| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mandeep Kaur | + 2113 word(s) | 2113 | 2020-10-08 17:29:34 | | | |
| 2 | Dean Liu | Meta information modification | 2113 | 2020-10-16 06:29:13 | | |
Video Upload Options
The advancement of micro-electro-mechanical systems and small-sized optical components enable the fabrication of imaging devices with promising performance as compared to the traditional medical devices. Such devices enable the detection of lesions/tumors at an earlier stage and act as a guide during surgical procedures, increasing diagnostic capabilities. A variety of different imaging techniques used in medical applications are reviewed in here.
1. Introduction
The growth and development of optical components and, in particular, the miniaturization of micro-electro-mechanical systems (MEMSs), has motivated and enabled researchers to design smaller and smaller endoscopes. The overarching goal of this work has been to image smaller previously inaccessible luminal organs in real time, at high resolution, in a minimally invasive manner that does not compromise the comfort of the subject, nor introduce additional risk. Thus, an initial diagnosis can be made, or a small precancerous lesion may be detected, in a small-diameter luminal organ that would not have otherwise been possible. Continuous advancement in the field has enabled a wide range of optical scanners.
2. Imaging Technologies
Imaging devices used in medical applications are characterized by their resolution. Non-invasive technologies, such as MRI, CT, and ultrasound, provide in vivo imaging of tissue structures with a large penetration depth, but the resolution is limited. Endoscopic imaging permits imaging body structures with finer details, however, they are invasive in some cases. Fiber optics allow the fabrication of imaging devices that are flexible and can image the target area via hollow cavities. The recent advances in the optical and mechanical fields allow in vivo imaging of tissue surfaces to be performed by using devices having a very fine resolution at the micrometer level.
In an endoscopic optical imaging device, the laser light is illuminated on the target surface using an optical fiber or by deviating the light by micro mirrors. The image can be either reconstructed by direct reflectance, or via detection of the fluorescence light using optical fibers, lenses, or CCD/CMOS cameras. In the case of direct reflectance imaging, the morphological information of the tissue structure is obtained, while fluorescence provides information about the inner cell or tissue structures by labelling them using fluorophores. Fluorophore materials are usually added from outside to stain the target sample and emit light at a wavelength higher than that of illumination, increasing the contrast in the images.
In biomedical imaging devices, OCT is one of the most used techniques. OCT is a direct reflectance imaging technique where the imaging contrast is provided by the change in the refractive index of the tissue media causing a variation of intensity of the reflected light[1]. Most of the current OCT systems are FD-OCT based, where the information about the depth scan is calculated by using the inverse Fourier transform of the backscattered light spectrum. The fast-axial scanning is obtained by sweeping the light source, which gives high-resolution images due to fast signal acquisition and provides images with a large signal to noise ratio. Given the current state of the art, ophthalmology is the predominating application for OCT due to the low laser output compatible with the ophthalmological structures permitting the imaging without damaging the tissue structures[2][3][4]. The transparency and low light scattering of the aqueous humor media allows imaging of the ocular surfaces at a greater depth with resolution independent of the surface depth. In addition to ophthalmology, deep tissue penetration and high resolution morphological and functional imaging make OCT a promising technology to assess the artery lumens in cardiology[5], tissue structures of the biliary tract, and the gastrointestinal (GI) tract, especially for Barrett’s esophagus[6]. Furthermore, the skin is a highly light-scattering tissue that contains a large number of inhomogeneities. OCT makes it possible to detect and diagnose various skin diseases and lesions[7]. OCT is also a useful method to monitor lesions in the enamel and dental structures of the teeth[8].
Another promising technology is confocal microscopy, which provides high-resolution imaging of thick specimens by virtually slicing them using optical sectioning[5]. It is possible to image the tissue structures in either confocal or fluorescence mode. In the latter case, the target tissue structure is stained using fluorescent dyes to enhance the contrast in the image. Combining confocal microscopy with the optical fibers in a fibered confocal fluorescence microscopy (FCFM), Cell-viZio-developed mini optical probes can be used for microcirculation imaging of the stomach, ear, mesentery, kidney, and conjunctive tissues[9]. Other applications of confocal endomicroscopy include functional imaging of the GI tract[10], liver, pancreas, and reproductive tracts after the application of a fluorescent agent that provides contrast to the image[11].
In addition to OCT and confocal microscopy, nonlinear microscopy also finds many applications in tissue structure imaging. Nonlinear microscopy comprises multiphoton imaging, higher order harmonic generation, and coherent Raman scattering microscopy. These techniques allow the structural and chemical changes of cells, tissues, or organs to be inspected with deep tissue penetration and minimal photobleaching and phototoxicity. The use of a pinhole and the signal generation from only a localized specific area provide high-contrast images. The cellular level resolution allows these techniques to be used for imaging and diagnosing tumors at early stages in the skin and ovary. In particular, TPEF is used to image endothelial cells, while SHG is used to image collagen fibers, and CARS is used for the detection of chemical changes at the cellular levels[12].
Another technique is photoacoustic imaging. In this case, laser pulses are emitted towards the target tissue surface, which absorbs the light and generates an ultrasonic emission detected by an ultrasonic transducer. Thus, the imaging contrast is based on the absorption spectra of the media. These devices find applications in measuring the oxygen level in the blood and guiding surgical tools during surgical procedures. Furthermore, photoacoustic imaging is particularly suitable to image the breast, brain, and GI tract for cancer diagnosis due to the high optical absorption contrast[13][14].
Apart from imaging techniques, optical devices can monitor the target sample either from a side of the distal end perpendicular to its axis, or from the front end of the device providing a peripheric or en face image, respectively. In side-viewing devices, the probe is rotated to visualize the circumferential structural area. A forward-viewing imaging probe provides an image of the front surface and can be used as a guide for surgical procedures. It should be noted that forward-viewing imaging requires a transverse scan of the light beam, which poses challenges in the miniaturization of the imaging device.
In advanced imaging systems, the resolution of images is improved by scanning the laser beam across the surface. Often the systems are vibrated at resonance to get maximum light displacement at the distal end of the scanner. Since it is difficult to get resonant scanners for applications requiring low-frequency scanning, imaging devices based on the use of non-resonant or semi-resonant frequencies have been developed for this specific requirement.
Optical fibers are mainly actuated using electrostatic, electrothermal, piezoelectric, electromagnetic, or shape memory alloys. The electrostatic actuators are easy to fabricate and rapidly responsive but have a limited scanning range and moderate actuation force. On the other hand, electrothermal actuators can be made in very small dimensions and can provide large forces. In this case, they can actuate at very high frequencies, but the heat dissipation can compromise the material performance. Piezoelectric actuators are widely used in optical fiber endoscopes because of their fast response. However, the manufacturing of very small-sized endoscopes based on these actuators pose some challenges. The electromagnetic and shape memory alloy actuators are better suited for larger dimensional scales.
Base on the type of the actuator and power/voltage profile provided as the input, it is possible to generate 2-D scanning patterns in a raster, spiral, Lissajous, circular, or propeller shape. Raster and Lissajous patterns provide uniform light intensity within the light scanning area. The spiral pattern is easy to obtain but has more light intensity in the center compared to the edges. Each pattern has its own advantages and disadvantages. Table 1 summarizes the pros and cons of the different scanning patterns in detail.
Table 1. Comparison table for different scanning patterns
|
|
Raster |
Spiral |
Lissajous |
Circular |
Propeller |
|
Scanning pattern |
 |
 |
 |
 |
 |
|
Actuation pattern |
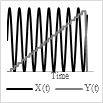 |
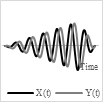 |
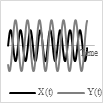 |
 |
Y(t) constant rotation |
|
Advantages |
Uniform light intensity |
Easy to get, area is swept by changing the driving voltage |
Uniform light intensity, most used |
Possible to get circular pattern with 1D actuation, area is swept by changing the driving voltage |
Easy to generate |
|
Disadvantages |
Points are scanned at different times can lead to motion artifacts |
Light intensity is higher in center |
Fill factor highly depends on the frequency ratio, quasi-random pattern |
Light intensity is higher in center |
Non uniform light intensity, the rotation of miniaturized structure requires complex and expensive devices |
A descriptive summary of some recently developed optical imaging devices for medical purposes using a variety of different imaging techniques, configurations, actuating methods, and scanning patterns is shown in Table 2. The scanning catheter developed by Aguirre et al. is a side-viewing OCT endoscope, where the light is transported to the distal end using an SMF, and the forward mirror used to deflect the light is vibrated at resonance using angular vertical electrostatic comb drives and generates a raster scanning pattern[15]. Park et al. developed a forward-viewing spectral domain OCT endomicroscope. In this case, an SMF is excited at resonance using piezoelectric tube actuators and describes a Lissajous scanning pattern[16]. Myaing et al. reported a forward-viewing two-photon fluorescence microscope. A double clad fiber (DCF) is used to deliver light to the target sample and collect the fluorescence signal. The spiral pattern is generated by the fiber tip using a piezoelectric actuator exciting the tip at resonance[17]. Recently, Li et al. developed a 2.4-mm confocal endomicroscope using electrostatic actuators for laser light scanning. Such a probe with a 1.5-µm lateral and 12-µm axial resolution is compatible with the finer operating channel of current endoscopes[10].
Table 2. Comparison of some advanced optical scanning devices.
| Working Principle | Frequency | FOV | Drive Voltage | Scanner Dimensions | Scanning Pattern | References | |
|---|---|---|---|---|---|---|---|
| OCT using rotational MEMS probe | Micromotor | 1 kHz | - | >1 V | 2.4 mm (in diameter) | Radial | [18] |
| MEMS fiber scanner for confocal microscopy | Electrothermal actuation | 239 Hz (x-axis) 207 Hz (y-axis) | 378 µm × 439 µm | 16 Vpp (duty cycle 13%) |
Diameter (1.65 mm) Rigid length (28 mm) |
Lissajous scanning | [19] |
| Fiber scanner for forward viewing endoscope | Piezoelectric tube | 86 Hz (x-axis) 97 Hz (y-axis) | 732 µm × 591.7 µm | 40 Vac | Diameter (3.2 mm) Rigid length (50 mm) |
Lissajous scanning | [16] |
| OCT based on 2D MEMS mirror | Electrothermal actuation | 1.25 Hz (fast scan actuator pair) 0.0125 Hz (longitudinal) |
2.3 mm × 2.3 mm | 0–4 V ramp (fast scan) 0.5–3.5 V ramp (slow axis) |
Diameter (5.8 mm) Rigid length (12 mm) |
Lissajous scanning | [20] |
| Scanning fiber endoscope | Piezoelectric tube | 5 kHz | 200 µm (in diameter) | <20 Vac | Diameter (1.2 mm -1.7 mm) Rigid length (9 mm) |
Spiral scan pattern | [21][22] |
| Multi-Photon Endoscope | Piezoelectric tube | 35 Hz | 900 µm (in diameter) | 40 Vac | Diameter (5 mm) Rigid length (4 cm) | Circular pattern | [23] |
| OmniVision camera | Chip on tip camera | - | 364 µm × 364 µm | 3.3 Vac | 650 µm × 650 µm × 1158 µm | - | [24] |
| FCFM using Cellvizio | Scanning mirrors at proximal end | 4 kHz | 160 µm × 120 µm ÷ 400 µm × 280 µm | - | 350 µm ÷ 1.8 mm diameter 20 mm Rigid length | - | [9] |
| Scanning confocal microscope | Electrostatic torsional mirrors | 4.3 kHz (fast scan) 1.07 kHz (slow scan) | 100 µm (in diameter) | 20 V | 1.2 mm × 2.5 mm × 6.5 mm | Lissajous scanning | [25][26] |
| Two-photon microscope | Electrostatically actuated mirrors | 1.08 kHz (fast scan) 0.65 kHz (slow scan) | 295 µm × 100 µm | 60 V | 2.0 cm × 1.9 cm × 1.1 cm | Raster scanning | [27] |
| Side view endomicroscope | Electrostatically driven mirror | 12 kHz (fast scan) 3 kHz (slow scan) | 350 µm × 350 µm | 60 Vpp | 2.4 mm (in diameter) | Lissajous scanning | [10] |
| Non-resonant MEMS scanner for OCT | Thin film piezoelectric | 50 Hz | 1 mm × 0.7 mm | 40 Vpp | 2.2 mm × 2.7 mm | Lissajous scanning | [28] |
In summary, the choice of an optical scanner over another type often depends on the target tissue and the constraints it poses. For example, some tissue surfaces, such as the eye, cannot tolerate shining a laser light on it for a long period of time due to the energy of the laser beam causing photoablation of the tissue. Thus, some scanners are better fit for imaging certain tissue surfaces than others. Similarly, some of the optical scanning devices are better at imaging the tissue surface by illuminating the light from the side of the probe, while others are designed for direct en face imaging. In addition, the selection of an imaging device for a medical purpose is also based on the size, the usage simplicity, and the ergonomics of the device. Another relevant consideration for endoscopic devices is their suitability for reprocessing; that is their suitability multiple uses. Reprocessing, which generally requires washing optical devices using chemicals to reduce risks of infection, can be a very expensive process. Endoscopes should also be mechanically robust; the handling of the endoscopes during reprocessing may damage the endoscopes. For example, a small crack could be initiated, leading to light leakage and the consequent considerable degradation of the performance of the device.
References
- Wang, L.V.; Wu, H.-I.. Biomedical Optics: Principles and Imaging; Wiley: Hoboken, NJ, USA, 2007; pp. 181-210.
- Huang, D.; Li, Y.; Jia, Y.. Clinical OCT Angiography Atlas; Lumbroso, B.; Huang, D.; Chen, C.J.; Jia, Y.; Rispoli, M.; Romano, A.; Waheed, N.K., Eds.; Jaypee Brothers Medical Publishers: New Delhi, India, 2015; pp. 39-41.
- Benjamin J. Kim; David J. Irwin; Delu Song; Ebenezer Daniel; Jennifer D. Leveque; Aaishah R. Raquib; Wei Pan; Gui-Shuang Ying; Tomas S. Aleman; Joshua L. Dunaief; et al.Murray Grossman Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 2017, 89, 1604-1611, 10.1212/wnl.0000000000004500.
- J. P. Cunha; Rita Proença; Arnaldo Dias-Santos; Rita Almeida; Helena Águas; Marta Alves; Ana Luísa Papoila; Carlota Louro; António Castanheira-Dinis; OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. European Journal of Applied Physiology 2017, 255, 1827-1835, 10.1007/s00417-017-3715-9.
- Boudoux, C.. Fundamentals of Biomedical Optics: From Light Interactions with Cells to Complex Imaging Systems; Pollux: Montreal, QC, Canada, 2017; pp. 261-403.
- Brett E Bouma; G J Tearney; Clinical imaging with optical coherence tomography.. Academic Radiology 2002, 9, 942-953, 10.1016/s1076-6332(03)80465-8.
- N.D. Gladkova; G. A. Petrova; N. K. Nikulin; S. G. Radenska-Lopovok; L. B. Snopova; Yu. P. Chumakov; V. A. Nasonova; V. M. Gelikonov; V. M. Gelikonov; R. V. Kuranov; et al.A. M. SergeevF. I. Feldchtein In vivo optical coherence tomography imaging of human skin: norm and pathology. Skin Research and Technology 2000, 6, 6-16, 10.1034/j.1600-0846.2000.006001006.x.
- Daniel Fried; John Xie; Sahar Shafi; John D. B. Featherstone; Thomas M. Breunig; Charles Q. Le; Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. Journal of Biomedical Optics 2001, 7, 618-628, 10.1117/1.1509752.
- Elisabeth Laemmel; Magalie Genet; Georges Le Goualher; Aymeric Perchant; Jean-François Le Gargasson; Eric Vicaut; Fibered Confocal Fluorescence Microscopy (Cell-viZio™) Facilitates Extended Imaging in the Field of Microcirculation. Journal of Vascular Research 2004, 41, 400-411, 10.1159/000081209.
- Gaoming Li; Xiyu Duan; Miki Lee; Mayur Birla; Jing Chen; Kenn R. Oldham; Thomas D. Wang; Haijun Li; Ultra-Compact Microsystems-Based Confocal Endomicroscope. IEEE Transactions on Medical Imaging 2020, 39, 2406-2414, 10.1109/tmi.2020.2971476.
- Martin Goetz; Confocal Laser Endomicroscopy: Applications in Clinical and Translational Science—A Comprehensive Review. ISRN Pathology 2011, 2012, 1-13, 10.5402/2012/387145.
- Adur, J.; Carvalho, H.F.; Cesar, C.L.; Casco, V.H.. Microscopy and Analysis; Stanciu, S.G., Eds.; IntechOpen: London, UK, 2016; pp. 121-149.
- Junjie Yao; Lihong V. Wang; Photoacoustic microscopy. Laser & Photonics Reviews 2013, 7, 758-778, 10.1002/lpor.201200060.
- Erich W. Stein; Konstantin Maslov; Noninvasive mapping of the electrically stimulated mouse brain using photoacoustic microscopy. Photons Plus Ultrasound: Imaging and Sensing 2008: The Ninth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-optics 2008, 6856, 68561J, 10.1117/12.769508.
- Aaron D. Aguirre; Paul R. Hertz; Yu Chen; James G. Fujimoto; Wibool Piyawattanametha; Li Fan; Ming C. Wu; Two-axis MEMS Scanning Catheter for Ultrahigh Resolution Three-dimensional and En Face Imaging.. Optics Express 2007, 15, 2445-2453, 10.1364/oe.15.002445.
- Hyeon-Cheol Park; Yeong-Hyeon Seo; Ki-Hun Jeong; Lissajous fiber scanning for forward viewing optical endomicroscopy using asymmetric stiffness modulation. Optics Express 2014, 22, 5818-5825, 10.1364/oe.22.005818.
- Mon Thiri Myaing; Daniel J. Macdonald; Xingde Li; Fiber-optic scanning two-photon fluorescence endoscope. Optics Letters 2006, 31, 1076-1078, 10.1364/ol.31.001076.
- Peter H. Tran; David S. Mukai; Matthew Brenner; Zhongping Chen; In vivo endoscopic optical coherence tomography by use of a rotational microelectromechanical system probe.. Optics Letters 2004, 29, 1236-1238, 10.1364/ol.29.001236.
- Yeong-Hyeon Seo; Kyungmin Hwang; Ki-Hun Jeong; 165 mm diameter forward-viewing confocal endomicroscopic catheter using a flip-chip bonded electrothermal MEMS fiber scanner. Optics Express 2018, 26, 4780-4785, 10.1364/oe.26.004780.
- Jingjing Sun; Shuguang Guo; Lei Wu; Lin Liu; Se-Woon Choe; Brian S. Sorg; Huikai Xie; 3D In Vivo optical coherence tomography based on a low-voltage, large-scan-range 2D MEMS mirror. Optics Express 2010, 18, 12065-12075, 10.1364/oe.18.012065.
- Cameron M. Lee; John E. Chandler; Eric J. Seibel; Wide field fluorescence imaging in narrow passageways using scanning fiber endoscope technology. BiOS 2010, 7558, 755806, 10.1117/12.842617.
- Eric J. Seibel; Richard S. Johnston; C. David Melville; A full-color scanning fiber endoscope. Biomedical Optics 2006 2006, 6083, 608303, 10.1117/12.648030.
- Farhad Akhoundi; Yukun Qin; N. Peyghambarian; Jennifer K. Barton; Khanh Kieu; Compact fiber-based multi-photon endoscope working at 1700 nm. Biomedical Optics Express 2018, 9, 2326-2335, 10.1364/boe.9.002326.
- Omnivision . OVM6948. Retrieved 2020-10-13
- D. L. Dickensheets; G. S. Kino; Micromachined scanning confocal optical microscope. Optics Letters 1996, 21, 764, 10.1364/ol.21.000764.
- D.L. Dickensheets; G.S. Kino; Dickensheets D.L.; Kino Gordon S.; Silicon-micromachined scanning confocal optical microscope. Journal of Microelectromechanical Systems 1998, 7, 38-47, 10.1109/84.661382.
- Wibool Piyawattanametha; Eric D. Cocker; Laurie D. Burns; Robert P. J. Barretto; Juergen C. Jung; Hyejun Ra; Olav Solgaard; Mark J. Schnitzer; In vivo brain imaging using a portable 29 g two-photon microscope based on a microelectromechanical systems scanning mirror. Optics Letters 2009, 34, 2309-2311, 10.1364/ol.34.002309.
- Takayuki Naono; Takamichi Fujii; Masayoshi Esashi; Shuji Tanaka; Non-resonant 2-D piezoelectric MEMS optical scanner actuated by Nb doped PZT thin film. Sensors and Actuators A: Physical 2015, 233, 147-157, 10.1016/j.sna.2015.06.029.