyclic peptides are active as antitumor, antimicrobial, antioxidant, and weight loss inducers, according to their amino acid contents and sequences [31]. Assessments of the amino acid contents, sequences, and concentrations of short peptides in food are carried out using LC–MS techniques [26][71] (Table 1).
References
- Codex Alimentarius. International Food Standards. CXS 12-1981. Standard for Honey 1981. Revised 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252Fcxs_012e.pdf (accessed on 17 July 2022).
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124.
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323.
- Erkmen, O.; Bozoglu, T.F. Enzymatic and Nonenzymatic Food Spoilage. In Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 401–406.
- Wang, J.; Li, Q.X. Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geographical Origins. Adv. Food Nutr. Res. 2011, 62, 89–137.
- Rossano, R.; LaRocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What Are the Proteolytic Enzymes of Honey and What They Do Tell Us? A Fingerprint Analysis by 2-D Zymography of Unifloral Honeys. PLoS ONE 2012, 7, e49164.
- Utzeri, V.J.; Schiavo, G.; Ribani, A.; Tinarelli, S.; Bertolini, F.; Bovo, S.; Fontanesi, L. Entomological signatures in honey: An environmental DNA metabarcoding approach can disclose information on plant-sucking insects in agricultural and forest landscapes. Sci. Rep. 2018, 8, 996.
- Borutinskaite, V.; Treigyte, G.; Čeksteryte, V.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J. Food Nutr. Res. 2018, 57, 57–69.
- Nolan, V.C.; Harrison, J.; Cox, J.A. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251.
- Bizerra, F.C.; Da Silva, P.I.; Hayashi, M.A.F. Exploring the antibacterial properties of honey and its potential. Front. Microbiol. 2012, 3, 398.
- Barboni, E.; Kemeny, D.; Campos, S.; Vernon, C. The purification of acid phosphatase from honey bee venom (Apis mellifica). Toxicon 1987, 25, 1097–1103.
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229.
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2018, 278, 692–699.
- Carter, C.; Thornburg, R.W. Tobacco Nectarin I. J. Biol. Chem. 2000, 275, 36726–36733.
- Carter, C.J.; Thornburg, R.W. Tobacco Nectarin V Is a Flavin-Containing Berberine Bridge Enzyme-Like Protein with Glucose Oxidase Activity. Plant Physiol. 2004, 134, 460–469.
- Silva, M.S.; Rabadzhiev, Y.; Eller, M.R.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in Honey; Intech Open: Vienna, Austria, 2017.
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448.
- Brudzynski, K.; Abubaker, K.; Laurent, M.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213.
- Gillette, C.C. Honey catalase. J. Econ. Entomol. 1931, 24, 605–606.
- Zámocký, M.; Gasselhuber, B.; Furtmüller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144.
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992.
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 36.
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.; Berghe, D.V. Structure-Activity Relationship of Flavonoids as Antioxidant and Pro-Oxidant Compounds. Stud. Nat. Prod. Chem. 2000, 22, 307–341.
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637.
- Hossain, L.; Lim, L.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159.
- Alaerjani, W.M.A.; Abu-Melha, S.A.; Khan, K.A.; Ghramh, H.A.; Alalmie, A.Y.A.; Alshareef, R.M.H.; Al-Shehri, B.M.; Mohammed, M.E.A. Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values. Open Chem. 2021, 19, 1162–1173.
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure–activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496.
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245.
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547.
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Rev. Nanomed. Nanobiotechnol. 2012, 5, 49–66.
- Joo, S.-H. Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2012, 20, 19–26.
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards. European Honey Standards 1999. Available online: https://www.apiservices.biz/en/articles/sort-by-date-up-online/768-honey-quality-and-international-regulatory-standards-1999 (accessed on 17 July 2022).
- Aušević, B.; Haurdic, B.; Jašić, M.; Bašić, M. Enzymatic activities in honey. In Proceedings of the Second Conference of Bee Keeping and Bee Products- with International Participation- Bee Keeping and Bee Products, Gradačac, Bosnia and Herzegovina, 20 August 2017; pp. 90–97.
- Alonso-Torre, S.; Cavia, M.M.; Fernández-Muiño, M.; Moreno, G.; Huidobro, J.; Sancho, M. Evolution of acid phosphatase activity of honeys from different climates. Food Chem. 2006, 97, 750–755.
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423.
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J. Fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J. Mol. Catal. B Enzym. 2009, 59, 76–81.
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Mc Sorley, E.M. Supplementation with Calcium and Short-Chain Fructo-Oligosaccharides Affects Markers of Bone Turnover but Not Bone Mineral Density in Postmenopausal Women. J. Nutr. 2013, 144, 297–304.
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and Oligofructose as Dietary Fiber: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362.
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12, 1178633717702869.
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8.
- Rizzi, G.P. The Strecker Degradation of Amino Acids: Newer Avenues for Flavor Formation. Food Rev. Int. 2008, 24, 416–435.
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143.
- Frischmann, M.; Bidmon, C.; Angerer, A.J.; Pischetsrieder, M. Identification of DNA Adducts of Methylglyoxal. Chem. Res. Toxicol. 2005, 18, 1586–1592.
- Kocadağlı, T.; Gökmen, V. Effects of Sodium Chloride, Potassium Chloride, and Calcium Chloride on the Formation of α-Dicarbonyl Compounds and Furfurals and the Development of Browning in Cookies during Baking. J. Agric. Food Chem. 2016, 64, 7838–7848.
- Cämmerer, B.; Jalyschko, W.; Kroh, L.W. Intact Carbohydrate Structures as Part of the Melanoidin Skeleton. J. Agric. Food Chem. 2002, 50, 2083–2087.
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2020, 28, 2188–2196.
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664.
- Svendsen, C.; Høie, A.H.; Alexander, J.; Murkovic, M.; Husøy, T. The food processing contaminant glyoxal promotes tumour growth in the multiple intestinal neoplasia (Min) mouse model. Food Chem. Toxicol. 2016, 94, 197–202.
- Krainer, S.; Brodschneider, R.; Vollmann, J.; Crailsheim, K.; Riessberger-Gallé, U. Effect of hydroxymethylfurfural (HMF) on mortality of artificially reared honey bee larvae (Apis mellifera carnica). Ecotoxicology 2015, 25, 320–328.
- Bailey, L. The Effect of Acid-Hydrolysed Sucrose on Honeybees. J. Apic. Res. 1966, 5, 127–136.
- Surh, Y.-J.; Liem, A.; Miller, J.A.; Tannenbaum, S.R. 5-Sulfooxymethylfurfural as a possible ultimate mutagenic and carcinogenic metabolite of the Maillard reaction product, 5-hydroxymethylfurfural. Carcinogenesis 1994, 15, 2375–2377.
- Bakhiya, N.; Monien, B.; Frank, H.; Seidel, A.; Glatt, H. Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the Maillard product 5-hydroxymethylfurfural. Biochem. Pharmacol. 2009, 78, 414–419.
- Morales, F.J. Hydroxymethylfurfural (HMF) and related compounds. In Process-Induced Food Toxicants: Occurrence, Formation, Mitigation, and Health Risks; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-07475-6.
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In Vitro Antioxidant and Antiproliferative Activities of 5-Hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611.
- Alizadeh, M.; Khodaei, H.; Abbasi, M.M.; Saleh-Ghadimi, S. Assessing the effect of 5-hydroxymethylfurfural on selected components of immune responses in mice immunised with ovalbumin. J. Sci. Food Agric. 2017, 97, 3979–3984.
- Lin, S.-M.; Wu, J.-Y.; Su, C.; Ferng, S.; Lo, C.-Y.; Chiou, R.Y.-Y. Identification and Mode of Action of 5-Hydroxymethyl-2-furfural (5-HMF) and 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid (MTCA) as Potent Xanthine Oxidase Inhibitors in Vinegars. J. Agric. Food Chem. 2012, 60, 9856–9862.
- Nagai, T.; Kai, N.; Tanoue, Y.; Suzuki, N. Chemical properties of commercially available honey species and the functional properties of caramelization and Maillard reaction products derived from these honey species. J. Food Sci. Technol. 2017, 55, 586–597.
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203.
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967.
- Brudzynski, K. Honey melanoidins: Emerging novel understanding on the mechanism of antioxidant and antibacterial action of honey. In Honey: Current Research and Clinical Applications; Majtan, J., Ed.; Nova Science Publisher, Inc.: Hauppauge, NY, USA, 2012; pp. 17–38.
- Antony, S.M.; Han, I.Y.; Rieck, J.R.; Dawson, P.L. Antioxidative Effect of Maillard Reaction Products Formed from Honey at Different Reaction Times. J. Agric. Food Chem. 2000, 48, 3985–3989.
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2012, 51, 1686–1696.
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379.
- Brenna, O.V.; Ceppi, E.L.; Giovanelli, G. Antioxidant capacity of some caramel-containing soft drinks. Food Chem. 2009, 115, 119–123.
- Tsai, P.-J.; Yu, T.-Y.; Chen, S.-H.; Liu, C.-C.; Sun, Y.-F. Interactive role of color and antioxidant capacity in caramels. Food Res. Int. 2009, 42, 380–386.
- Rawlings, N.; Barrett, A. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218.
- Rawlings, N.D. Twenty-five years of nomenclature and classification of proteolytic enzymes. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1868, 140345.
- Simpson, R.J. Fragmentation of Protein Using Trypsin. Cold Spring Harb. Protoc. 2006, 2006, prot4550.
- Gráf, L.; Szilágyi, L.; Venekei, I. Chymotrypsin. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2626–2633.
- Cotten, S.W. Evaluation of exocrine pancreatic function. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Marzinke, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 573–585.
- Gillette, M.A.; Carr, S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods 2013, 10, 28–34.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
10.3K
Entry Collection:
Chemical Bond
Revisions:
2 times
(View History)
Update Date:
19 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





