Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Honey is known for its content of biomolecules, such as enzymes. The enzymes of honey originate from bees, plant nectars, secretions or excretions of plant-sucking insects, or microorganisms such as yeasts. Honey can be characterized by enzyme-catalyzed and non-enzymatic reactions. Notable examples of enzyme-catalyzed reactions are the production of hydrogen peroxide through glucose oxidase activity and the conversion of hydrogen peroxide to water and oxygen by catalase enzymes.
- glucose oxidase
- catalase
- carbohydrates
- amino acids
- honey
1. Introduction
Honey is a sweet honeybee product which is mainly composed of sugars and water. Biomedical activities of honey are mostly due to its minor components, which include proteins, amino acids, organic acids, dicarbonyl molecules, hydrogen peroxide, phenolic acids, flavonoids, and enzymes [1,2,3]. Honey enzymes originate from three major sources: plant nectars and secretions, honeybees, and excretions of plant-sucking insects. Biochemical reactions can be divided to two types: enzyme-catalyzed and non-enzymatic reactions [4]. Enzyme-catalyzed reactions in honey are known to affect its quality and biological activities [5,6,7]. Enzymes present in honey include the diastase, invertase, glucose oxidase, catalase, glucosylceramidase, α-amylase, α-glucosidase, β-glucosidase, and proteases [6,8].
Enzymatic reactions in honey include the conversion of oligosaccharides and disaccharides (sucrose and maltose) to glucose and fructose by diastase and invertase enzyme activity. Glucose is converted to gluconic acid and hydrogen peroxide by glucose oxidase. Moreover, hydrogen peroxide is degraded to water and oxygen by catalase enzymes. Honey samples which exhibit high catalase activity are low in hydrogen peroxide [9]. Honey samples with high hydrogen peroxide concentrations are known to be useful for the treatment of wounds, and are characterized by a high activity of glucose oxidase and low activity of catalase [10]. Furthermore, honey and other honeybee products are characterized by enzyme-catalyzed reactions such as the proteases, glucosylceramidase and acid phosphatase [6,11].
2. Production and Degradation of Hydrogen Peroxide
Hydrogen peroxide is produced as a product of glucose oxidation by glucose oxidase and by the non-enzymatic autoxidation of polyphenols [16,17]. Glucose oxidase is secreted by honeybees; some studies have reported its production by plants, honey fungi, yeast, and bacteria [18,19,20,21]. Glucose oxidase catalyzes the conversion of glucose to gluconic acid and hydrogen peroxide, using molecular oxygen and vitamin B2 as cofactors (FAD) (Figure 1).
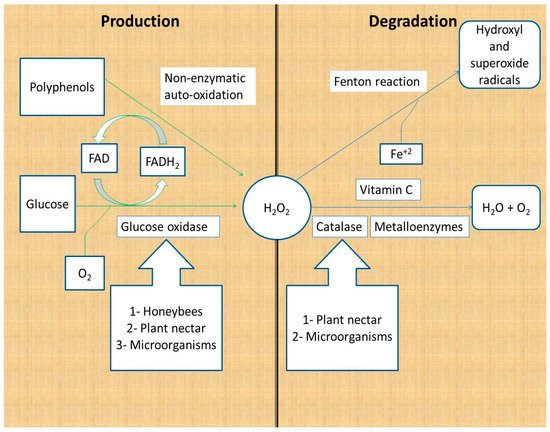
Figure 1. Production and degradation of hydrogen peroxide in honey. Hydrogen peroxide is produced from glucose by the action of glucose oxidase and non-enzymatically by polyphenols. Hydrogen peroxide is degraded to water and oxygen by enzymes and vitamin C, whereas it is degraded to hydroxyl and superoxide radicals through the Fenton reaction [16,17,18,19,20,21,22,23].
Honey hydrogen peroxide is degraded by enzymatic and non-enzymatic reactions. The major catabolic enzyme of hydrogen peroxide is Fe+3-dependent catalase, which catalyzes the conversion of two H2O2 molecules to two water molecules and one oxygen molecule. Plant nectar and the microorganisms in honey are the major sources of catalase in honey [16,22,23]. Other metal-containing enzymes (metalloenzymes) are capable of converting hydrogen peroxide to water following a different mechanism of action. Metalloenzymes include peroxidases and superoxide dismutase (Figure 1) [24].
Non-enzymatic reactions responsible for the degradation of hydrogen peroxide are the vitamin C and Fenton reactions. Vitamin C (hydro ascorbic acid) donates two protons and two electrons to hydrogen peroxide, reducing it to water and oxygen. It is reported that the addition of vitamin C to honey samples leads to decreased hydrogen peroxide levels, which reinforces the suggestion that vitamin C degrades hydrogen peroxide [16,25]. The second non-enzymatic reaction that depletes hydrogen peroxide from honey is the Fenton reaction. Fenton reactions involve the reaction of hydrogen peroxide with Fe+2 and/or polyphenols to produce hydroxide ions, oxygen, and hydroxyl and superoxide radicals. The hydroxyl and superoxide free radicals contribute to the antibacterial activity of honey because of their powerful damaging effect on the bacterial cells and DNA (Figure 1) [26,27].
Hydrogen peroxide generation in honey is influenced by its colloidal structure, which is due to the interactions between its macromolecules. Macromolecules in honey involved in the formation of the colloidal structure include oligo-sugars, proteins, and polyphenols. Colloidal particles of honey are composed of complexes of proteins, polyphenols, and melanoidins. The colloids of honey are compact and stable, with multiple layers. Colloidal honey samples are characterized by the generation of hydrogen peroxide, and antibiotic and antioxidant activities. Moreover, the colloidal structure of honey increases in dark and medium-color honeys, whereas it is not a character of the light color honeys [16,28].
Most of the antibacterial activity of honeys is due to the hydrogen peroxide concentration, high sugar concentration, and the low pH, due to the content of organic acids in honey, such as gluconic acid. Hydrogen-peroxide-producing honeys are used in wound and burn dressings, either alone or in combination with other medicines such as calcium alginate [16,28,29] (Table 1).
Table 1. Honey reactions and their biological activities.
| Reaction | Enzyme | Products | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| 1 | Production of hydrogen peroxide | Glucose oxidase | Hydrogen peroxide and gluconic acid | Antibacterial and wound and burn dressings | [16,28,29] |
| 2 | Production of short peptides | Proteases | Short peptides | Antimicrobial, antioxidant, antitumor and weight loss inducers | [30,31,32,33,34,35] |
| 3 | Degradation of amylose | Diastase | Glucose and maltose | Honey quality parameter that indicates storage conditions | [1] |
| 4 | Degradation of sucrose | Invertase | Glucose and fructose | Indicator for honey storage conditions | [36] |
| 5 | Degradation of organic phosphates | Acid phosphatase | Inorganic phosphate | Marker of honey floral origin and indicator of honey fermentation | [37,38,39] |
| 6 | Trans-glycosylation | Non-enzymatic | Disaccharides Oligosacharides |
Artificial sweeteners, increase bone mineral density in postmenopausal women (fructooligosaccharides), and classified as prebiotic molecules |
[40,41,42,43] |
| 7 | Production and degradation of dicarbonyls | Enzymatic (dihydroxyacetone phosphatase) and non-enzymatic | Dicarbonyls (glyoxal, methylglyoxal and 3-deoxyglucosone), methional and methylbutanal, AGEs and nucleoside AGEs and melanoidins | 1-Antibacterial, antitumor, antioxidant and contribution to the honey color, flavor and odorant. 2-High level of dicarbonyl molecules are reported to be with some toxicity to the humans such as tumerigenic, negative impact on blood vessels and induction of diabtes and uremia |
[44,45,46,47,48,49,50,51,52] |
| 8 | Production and degradation of HMF | Non-enzymatic | HMF, formic and levulinic acids, soluble HMF polymers and insoluble humins | Threatening honeybee life, human hepatorenal toxicity, induction of neoplastic changes, and irritation of mucous membranes HMF has positive impacts on human health, such as antioxidant, anti-carcinogen, anti-allergenic, and anti-hyperuricemic activities |
[53,54,55,56,57,58,59,60] |
| 9 | Maillard reaction | Non-enzymatic | Complexes of sugars and amino acids, amino aldoses and ketoses, dicarbonyls, enediols, 2-amino-2-deoxy-ald-oses and melanoidins | Antibacterial and antioxidant | [14,61,62,63,64] |
| 10 | Caramelization | Non-enzymatic | Deoxyosones, furan and pyran derivatives, HMF, hydroxydimethylfuranone (HDF) and hydroxyacetilfuran (HAF) | Contribution to the color, aroma, and flavor of honey and antioxidants | [65,66,67,68] |
3. Proteases
Proteases are responsible for the degradation of proteins to produce amino acids and short peptides according to the type of protease. According to their substrate preference, proteases are classified as endopeptidases and exopeptidases [69,70]. Unifloral honey samples are reported to contain serine protease enzymes, which contribute to their quality and biological activities [6]. A Croatian study reported the presence of three serine proteases in honey: trypsin, chymotrypsin, and elastase [37]. Trypsin cleaves the peptide bonds formed by the carboxyl groups of arginine and lysine [71]. The sites of chymotrypsin cleavage are peptide bonds formed by the carboxyl groups of aromatic amino acids (tyrosine, phenylalanine, and tryptophan) and leucine [72]. Elastase is associated with breakdown of the peptide bonds formed by the short aliphatic amino acids such as glycine, alanine, and valine [73]. The major products of honey proteases are short peptides that function as antioxidants, antitumor, and antimicrobials, and are used as weight loss inducers (Figure 2). Alaerjani et al. (2021) published an article which proved the presence of short peptides in honey samples from Saudi Arabia. They analyzed five honey samples using LC–MS and concluded that short peptides in honey samples are of floral origin and storage-dependent [30] (Table 1).
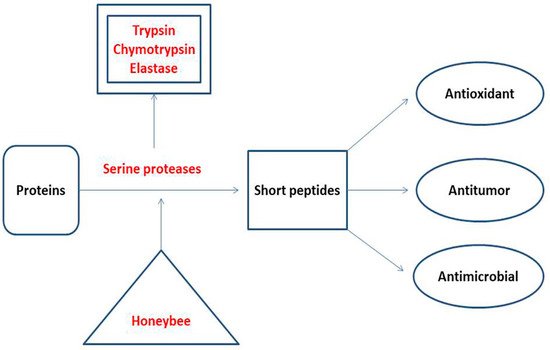
Amino acids and their sequences in short peptides are responsible for their activity. Short peptides that contain cysteine, methionine, tyrosine, lysine, histidine, and tryptophan are known to act as antioxidants [31,32,33]. Short peptides rich in hydrophobic amino acids, such as glysine, alanine, valine, leucine, and isoleucine, are active antimicrobial peptides because of their ability to disrupt the plasma membrane of the microbes [30,34]. Short and cyclic peptides are active as antitumor, antimicrobial, antioxidant, and weight loss inducers, according to their amino acid contents and sequences [35]. Assessments of the amino acid contents, sequences, and concentrations of short peptides in food are carried out using LC–MS techniques [30,74] (Table 1).
This entry is adapted from the peer-reviewed paper 10.3390/molecules27154719
This entry is offline, you can click here to edit this entry!
