Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | PEDRO NUNEZ ABADES | -- | 2721 | 2022-08-14 11:43:05 | | | |
| 2 | Camila Xu | Meta information modification | 2721 | 2022-08-15 04:51:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pardillo-Díaz, R.; Pérez-García, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. Oxidative Stress in Neurodegenerative Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/26128 (accessed on 07 February 2026).
Pardillo-Díaz R, Pérez-García P, Castro C, Nunez-Abades P, Carrascal L. Oxidative Stress in Neurodegenerative Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/26128. Accessed February 07, 2026.
Pardillo-Díaz, Ricardo, Patricia Pérez-García, Carmen Castro, Pedro Nunez-Abades, Livia Carrascal. "Oxidative Stress in Neurodegenerative Diseases" Encyclopedia, https://encyclopedia.pub/entry/26128 (accessed February 07, 2026).
Pardillo-Díaz, R., Pérez-García, P., Castro, C., Nunez-Abades, P., & Carrascal, L. (2022, August 14). Oxidative Stress in Neurodegenerative Diseases. In Encyclopedia. https://encyclopedia.pub/entry/26128
Pardillo-Díaz, Ricardo, et al. "Oxidative Stress in Neurodegenerative Diseases." Encyclopedia. Web. 14 August, 2022.
Copy Citation
Neurodegenerative diseases are characterized by gradually progressive, selective loss of anatomically or physiologically related neuronal systems that produce brain damage from which there is no recovery. Neurodegenerative diseases, bridging their clinical differences, are characterized by the gradual and irreversible damage and loss of specific neuronal networks.
oxidative stress
neurodegenerative diseases
hyperexcitability
1. Introduction
Degenerative diseases of the central nervous system (CNS) impose substantial medical and public health burdens on populations throughout the world. The overwhelming rise in the prevalence of these disorders parallels the rapid increase in human lifespan. Neurodegenerative diseases, bridging their clinical differences, are characterized by the gradual and irreversible damage and loss of specific neuronal networks. Different brain areas affected will trigger different diseases, with the most common being Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) [1]. Among these neurodegenerative diseases AD is the most prevalent, causing between 60% and 80% of dementia cases globally. This disease, derived from an initial deterioration of the hippocampus and a posterior deterioration of the cortex and brainstem regions, is manifested by psychological, motor and specially memory alterations [2]. In the case of PD, the degeneration affects the dopaminergic neurons from the substantia nigra pars compacta, notably producing motor disorders such as bradykinesia, akinesia, stiffness, resting tremor and postural instability as well as non-motor manifestations such as depression, anxiety, sleep disorders, and cognitive and autonomic function alterations [3]. With a lower incidence (2–11 cases per 100,000 people) [4], ALS is the most common disease affecting the motoneurons, and it is triggered by the gradual, irreversible loss of the motoneurons located in the corticospinal tract. Although in its early stages is commonly manifested as muscular weakness, in 25% of patients ALS may be first manifested as bulbar symptoms, characterized by speech and swallowing difficulties. These symptoms constantly evolve until the event of the death of the patient, usually produced by respiratory arrest [5]. Finally, MS is the most prevalent non-traumatic disabling disease among the young adult population. It is an autoimmune inflammatory disease that has been classically attributed to a demyelinating pathology of the CNS. Although optic neuritis is the first symptom of MS for many people, the late stages of this disorder are accompanied with brain and spinal atrophy from which the brainstem and spinal cord syndromes derive [6]. All these neurodegenerative disorders seem to have common etiopathogenic pathways, albeit each of them affect different regions of the CNS, leading to different clinical manifestations. Thus, protein misfolding and aggregation as well as oxidative stress are highly recognizable common features in all these pathologies. In terms of neuronal functionality, hyperexcitability appears to be the common hallmark among all of them and often the degree of excitability of patient’s derived motor neurons correlates with the patient’s rate of survival, especially in ALS [7][8][9]. However, the molecular and cellular mechanisms that lead to the onset of these malfunctions and thus to the development of the disease are complex and still poorly understood. Therefore, an in-depth understanding of the mechanisms underlying the neurodegenerative processes is a necessary step in the development of new treatments capable of preventing the emergence of these diseases or, at least, of delaying their progression [1][10][11].
2. Pathogenesis of Neurodegenerative Disease: Oxidative Stress and Inflammation
Irreversible neuronal deterioration is the most common consequence of neurodegenerative diseases -either AD, PD, ALS, MS or others. Although the pathogenesis of these diseases is not fully understood, several neurological components have been already identified as responsible for the neuronal pathology such as increased protein aggregates, glial activation/inflammation, mitochondrial dysfunction, disbalance of neurotransmitters, autophagia or oxidative stress (Figure 1). A comprehensive review of the molecular features and histopathology of all of these pathogenic mechanisms in neurodegenerative diseases is beyond the scope of this research except for oxidative stress but it is worth highlighting several aspects of the role of neuroinflammation because of its interrelation with oxidative stress and hyperexcitability in motor cortex neurons.
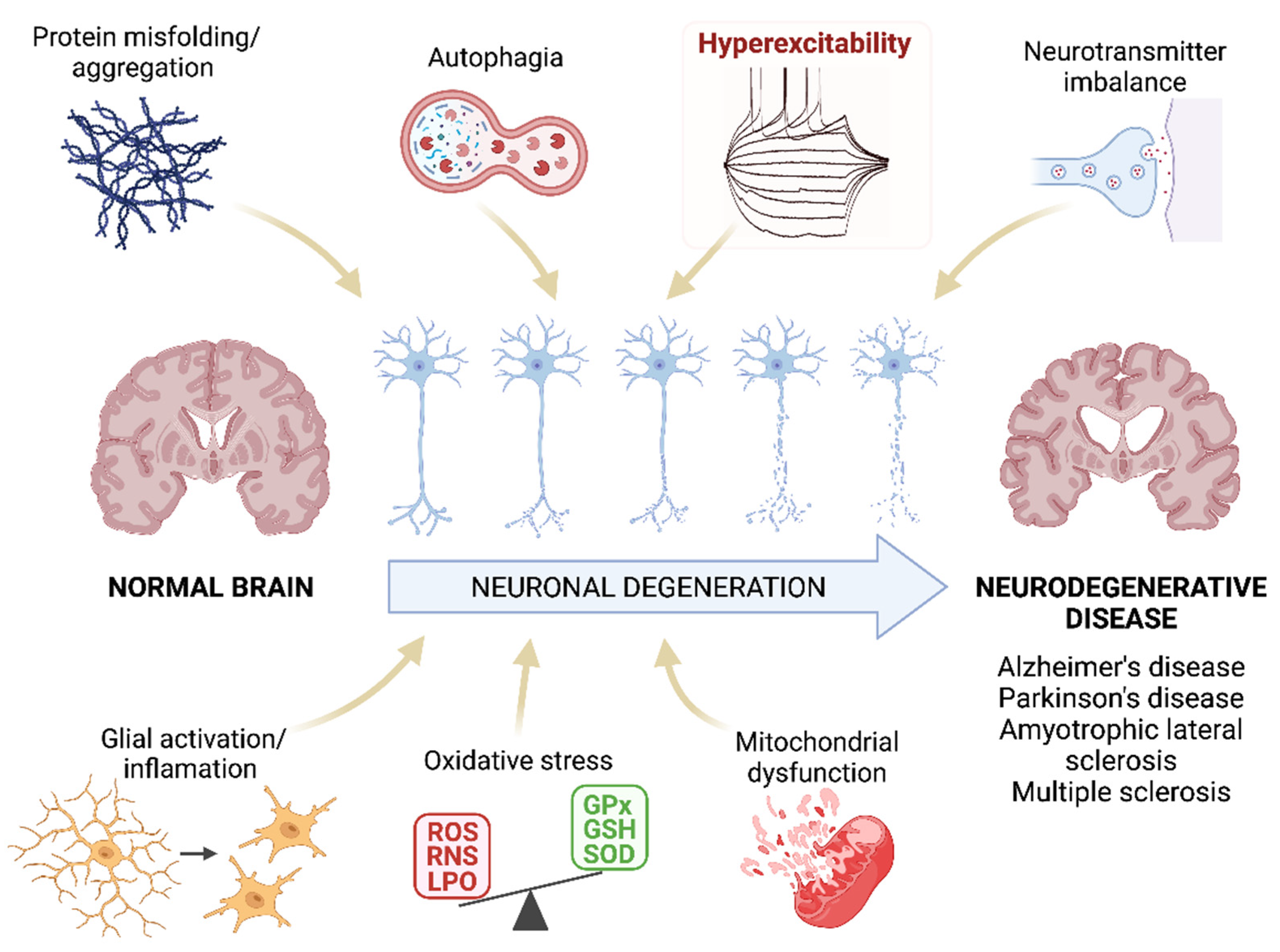
Figure 1. Pathogenesis of neurodegenerative diseases. Schematic representation of the mechanisms that are proposed as the main pathogenic agents of neurodegenerative diseases. Researchers highlight the hyperexcitability as this parameter will be the main focus of this research. ROS: reactive oxygen species; RNS: reactive nitrogen species; LPO: lipid peroxidation; GPx: glutathione peroxidase; GSH: glutathione; SOD: superoxide dismutase. Created with BioRender.com [12].
2.1. Inflammation Triggers Neurodegenerative Diseases
The blood-brain barrier (BBB) is the main protective barrier of the CNS. It has a highly selective permeability and, in addition of selectively allowing the entrance of essential nutrients such as glucose and amino acids, it also regulates the controlled access of immune cells into the brain. An increased BBB permeability to immune cells can disbalance the homeostasis and provoke serious neurological deficits. Thus, a common feature of these CNS diseases is a chronic immune activation, affecting innate and adaptative immune responses. In addition, microglia, astrocytes, endothelial cells, and peripheral immune cells release inflammatory cytokines and chemokines, causing a prolonged proinflammatory state of microglia, increasing the permeability of the BBB and facilitating the infiltration of immune cells into the CNS [13][14].
Microgliosis. Microglia composes the immune system of the CNS. In addition to their phagocytic capacity, active microglia releases proinflammatory molecules such as nitric oxide (NO), tumor necrosis factor α (TNFα), major histocompatibility complex class II (MHC II), prostaglandin-endoperoxide synthase 2 (Cox-2), monocyte chemotaxic protein 1 (MCP-1), and interleukins 1β and 6 (IL-1β and IL-6), playing a crucial role in the inflammatory response. Although microglia is necessary for the elimination of dead neurons, its overactivation is related to a faster progression of neurodegenerative diseases such as AD, PD, ALS and MS [15][16][17][18][19][20]. Under normal conditions, microglia is in a “resting” state morphologically characterized by ramifications that extend and retract so that these cells can constantly monitor the state of the microenvironment that surrounds them. When an abnormality is detected, the resting microglia transitions to an activated state in which the morphology changes to an ameboid shape. Active microglia can be observed, for example, surrounding senile plaques in AD. In addition to activation markers, this microglia expresses proinflammatory mediators such as MCP-1 that contribute to the recruitment of astrocytes in senile plaques [20]. In PD it has been suggested that microgliosis is caused by α-Syn aggregates, and that this active microglia contributes to the death of dopaminergic neurons [21]. Likewise, microgliosis is also a hallmark of neuroinflammation in ALS. Active microglia has been observed by positron emission tomography in the brains of living patients in which, the intensity of activation correlates with the severity of the disease, depicting the role of active microglia in the course of the disease [22]. Microglial activation is also evident in MS, both in early and later stages of the disease. Its activation correlates with axon and oligodendrocyte pathology, in which myelin remnants and cell fragments can be found. In addition to the production of various inflammatory neurotoxic mediators, microglia secretes the proteolytic lipolytic enzymes which ultimately lead to the destruction of myelin [23].
Reactive Astrocytes. Astrocytes are the main components of the glia and perform numerous functions of support and maintenance of the nervous system [24]. Activated astrocytes can give rise to two different forms, known as A1 and A2. While A2 favors tissue repair, A1 favors its degradation. The activation of the latter contributes to neuroinflammation through the release of proteins and inflammatory mediators. Thus, in neurodegenerative diseases astroglia lose its protective function which may cause the collapse of the BBB and neuronal death. For example, in the case of AD, astroglia A1 causes an imbalance in the release of calcium, glutamate and GABA, contributing to the development of the disease [25]. In fact, in many cases the cognitive impairment associated with AD correlates with the number of reactive astrocytes present in senile plaques and with the concentration of proinflammatory mediators produced by them [26]. Moreover, A1 astrocytes promote the formation of amyloid beta (Aβ) plaques [24]. A1 reactivity has also been detected in the substantia nigra of PD patients [27]. At the beginning of the disease, α-Syn inclusions are found inside the astrocytes (also increased in number), which leads to the mobilization of phagocytic microglia and the destruction of cholinergic neurons, producing the clinical symptoms of the disease [28]. Similarly, the degree of astrocytic reactivity correlates directly with the rate of neuronal death in ALS [24][29]. Finally, the existence of reactive astrocytes has also been demonstrated in MS, being the main source of proinflammatory cytokines. These cytokines are the cause of uncontrolled inflammatory reactions with T cell infiltration and associated damage [24][30][31].
Pattern-recognition receptors (PRRs). Innate immune responses are triggered by PRRs. The best known PRRs are the Toll-like receptors (TLRs). It has been proved that CNS cells can activate the innate immune system via motifs related to damage and/or stress, to which TLRs bind. In fact, several studies show that TLRs are upregulated in the brains of patients with neurodegenerative disorders such as AD, PD, ALS or MS [32][33][34]. For example, TLR2, TLR3, and TLR4 are overexpressed in microglia of patients with PD and ALS, activating pathways that lead to the production of proinflammatory cytokines that contribute to neuronal damage [13]. Likewise, the expression of TLR2 and TLR4 is also increased in AD, and it has been shown in vitro that Aβ induces TLR synthesis. However, this increase could also play a beneficial role in neurodegenerative diseases, since TLRs help the absorption and elimination of protein aggregates, including Aβ [35][36][37]. Thus, these receptors seem to play a dual role in the development of neurodegenerative disorders, being able to cause neuronal damage but also favoring tissue regeneration. This suggests that enhancing their beneficial functions may be useful as a therapeutic strategy in the treatment of these pathologies [13].
2.2. Role of Oxidative Stress in Neurodegenerative Diseases
Another essential mechanism in neurodegenerative processes is oxidative stress [38]. Oxidative stress is known as the cellular situation in which the free radicals -reactive oxygen and/or nitrogen species (ROS and RNS, respectively)- exceed the natural antioxidant defenses of the organism [39]. The importance of these mechanisms in the development of AD, PD, ALS, and MS lies in the fact that neurons are especially vulnerable to ROS and RNS since they contain high levels of unsaturated lipids susceptible to oxidation and high levels of transition metals that catalyze the synthesis of free radicals [40]. Besides, the CNS has a high metabolic rate and, hence, a high oxygen demand which favors free radical formation. The metabolism of neurotransmitters is also a source of free radicals in the CNS. In addition, the antioxidant defense of the CNS is weaker than in other organs making it even more susceptible to oxidative stress. Under oxidative stress conditions, dysfunctional mitochondria are unable to produce the high energy levels required by neuronal cells to perform their normal biochemical and physiological functions. This mitochondrial impairment makes them vulnerable to rapid cell death [41]. Under physiological concentrations ROS play a crucial role as regulators of various cellular functions such as growth, proliferation, differentiation and survival; they participate in cognitive function and in the maintenance of antioxidant mechanisms [42][43]. However, an excess of ROS and/or RNS can damage cells modifying lipids, proteins, and DNA [38]. ROS produced by mitochondria cause lipid peroxidation (LPO), which alters the cell membrane structure, and integrity affecting cell signaling. In here, researchers review the evidences that demonstrate the presence of oxidative stress in neurodegenerative diseases and its impact in AD, PD, MS and ALS (Figure 2).
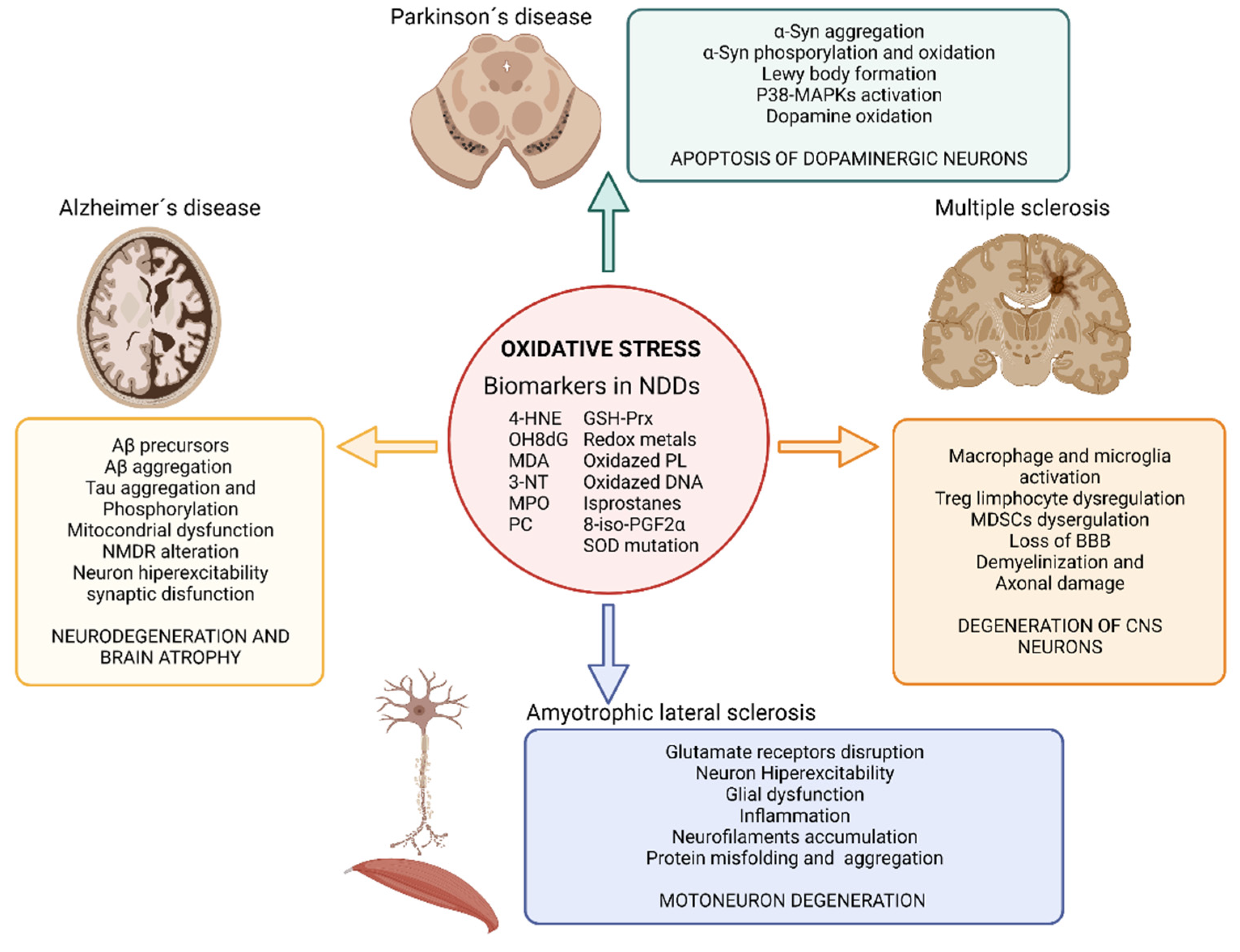
Figure 2. Impact of oxidative stress on neurodegenerative diseases. Illustration showing the impact of oxidative stress on Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and multiple sclerosis and the main biomarkers of oxidative stress found in neurodegenerative disease’s studies. Abbreviatures: BBB, blood brain barrier; GSH-Prx, glutathione-peroxiredoxins; 4-HNE, 4-hidroxinonenal; NDDs, neurodegenerative diseases; NMDR, N-metil-D-aspartato receptors; MDA, malondialdehyde; MAPKs, Mitogen-activated protein kinases; MPO, Myeloperoxidase; 3-NT, 3-nitrotyrosine; OH8dG, 8-hydroxy-2′-deoxyguanosine; PC, protein carbonyl; 8-iso-PGF2α,8-iso-prostaglandin F2α; α-Syn, α-Synuclein; SOD, superoxide dismutase. Created with BioRender.com [12].
The increased levels of LPO are strongly associated with neurotoxicity in AD [44][45]. Oxidative stress has been detected even before the development of Aβ plaques, thus being proposed as the key player in the etiology of this disease. Oxidative stress contributes to mitochondrial dysfunction, neuronal bodies, and synapsis dysfunctions, as well as to Aβ production [46]. Mitochondrial dysfunction in AD involves impaired mitochondrial complexes that control ATP generation, producing 4-hidroxinonenal (4-HNE) and leading to Aβ accumulation. In addition, increased ROS levels drive to tau aggregates, tau hyperphosphorylation and cell death [38][41]. The imbalance of bioactive metals has also been proposed as one of the mechanisms by which oxidative stress influences AD. Thus, zinc directly affects Aβ precursors [47], and aluminum, zinc, iron, and copper promote Aβ aggregation [48][49]. Similarly, redox-active transition metals could promote Tau phosphorylation [50]. Finally, ROS, amyloid, and Tau protein affect the activity of glutamate receptors and uptake leading to an exacerbate influx of Ca2+ in postsynaptic neurons that increase ROS production, oxidative stress, Tau phosphorylation, LPO and hyperexcitability driving to synaptic dysfunction responsible for AD [51][52].
In PD, oxidative stress is associated with α-Syn aggregation and Lewy body formation. Elevated levels of oxidative stress markers such as 4-HNE, protein carbonyl, 8-hydroxy-2′-deoxyguanosine (OH8dG), and 8-hydroxyguanosine have been found in postmortem samples of brains from PD patients. The cascade of events that lead to the degeneration of dopaminergic neurons in PD is also linked to oxidative stress due to the fact that it activates the p38 mitogen activated protein kinase pathway, ultimately leading to apoptosis of the dopaminergic brain cells [53][54]. Furthermore, iron accumulates in the substantia nigra of PD patients enhancing pro-oxidant interactions between iron and dopamine. This imbalance in iron metabolism may also increases the phosphorylation or oxidation of α-Synuclein (α-Syn) in the substantia nigra [55][56]. Iron accumulation in PD patients correlates with the specific spatiotemporal progression of neuronal loss [38][57].
Related to ALS, many studies have reported high levels of oxidative stress during the pathogenesis of the disease. The serum of patients with ALS also shows elevated concentrations of oxidative stress markers such as 3-nitrotyrosine (3-NT), 4-HNE, OH8dG and high activity of the antioxidants defenses such as glutathione peroxidase, superoxide dismutase (SOD), glutathione reductase, and malondialdehyde (MDA) [58][59]. Oxidative stress in ALS has been proposed as a disruptor of glutamate receptors that lead to an increased concentration of glutamate in the synaptic cleft [60][61][62]. This increased glutamate produces motor neurons hyperexcitability and an increment in calcium influx in cells (neurons and glia) as well as mitochondria thus, causing cell damage [63]. ROS also activate glial cells that produce proinflammatory cytokines and more ROS, thus spreading the neurodegeneration toward the whole population [62][64][65]. In addition, ROS has an important impact on the neurocytoskeleton, promoting abnormal accumulation of neurofilaments [66]. Other evidence on the importance of oxidative stress in this disease is the presence of mutations in genes involved in removing ROS. Notably, most of the ALS patients that display the familiar form of the disease (15–20%) present mutations in the SOD1 gene, which plays an important role in the defense against oxidative stress. It has been described more than 150 ALS-related SOD1 gene mutations, affecting various parts of the enzyme. This results in protein misfolding and aggregation, increased ROS production, and redox system disequilibrium, which ultimately leads to nerve cell loss [67]. In sporadic form of ALS, the overexpression of the cystine/glutamate antiporter causes increased levels of ROS and RNS and extracellular glutamate accumulation, which translates into an increase of neuron excitability [41][67].
Oxidative stress is present since the early stages of MS, participating in the demyelination and axonal damage typical of this disease. Postmortem studies demonstrate that in active lesions of the white matter and cerebral cortex, demyelination and neurodegeneration are closely associated with the presence of oxidized lipids (such as oxidized phospholipids and MDA) in myelin membranes and in apoptotic oligodendrocytes and neurons, in which oxidized DNA can be found within their nuclei [68][69][70]. Additional evidence of oxidative stress in MS are the presence of increased protein carbonyls, NO metabolites, SOD, catalase, glutathione reductase, inducible nitric oxide synthase (iNOS), protein carbonyl, 3-NT, isoprostanes, MDA, 8-iso-prostaglandin F2α and products of DNA oxidation in blood, serum, urine and postmortem brain of MS patients [71]. In MS, free radicals trigger an inflammatory response (activating macrophage and microglia) that in turn favors the synthesis of more ROS and RNS, aggravating the problem in a self-reinforcing feedback. The overproduction of free radicals overpasses the antioxidant capacity of the body and produces damage to the nervous tissue [72]. Oxidative stress is also linked in MS to the impairment of regulatory T cells [73] and myeloid-derived suppressor cells (MDSCs) [74] that interfere in the adaptive immune response and in the loss of the BBB [68][75].
References
- Kirmani, B.F.; Shapiro, L.A.; Shetty, A.K. Neurological and Neurodegenerative Disorders: Novel Concepts and Treatment. Aging Dis. 2021, 12, 950.
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408.
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42.
- Zapata-Zapata, C.H.; Franco-Dáger, E.; Solano-Atehortúa, J.M.; Ahunca-Velásquex, L.F. Esclerosis Lateral Amiotrófica: Actualización. IATREIA 2016, 29, 194–205.
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic Lateral Sclerosis. Lancet 2011, 377, 942–955.
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40.
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.W.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G. Intrinsic Membrane Hyperexcitability of Amyotrophic Lateral Sclerosis Patient-Derived Motor Neurons. Cell Rep. 2014, 7, 1–11.
- Kanai, K.; Shibuya, K.; Sato, Y.; Misawa, S.; Nasu, S.; Sekiguchi, Y.; Mitsuma, S.; Isose, S.; Fujimaki, Y.; Ohmori, S. Motor Axonal Excitability Properties Are Strong Predictors for Survival in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 734–738.
- Geevasinga, N.; Menon, P.; Özdinler, P.H.; Kiernan, M.C.; Vucic, S. Pathophysiological and Diagnostic Implications of Cortical Dysfunction in ALS. Nat. Rev. Neurol. 2016, 12, 651–661.
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative Diseases and Oxidative Stress. Biomed. Pharmacother. Biomed. Pharmacother. 2004, 58, 39–46.
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795.
- BioRender. Available online: https://biorender.com (accessed on 30 June 2022).
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in Neurodegenerative Diseases. Immunology 2010, 129, 154–169.
- Bogár, F.; Fülöp, L.; Penke, B. Novel Therapeutic Target for Prevention of Neurodegenerative Diseases: Modulation of Neuroinflammation with Sig-1R Ligands. Biomolecules 2022, 12, 363.
- Fuhrmann, M.; Bittner, T.; Jung, C.K.E.; Burgold, S.; Page, R.M.; Mitteregger, G.; Haass, C.; LaFerla, F.M.; Kretzschmar, H.; Herms, J. Microglial Cx3cr1 Knockout Prevents Neuron L.Loss in a Mouse Model of Alzheimer’s Disease. Nat. Neurosci. 2010, 13, 411–413.
- Xu, L.; He, D.; Bai, Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol. Neurobiol. 2016, 53, 6709–6715.
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397.
- Kocur, M.; Schneider, R.; Pulm, A.-K.; Bauer, J.; Kropp, S.; Gliem, M.; Ingwersen, J.; Goebels, N.; Alferink, J.; Prozorovski, T.; et al. IFNβ Secreted by Microglia Mediates Clearance of Myelin Debris in CNS Autoimmunity. Acta Neuropathol. Commun. 2015, 3, 20.
- Lue, L.F.; Walker, D.G.; Rogers, J. Modeling Microglial Activation in Alzheimer’s Disease with Human Postmortem Microglial Cultures. Neurobiol. Aging 2001, 22, 945–956.
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934.
- Béraud, D.; Hathaway, H.A.; Trecki, J.; Chasovskikh, S.; Johnson, D.A.; Johnson, J.A.; Federoff, H.J.; Shimoji, M.; Mhyre, T.R.; Maguire-Zeiss, K.A. Microglial Activation and Antioxidant Responses Induced by the Parkinson’s Disease Protein α-Synuclein. J. Neuroimmune Pharmacol. 2013, 8, 94–117.
- Komine, O.; Yamanaka, K. Neuroinflammation in Motor Neuron Disease. Nagoya J. Med. Sci. 2015, 77, 537–549.
- Bogie, J.F.J.; Stinissen, P.; Hendriks, J.J.A. Macrophage Subsets and Microglia in Multiple Sclerosis. Acta Neuropathol. 2014, 128, 191–213.
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675.
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte Dysfunction in Alzheimer Disease. J. Neurosci. Res. 2017, 95, 2430–2447.
- Kashon, M.L.; Ross, G.W.; O’Callaghan, J.P.; Miller, D.B.; Petrovitch, H.; Burchfiel, C.M.; Sharp, D.S.; Markesbery, W.R.; Davis, D.G.; Hardman, J.; et al. Associations of Cortical Astrogliosis with Cognitive Performance and Dementia Status. J. Alzheimers Dis. 2004, 6, 581–595.
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s Disease. Park. Relat. Disord. 2012, 18 (Suppl. 1), S210–S212.
- Gu, X.-L.; Long, C.-X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic Expression of Parkinson’s Disease-Related A53T Alpha-Synuclein Causes Neurodegeneration in Mice. Mol. Brain 2010, 3, 12.
- Martorana, F.; Brambilla, L.; Valori, C.F.; Bergamaschi, C.; Roncoroni, C.; Aronica, E.; Volterra, A.; Bezzi, P.; Rossi, D. The BH4 Domain of Bcl-XL Rescues Astrocyte Degeneration in Amyotrophic Lateral Sclerosis by Modulating Intracellular Calcium Signals. Hum. Mol. Genet. 2012, 21, 826–840.
- Perriard, G.; Mathias, A.; Enz, L.; Canales, M.; Schluep, M.; Gentner, M.; Schaeren-Wiemers, N.; Du Pasquier, R.A. Interleukin-22 Is Increased in Multiple Sclerosis Patients and Targets Astrocytes. J. Neuroinflammation 2015, 12, 119.
- Brosnan, C.F.; Raine, C.S. The Astrocyte in Multiple Sclerosis Revisited. Glia 2013, 61, 453–465.
- Arroyo, D.S.; Soria, J.A.; Gaviglio, E.A.; Rodriguez-Galan, M.C.; Iribarren, P. Toll-like Receptors Are Key Players in Neurodegeneration. Int. Immunopharmacol. 2011, 11, 1415–1421.
- Owens, T. Toll-like Receptors in Neurodegeneration. Curr. Top. Microbiol. Immunol. 2009, 336, 105–120.
- Okun, E.; Griffioen, K.J.; Lathia, J.D.; Tang, S.-C.; Mattson, M.P.; Arumugam, T.V. Toll-like Receptors in Neurodegeneration. Brain Res. Rev. 2009, 59, 278–292.
- Marta, M.; Meier, U.C.; Lobell, A. Regulation of Autoimmune Encephalomyelitis by Toll-like Receptors. Autoimmun. Rev. 2009, 8, 506–509.
- Tahara, K.; Kim, H.-D.; Jin, J.-J.; Maxwell, J.A.; Li, L.; Fukuchi, K. Role of Toll-like Receptor Signalling in Abeta Uptake and Clearance. Brain 2006, 129, 3006–3019.
- Prinz, M.; Garbe, F.; Schmidt, H.; Mildner, A.; Gutcher, I.; Wolter, K.; Piesche, M.; Schroers, R.; Weiss, E.; Kirschning, C.J.; et al. Innate Immunity Mediated by TLR9 Modulates Pathogenicity in an Animal Model of Multiple Sclerosis. J. Clin. Investig. 2006, 116, 456–464.
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325.
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, Oxidative Stress and Neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911.
- Urano, S.; Asai, Y.; Makabe, S.; Matsuo, M.; Izumiyama, N.; Ohtsubo, K.; Endo, T. Oxidative Injury of Synapse and Alteration of Antioxidative Defense Systems in Rats, and Its Prevention by Vitamin E. Eur. J. Biochem. 1997, 245, 64–70.
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408.
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049.
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2019, 37, 113–139.
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147.
- Padurariu, M.; Ciobica, A.; Lefter, R.; Serban, I.L.; Stefanescu, C.; Chirita, R. The Oxidative Stress Hypothesis in Alzheimer’s Disease. Psychiatr. Danub. 2013, 25, 401–409.
- Sharma, C.; Kim, S.R. Linking Oxidative Stress and Proteinopathy in Alzheimer’s Disease. Antioxidants 2021, 10, 1231.
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and Regulated α-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by a Disintegrin Metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927.
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; Paradis, M.D.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid Induction of Alzheimer Aβ Amyloid Formation by Zinc. Science 1994, 265, 1464–1467.
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, Iron, and Zinc Ions Promote Aggregation of Physiological Concentrations of Β-amyloid Peptide. J. Neurochem. 1993, 61, 1171–1174.
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661.
- Tong, H.; Zhang, X.; Meng, X.; Lu, L.; Mai, D.; Qu, S. Simvastatin Inhibits Activation of NADPH Oxidase/P38 MAPK Pathway and Enhances Expression of Antioxidant Protein in Parkinson Disease Models. Front. Mol. Neurosci. 2018, 11, 165.
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031.
- Hare, D.J.; Double, K.L. Iron and Dopamine: A Toxic Couple. Brain 2016, 139, 1026–1035.
- Hare, D.J.; Kysenius, K.; Paul, B.; Knauer, B.; Hutchinson, R.W.; O’Connor, C.; Fryer, F.; Hennessey, T.P.; Bush, A.I.; Crouch, P.J. Imaging Metals in Brain Tissue by Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS). JoVE (J. Vis. Exp.) 2017, 119, e55042.
- Duce, J.A.; Wong, B.X.; Durham, H.; Devedjian, J.-C.; Smith, D.P.; Devos, D. Post Translational Changes to α-Synuclein Control Iron and Dopamine Trafficking; a Concept for Neuron Vulnerability in Parkinson’s Disease. Mol. Neurodegener. 2017, 12, 45.
- Park, H.R.; Yang, E.J. Oxidative Stress as a Therapeutic Target in Amyotrophic Lateral Sclerosis: Opportunities and Limitations. Diagnostics 2021, 11, 1546.
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metalloenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437.
- Foran, E.; Trotti, D. Glutamate Transporters and the Excitotoxic Path to Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. Antioxid. Redox Signal. 2009, 11, 1587–1602.
- Trotti, D.; Danbolt, N.C.; Volterra, A. Glutamate Transporters Are Oxidant-Vulnerable: A Molecular Link between Oxidative and Excitotoxic Neurodegeneration? Trends Pharm. Sci. 1998, 19, 328–334.
- Rao, S.D.; Yin, H.Z.; Weiss, J.H. Disruption of Glial Glutamate Transport by Reactive Oxygen Species Produced in Motor Neurons. J. Neurosci. 2003, 23, 2627–2633.
- Heath, P.R.; Shaw, P.J. Update on the Glutamatergic Neurotransmitter System and the Role of Excitotoxicity in Amyotrophic Lateral Sclerosis. Muscle Nerve 2002, 26, 438–458.
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxidative Med. Cell. Longev. 2020, 2020, 5021694.
- Rao, S.D.; Weiss, J.H. Excitotoxic and Oxidative Cross-Talk between Motor Neurons and Glia in ALS Pathogenesis. Trends Neurosci. 2004, 27, 17–23.
- Hirano, A.; Nakano, I.; Kurland, L.T.; Mulder, D.W.; Holley, P.W.; Saccomanno, G. Fine Structural Study of Neurofibrillary Changes in a Family with Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 1984, 43, 471–480.
- Pansarasa, O.; Bordoni, M.; Diamanti, L.; Sproviero, D.; Gagliardi, S.; Cereda, C. SOD1 in Amyotrophic Lateral Sclerosis: “Ambivalent” Behavior Connected to the Disease. Int. J. Mol. Sci. 2018, 19, 1345.
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative Stress in Multiple Sclerosis: Central and Peripheral Mode of Action. Exp. Neurol. 2016, 277, 58–67.
- Fischer, M.T.; Wimmer, I.; Höftberger, R.; Gerlach, S.; Haider, L.; Zrzavy, T.; Hametner, S.; Mahad, D.; Binder, C.J.; Krumbholz, M. Disease-Specific Molecular Events in Cortical Multiple Sclerosis Lesions. Brain 2013, 136, 1799–1815.
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative Damage in Multiple Sclerosis Lesions. Brain 2011, 134, 1914–1924.
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. Oxidative Stress-Related Biomarkers in Multiple Sclerosis: A Review. Biomark. Med. 2016, 10, 889–902.
- Olla, S.; Steri, M.; Formato, A.; Whalen, M.B.; Corbisiero, S.; Agresti, C. Combining Human Genetics of Multiple Sclerosis with Oxidative Stress Phenotype for Drug Repositioning. Pharmaceutics 2021, 13, 2064.
- Corthay, A. A Three-cell Model for Activation of Naive T Helper Cells. Scand. J. Immunol. 2006, 64, 93–96.
- Zhang, Q.; Fujino, M.; Xu, J.; Li, X. The Role and Potential Therapeutic Application of Myeloid-Derived Suppressor Cells in Allo-and Autoimmunity. Mediat. Inflamm. 2015, 2015, 421927.
- Imaizumi, S.; Kondo, T.; Deli, M.A.; Gobbel, G.; Joó, F.; Epstein, C.J.; Yoshimoto, T.; Chan, P.H. The Influence of Oxygen Free Radicals on the Permeability of the Monolayer of Cultured Brain Endothelial Cells. Neurochem. Int. 1996, 29, 205–211.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.3K
Revisions:
2 times
(View History)
Update Date:
15 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




