Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Turkan KOPAC | -- | 3065 | 2022-08-10 17:05:27 | | | |
| 2 | Conner Chen | Meta information modification | 3065 | 2022-08-11 02:33:48 | | | | |
| 3 | Conner Chen | + 14 word(s) | 3079 | 2022-08-11 02:43:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Özdemir, O.; Kopac, T. Applications for Nanotechnology in Endodontics. Encyclopedia. Available online: https://encyclopedia.pub/entry/26047 (accessed on 08 February 2026).
Özdemir O, Kopac T. Applications for Nanotechnology in Endodontics. Encyclopedia. Available at: https://encyclopedia.pub/entry/26047. Accessed February 08, 2026.
Özdemir, Olcay, Turkan Kopac. "Applications for Nanotechnology in Endodontics" Encyclopedia, https://encyclopedia.pub/entry/26047 (accessed February 08, 2026).
Özdemir, O., & Kopac, T. (2022, August 10). Applications for Nanotechnology in Endodontics. In Encyclopedia. https://encyclopedia.pub/entry/26047
Özdemir, Olcay and Turkan Kopac. "Applications for Nanotechnology in Endodontics." Encyclopedia. Web. 10 August, 2022.
Copy Citation
Nanotechnology has been utilized in several different parts of dentistry. Nanomaterials can be employed as irrigation, intracanal medicament, obturation materials and sealers.
nanotechnology
nanomaterials
nano-characterization techniques
nano-dentistry
endodontics
nano-endodontology
1. Applications for Nanotechnology in Endodontics
Nanotechnology has been utilized in several different parts of dentistry [1]. Nanomaterial studies have resulted in the development of novel materials which improved the clinical outcomes significantly [2]. The scope and applications of nanotechnology in endodontics have been described in a number of studies in the literature [1][2][3][4][5][6].
Successful endodontic treatment involves some major procedures such as biomechanical steps, disinfection, 3D sealing, and obturation of the root canal system [7][8]. Failure might occur owing to the insufficiency of biomechanical steps related with the root canal system anatomy, also the microleakage of sealing materials, in spite of the many successful root canal treatment applications. The microleakage arises mostly due to the probable deficiencies in the quality [9][10]. The irrigant activation and cleaning plays an important role in the success and failure of endodontic treatment. It was reported that irrigant activation promoted better pulp tissue dissolution in comparison to syringe/needle protocol, and pulp tissue dissolution was significantly higher when heating was followed by sonic/ultrasonic activation [11][12]. Some materials used in endodontics might have specific shortcomings such as shrinkage, moisture sensitivity, and dissolution in the oral medium [13]. Progress in the synthesis of novel materials having better quality sealing and biomechanical features will enable the endurance of the success of endodontic treatment [2].
Research related to the nano-applications in endodontics has been inaugurated in several treatment fields. Nanomaterials having superior resistance toward wear and fatigue are proposed for the surface modification of rotary nickel–titanium (Ni-Ti) files presently used in root canal treatment. Nanoparticles (NPs) would be effective for the improvement of the medicament, irrigant, sealers, and obturating materials, and drug elutions would help for the improvement of sealing and disinfection of root canal systems. Regenerative endodontics applications are currently in progress, generating enhanced scaffolds [1].
There are a considerable amount of successful research activities progressing in the field of endodontics which attempt to amend several clinical directions such as files and filling constituents. Some NPs show better antimicrobial characteristics, which can improve the efficacy of endodontic materials, intracanal medicaments and irrigation solutions, because of their capability to spread better into the complex anatomical parts of the root canal systems owing to their particle size [2][14][15]. Most studies have continuously concentrated on the synthesis and utilization of nanocomposites obtained by the modification of nanomaterials. These NPs could fortify the sealing characteristics during obturation, which could be applied as root repair and root-end filling materials [2][15][16][17].
2. Nanomaterials in Endodontics
Nanomaterials can be employed as irrigation, intracanal medicament, obturation materials and sealers [4]. The functional applications of nanomaterials in endodontics are shown in Figure 1. The types of nanomaterials in endodontic applications can be mainly classified as organic NPs and inorganic NPs. Graphene, chitosan, and poly (lactic) co-glycolic acid are among the organic nanomaterials that are used. Inorganic nanomaterials involve bioactive glass, calcium silicate (Ca2SiO4 or 2CaO·SiO2), hydroxyapatite (Ca5(PO4)3(OH)), silver compounds, and metal oxides. Bioactive glass can be composed of silicon dioxide (SiO2), sodium oxide (Na2O), and phosphorus pentoxide (P2O5). Metal oxides may involve the oxides of iron (FeOx), zirconium (ZrO2), titanium (TiO2), calcium (CaO), magnesium (MgO), copper (CuO), and silicon (SiO2). Such nanomaterials have shown promising results in endodontics [4][18]. Figure 2 shows the main types of nanomaterials employed in endodontic applications. However, it should be noted that the materials might somehow be subject to long-term degradation. Thus, the issues related with the problems of deterioration that might occur simultaneously need to be taken into consideration. In addition, the problems of aging or long-term degradation due to either radiation degradation manifesting during radiation treatments or surface degradation might also be significant [19][20].
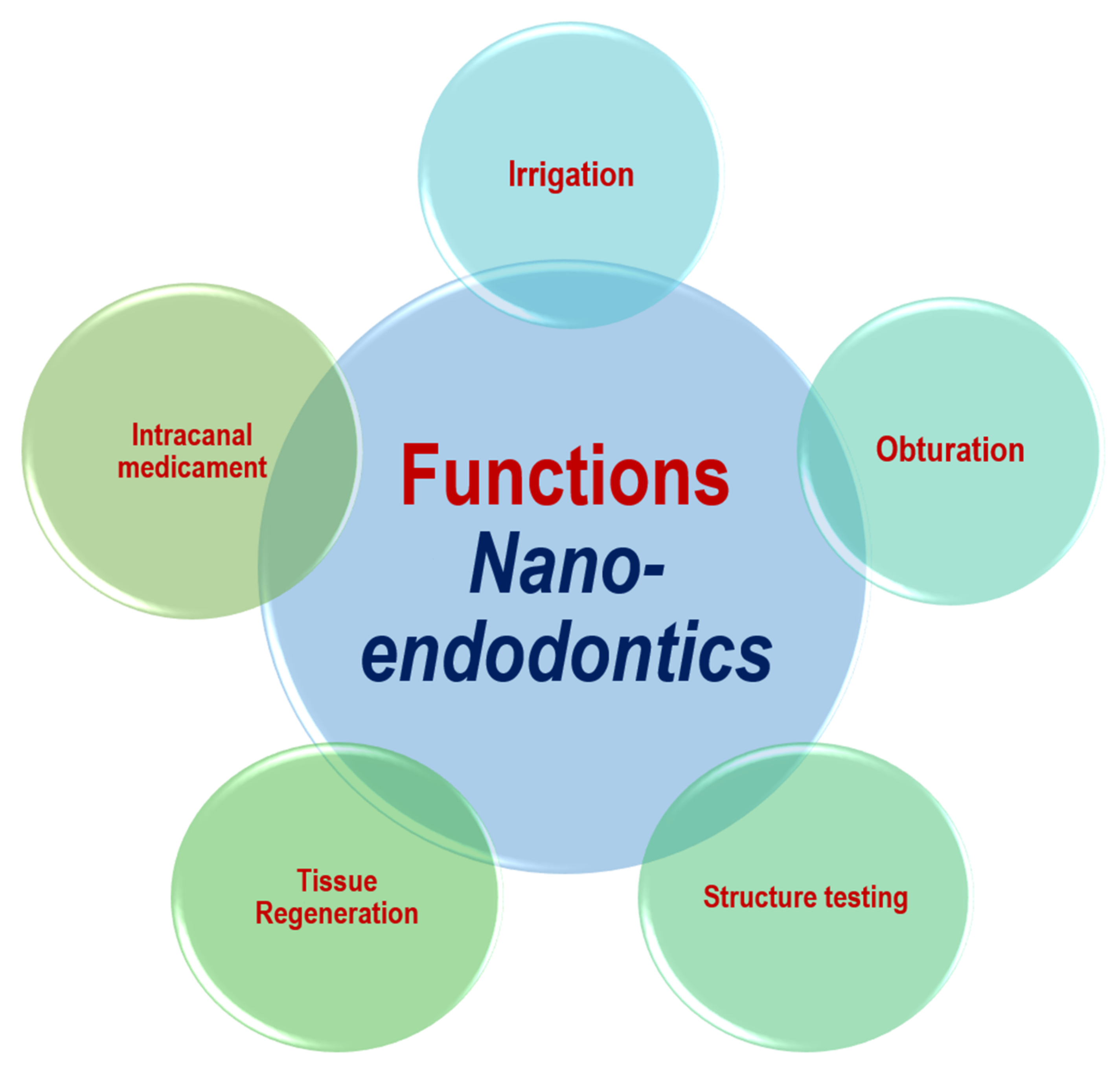
Figure 1. Functional applications of nanotechnology in endodontics.
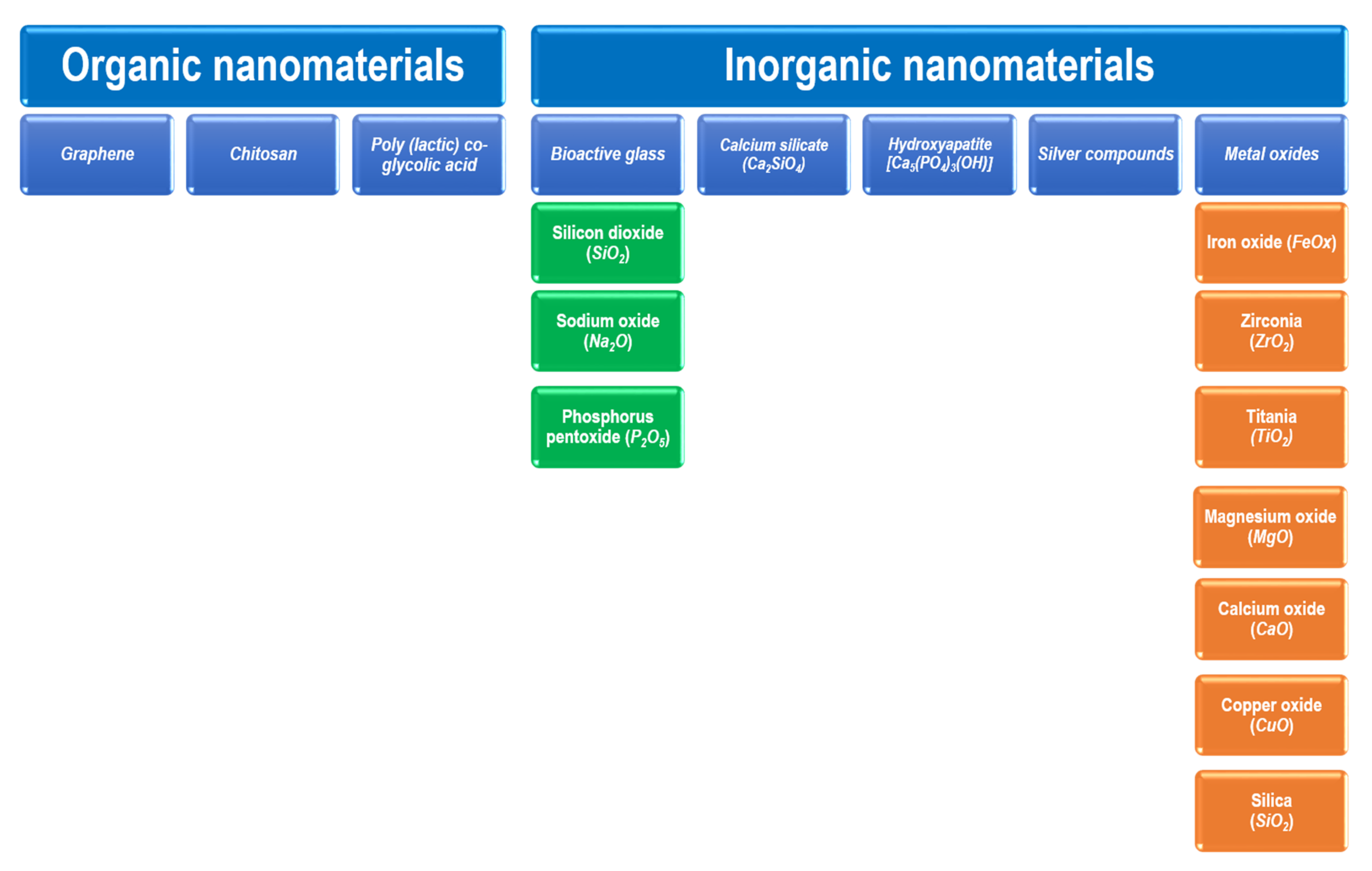
Figure 2. Types of nanomaterials employed in endodontic applications.
It has been confirmed that the most efficient disinfection of root canals could be through the use of NPs owing to their broad-spectrum antibacterial activities [3][18][21]. The nanomaterials evaluated for disinfection in endodontics practice include zinc oxide, silver chloride and chitosan nanoparticles [18]. The efficacy of zinc oxide and chitosan against Enterococcus faecalis (E. faecalis) was owing to their capability to break up the cell wall. Additionally, these have the ability to break down the biofilms within the root canals [21][22][23]. Silver NPs are effective as root canal disinfection agents. It was indicated that a silver NP (0.02%) gel was able to disrupt and kill the E. faecalis biofilm [24][25].
A kind of bioactive glass NPs have been employed efficiently as an antimicrobial agent for root canal disinfection [26][27]. Its antimicrobial effect is owing to its capability to sustain the alkaline environment for a longer time. Studies have shown that an increase in silica release and pH level was possible via the employment of a specific type of a nanometric bioactive suspension and micrometric hybrid material [6][28]. Fioretti et al. [29] reported the use of multilayered functional groups involving nanostructured films which contain a melanocortin peptide as a novel biomaterial for endodontic regeneration.
Mineral trioxide aggregate (MTA) has been utilized commonly in most endodontics applications. Some studies concerned the nano-modification of this material. For example, Saghiri et al. [30] investigated the nano-modification of MTA and investigated the enhancement of its physiochemical properties. They analyzed the properties of WMTA (white) and NWMTA (nano white) and made a comparison. After preparation, WMTA and NWMTA were mixed together. The specific surface area before hydration, setting time, X-ray crystallography (XRD) and microhardness at different pH conditions were evaluated by BET, ISO 6876, Vickers hardness and EDS analysis techniques, respectively, for both materials. Significant differences in specific surface area, surface hardness and setting time were observed for the two materials. It was reported that an increase in specific surface area resulted in reduced setting time and increased microhardness even at lower pH conditions [30]. In another study, the changes of material physical properties and setting time were studied by Akbari et al. [31] when nano-SiO2 was added to WMTA. It was observed that nano-SiO2, which acted as a filler in cement, improved the microstructure and accelerated the process of hydration. The influence of a nano-BG (58S) for the odontogenic differentiation and mineralization of human dental pulp cells (hDPCs) was investigated by Gong et al. [32] using an in vitro analysis. Their study involved the preparation of extractions from the incubation of various particulates (58S, 45S5, nano-58S BG) in Dulbecco modified Eagle medium. They used the BG extractions in which the hDPCs were cultured as supernatants, and they studied the proliferation of hDPCs and the odontogenic differentiation depending on the polymerase chain reaction of genes related with differentiation and mineralization (ALP, collagen type I, DSPP, dentin matrix protein 1). They examined the gene expressions through ALP activity evaluation, osteocalcin and DSPP immunocytochemistry staining, and mineralization assay. They reported that the nano-bioactive glass induced the differentiation and mineralization of hDPCs more effectively, and it could be proposed as a potential candidate for the regeneration of the dentin–pulp complex [32].
Saghiri et al. [33] evaluated the impact of bismuth oxide as a radiopaque additive, investigating the influence of particle size and radiopacity of some type of cements based on tricalcium silicate (CSC). Different CSC kinds were used in their study (CSC, CSC + 10% bismuth oxide (10 μm), CSC + 20% bismuth oxide, CSC + 10% nano-bismuth oxide (50–80 nm), CSC + 20% nano-bismuth oxide, nano-WMTA (40–100 nm)). The radiopacity, compressive strength and surface microhardness analysis were carried out on the samples. It was reported that the 20% nano-bismuth oxide addition improved the physical properties without any considerable change of radiopacity, and lower values of physical properties were observed with the 10 μm sized bismuth oxide-containing CSC material. In another study, the biocompatibility of the NPs based on active CS and hydroxyapatite (HA-CS) systems was studied by Petrović et al. [34], in which the in vitro cytotoxic and in vivo inflammatory responses to the materials were evaluated. In the followed methodology, the cytotoxicity of the eluates of the materials was examined employing the MTT assay on MRC-5 cells. Test samples involving polyethylene tubes were implanted in the subcutaneous tissue of Wistar rats, and histopathological evaluation was carried out. According to the results, HA-CS caused rather thick capsules, while MTA (control) resulted in thin capsule formation. Cytotoxic and inflammatory response evaluations showed the more effective biocompatibility of CS and HA-CS as compared to MTA [34]. According to the literature reports, the outcome of HA NPs indeed depends on their resistance to aging, including radiation [19][20].
The evaluation of the angiogenic properties of some vital pulp therapy materials such as WMTA, calcium hydroxide (CH), Geristore, and nano-WMTA was investigated in a study conducted by Saghiri et al. [35]. In their study, materials were prepared in the form of disks dispersed into water; then, they were centrifuged for obtaining supernatant elution. Mice molar endothelial cells (ECs) were left on the prepared hydrogel arrays. For the evaluation of the investigations according to the choroidal neovascularization (CNV) model, female mice (6 weeks old) were laser treated, and elution from samples were taken by injection on the laser running day and after 1 week. It was reported that the results indicated minimum antiangiogenic activity, while Geristore and nano-WMTA confirmed more significant proangiogenic activities [35].
Naseri et al. [36] evaluated the microhardness and superficial chemical structure of radicular dentin by the addition of nano-CH by an in vitro work. It was reported that CH and NCH were effective in intracanal medicaments on the chemical and physical features of dentin. In the trial, a number of dentin discs were prepared as control and treatment groups using pastes of CH and NCH. Dentin microhardness was assessed after a certain period of time using the Vickers test, and the Fourier transform infrared (FTIR) analysis was used for chemical characterization. The use of CH in intracanal medicament reduced the microhardness of dentin (4 weeks), while NCH did not cause any change; on the other hand, a chemical structure change was observed 1 week later for both materials [36].
In a study, Yang et al. [37] investigated the antibiofilm effect of an auxiliary irrigant solution (proanthocyanidin PA) on E. faecalis along with the effects on the biodegradation and mechanical resistance properties of demineralized root dentine. In their followed method, E. faecalis was added into human root dentine tubules applying a series of centrifugation procedures and then left to grow for a period of 1 week. Dentine blocks affected by E. faecalis were processed with various irrigant solutions such as sterile water, chlorhexidine (CHX), and PA. Then, the bacteria within E. faecalis biofilms (live, dead) were identified using a confocal laser scanning microscope. The hydroxyproline release and elastic modulus of human dentine were characterized for the evaluation of the biostability. The demineralized dentine collagen was analyzed by X-ray photoelectron spectroscopy (XPS) for surface chemical characterization. According to the results, PA was effective at killing E. faecalis within biofilms and improved the biostability of the demineralized root dentine collagen matrix. It was proposed that PA could be applied effectively as an auxiliary endodontic irrigant for antibiofilm and collagen stabilizing [37]. In another study, the regeneration of copper–calcium hydroxide (Cupral)-endodontically treated teeth with apical periodontitis was evaluated via the employment of an electrophoresis technique by Meto et al. [38]. It was reported that the Cupral-electrophoresis methodology was effective in treating destructive periodontitis of teeth with problematic canals up to 18 months allowing teeth preservation.
3. Nano-Testing of Structures in Endodontic Applications
The Ni-Ti endodontic rotary file is one of the widely utilized instruments in dental applications. The alloys employed have many advantages such as high resistance to corrosion and superelasticity that grant them with good shape memory. So, exploring the complicated anatomy of the root canal for a suitable endodontic treatment would be possible. It was reported that cobalt coatings of a Ni-Ti file with fullerene-like WS2 impregnation on NPs resulted in considerable improvements in the breakage time and fatigue resistance [2].
External cervical resorption (ECR), which is the dental hard tissue loss due to the action of odontoclasts, associates dental, periodontal and pulpal tissues in the following phases and involves a dynamic mechanism. Over the recent years, ECR has drawn increased attention, owing to the advanced micro-CT, histopathological and radiographic CBCT detection techniques. However, it is reported that further work is necessary for the confirmation of the causes and effects of some possible influencing factors. The maxillary central incisor, maxillary canine, maxillary lateral incisor, mandibular first molar and maxillary first molar teeth are mostly affected, respectively. The corresponding steps in the ECR process involve initiation, following progression and resorption, then reparative phases. Resorption, repair or remodeling might develop concurrently at varying parts of the infected tooth. The improved accuracy of CBCT analysis leads to the more accurate identification and evaluation of ECR along with determination of the best treatment procedure [39].
In a study conducted by Zinelis et al. [40], a nano-indentation technique was used for the evaluation of the in-depth hardness profiles of Ni-Ti and stainless steel (SS) instrument cross-sections, utilizing three instruments of each kind. Hardness profiles were determined toward the center (2000 nm) by an MTS XP nanoindenter. According to the results, a drop in hardness was observed as moving to the center for the endodontic instruments, which implied that the surface hardness was considerably improved depending on the applied techniques. The cyclic fatigue effect on Ni-Ti endodontic instruments was investigated by Jamleh et al. [41] using a nano-indentation analysis. In the investigation, several numbers of fractured and new Ni-Ti rotary instruments were analyzed, and they reported that the technique could be utilized for the determination of the performance along with the failure mechanism of the instruments. The fatigue analysis indicated a considerable decrease in the elastic modulus and hardness of the instruments.
A methodology composed of a combination of techniques for the investigation of a central incisor with a large ECR case was introduced in a study by Mavridou et al. [42]. The diagnosis was based on clinical inspection, cone-beam computed tomography (CBCT) and digital radiography. The tooth was examined by micro-CT, nano-CT and hard tissue histology after extraction. It was reported that the nano-CT was more efficient than the other techniques. The reparative tissue, pulp tissue reactions, pericanalar resorption-resistant sheet, resorbed canals and their interconnection with the periodontal ligament space were determined by nano-CT technique. The methodology was proposed as a fast and minimal invasive method to study the ex vivo evaluation of ECR together with the hard tissue histology. The approach of combining clinical and CBCT along with the nano-CT and histological mapping analysis was proposed as an ideal method for ECR identification. Mavridou et al. [43] studied the mechanisms and properties of ECR patterns in endodontically treated teeth and teeth with vital pulps (seven cases of each). The diagnosis depended on clinical and radiographic CBCT analysis results. The extracted teeth were also examined by scanning electron microscopy (SEM), hard-tissue histology and nano-CT. They observed similar ECR patterns consisting of initiation, resorption, and then reparative steps in all the teeth, while some differences were observed in the resorption and reparative stages between the two types of teeth. The resorption stage in root canal-filled teeth was more intense than the repair stage due to the clastic cells and the presence of granulation tissues as well as the absence of the pulp and protective PRRS layer and due to the chemical composition change of the root dentine after root canal treatment [43].
A study was conducted by Iacono et al. [44] for the comparison of the phase transformation behavior, microstructure, nano-hardness, elasticity modulus and the surface chemistry of HyFlex EDM and conventional HyFlex CM instruments, using analysis techniques such as XRD, DSC, Raman spectroscopy and FE-SEM. Considerable differences in measurement results of elasticity modulus and nano-hardness were determined between the two files. HyFlex EDM revealed enhanced phase transformation temperatures and hardness. Analysis results confirmed the enhanced mechanical behavior of the instruments [44].
A study was carried out by Petitjean et al. [45] on the assessment of a calcified extraradicular deposit on the apical root surfaces of both roots by the application of a multimodular approach involving a combination of multiple investigation methods. The root contained an apical periodontitis lesion, and a sinus tract was the only connection with the oral cavity. The related diagnosis and treatment were achieved using clinical, ultrasound and radiographic (2, 3D) examinations. Microscopic imaging, electron probe microanalysis (EPMA), hard/soft tissue histology and nano-CT were also used for the analysis of the structure and composition of the extraradicular deposit [45]. In a review paper presented by Patel et al. [39] on the histopathology and distribution of ECR, it was emphasized that CBCT appeared as a better technique because of the limited performance of regular periapical radiography in the detection and assessment of ECR.
Contrast-enhanced nano-CT was used by Hildebrand et al. [46] to investigate the dental ultrastructures (soft dental tissues, cellular layers) utilizing phosphotungstic acid (PTA) as an agent. In the method, sound third molars from healthy adults were put in paraformaldehyde buffered solutions, and the influence of PTA concentration on dental hard and soft tissues for CT identification was evaluated. The samples were also analyzed using a high-resolution nano-CT for the examination of the cementum and pulpal sections. A 3D investigation along with quantitative analysis of the dentine composition was obtained via the segmentation of the sigmoidal dentinal tubules and the surrounding dentine. It was reported that the staining protocol allowed the visualization of hard and soft tissues along with cellular layers in teeth using nano-CT imaging, and the protocol depended on the tissue type and size. The method offered an improved opportunity for the concomitant visualization of hard and soft dental tissues [46].
In a study, nano-CT analysis was utilized in the evaluation of the total obturation volumes and voids for various obturation techniques by Holmes et al. [47]. Their study was based on the consideration that the material and the technique used did not have any effect on the total obturation volume or voids. Using maxillary left central incisor 3D-printed replicas and different obturation groups in the investigation, nano-CT along with volumetric analysis were performed after obturation. It was reported that the materials and the obturation technique considerably affected the voids and the total volume of obturation material [47]. The disinfecting and shaping characteristics of some preparation protocols in C-shaped root canals were evaluated by Gazzaneo et al. [48] employing a correlative microcomputed tomographic and molecular microbiology work. The BioRaCe and XP-endo Shaper systems were reported to have similar disinfection and shaping properties in mandibular molars having C-shaped canals. In addition, the supplementary steps with the Hedström file and the XP-endo Finisher promoted similar decreases in the unprepared canal surfaces, whereas the effects were not sufficient to have significant improvements in bacterial elimination; thus, the development of more effective strategies would be needed for the disinfection of the mentioned canals [48].
References
- Chogle, S.M.A.; Kinaia, B.M.; Goodis, H.E. Scope of nanotechnology in endodontics. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–539.
- Alenazy, M.S.; Mosadomi, H.A.; Al-Nazhan, S.; Rayyan, M.R. Clinical considerations of nanobiomaterials in endodontics: A systematic review. Saudi Endod. J. 2018, 8, 163–169.
- Kishen, A.; Shrestha, A. Nanoparticles for Endodontic Disinfection. In Nanotechnology in Endodontics: Current and Potential Clinical Applications; Kishen, A., Ed.; Springer International Publishing Switzerland: Cham, Switzerland, 2015; pp. 97–119.
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21.
- Akbarianrad, N.; Mohammadian, F.; Nazari, M.A.; Nobar, B.R. Applications of nanotechnology in endodontic: A Review. Nanomed. J. 2018, 5, 121–126.
- Markan, S.; Lehl, G.; Kapoor, S. Recent Advances of Nanotechnology in Endodontics, Conservative and Preventive Dentistry-A Review. J. Dent. Oral Biol. 2017, 2, 1067.
- Sjögren, U.; Hägglund, B.; Sundqvist, G.; Wing, K. Factors affecting the long-term results of endodontic treatment. J. Endod. 1990, 16, 498–504.
- Carrotte, P.V. Endodontics: Part 8 Filling the root canal system. Br. Dent. J. 2004, 197, 667–672.
- Lin, L.M.; Skribner, J.E.; Gaengler, P. Factors associated with endodontic treatment failures. J. Endod. 1992, 18, 625–627.
- Ray, H.A.; Trope, M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int. Endod. J. 1995, 28, 12–18.
- Iandolo, A.; Simeone, M.; Orefice, S.; Rengo, S. 3D cleaning, a perfected technique: Thermal profile assessment of heated NaOCl. Detersione 3D, una tecnica perfezionata: Valutazione dei profili termici dell’NaOCl riscaldato. G. Ital. Endod. 2017, 31, 58–61.
- Iandolo, A.; Amato, M.; Abdellatif, D.; Barbosa, A.F.A.; Pantaleo, G.; Blasi, A.; Franco, V.; Silva, E.J.N.L. Effect of different final irrigation protocols on pulp tissue dissolution from an isthmus model. Aust. Endod. J. 2021, 47, 538–543.
- Gandolfi, M.G.; Spagnuolo, G.; Siboni, F.; Procino, A.; Rivieccio, V.; Pelliccioni, G.A.; Prati, C.; Rengo, S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral Investig. 2015, 19, 2075–2089.
- Shrestha, A.; Fong, S.-W.; Khoo, B.-C.; Kishen, A. Delivery of Antibacterial Nanoparticles into Dentinal Tubules Using High-intensity Focused Ultrasound. J. Endod. 2009, 35, 1028–1033.
- Damas, B.A.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity Comparison of Mineral Trioxide Aggregates and EndoSequence Bioceramic Root Repair Materials. J. Endod. 2011, 37, 372–375.
- Özdemir, O.; Kopac, T. Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. J. Appl. Biomater. Funct. Mater. 2022, 20, 1–9.
- Al-Haddad, A.; Ab Aziz, Z.A.C. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210.
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852.
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.-J. The Influence of X-ray Radiation on the Mineral/Organic Matrix Interaction of Bone Tissue: An FT-IR Microscopic Investigation. Int. J. Artif. Organs 2005, 28, 66–73.
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified Hydroxyapatite Structure and Properties: Modeling and Synchrotron Data Analysis of Modified Hydroxyapatite Structure. Ferroelectrics 2015, 475, 135–147.
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465.
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182.
- Shrestha, A.; Zhilong, S.; Gee, N.K.; Kishen, A. Nanoparticulates for Antibiofilm Treatment and Effect of Aging on Its Antibacterial Activity. J. Endod. 2010, 36, 1030–1035.
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353.
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis Biofilm. J. Endod. 2014, 40, 285–290.
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311.
- Waltimo, T.; Brunner, T.; Vollenweider, M.; Stark, W.; Zehnder, M. Antimicrobial Effect of Nanometric Bioactive Glass 45S5. J. Dent. Res. 2007, 86, 754–757.
- Waltimo, T.; Möhn, D.; Paqué, F.; Brunner, T.J.; Stark, W.J.; Imfeld, T.; Schätzle, M.; Zehnder, M. Fine-tuning of Bioactive Glass for Root Canal Disinfection. J. Dent. Res. 2009, 88, 235–238.
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Al Alam, D.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haïkel, Y.; Gangloff, S.C.; et al. Nanostructured Assemblies for Dental Application. ACS Nano 2010, 4, 3277–3287.
- Saghiri, M.A.; Asgar, K.; Lotfi, M.; Garcia-Godoy, F. Nanomodification of mineral trioxide aggregate for enhanced physiochemical properties. Int. Endod. J. 2012, 45, 979–988.
- Akbari, M.; Zebarjad, S.M.; Nategh, B.; Rouhani, A. Effect of Nano Silica on Setting Time and Physical Properties of Mineral Trioxide Aggregate. J. Endod. 2013, 39, 1448–1451.
- Gong, W.; Huang, Z.; Dong, Y.; Gan, Y.; Li, S.; Gao, X.; Chen, X. Ionic Extraction of a Novel Nano-sized Bioactive Glass Enhances Differentiation and Mineralization of Human Dental Pulp Cells. J. Endod. 2013, 40, 83–88.
- Saghiri, M.A.; Gutmann, J.L.; Orangi, J.; Asatourian, A.; Sheibani, N. Radiopacifier Particle Size Impacts the Physical Properties of Tricalcium Silicate–based Cements. J. Endod. 2014, 41, 225–230.
- Petrović, V.; Opačić-Galić, V.; Živković, S.; Nikolić, B.; Danilović, V.; Miletić, V.; Jokanović, V.; Mitić-Ćulafić, D. Biocompatibility of new nanostructural materials based on active silicate systems and hydroxyapatite: in vitro and in vivo study. Int. Endod. J. 2015, 48, 966–975.
- Saghiri, M.A.; Asatourian, A.; Nguyen, E.H.; Wang, S.; Sheibani, N. Hydrogel Arrays and Choroidal Neovascularization Models for Evaluation of Angiogenic Activity of Vital Pulp Therapy Biomaterials. J. Endod. 2018, 44, 773–779.
- Naseri, M.; Eftekhar, L.; Gholami, F.; Atai, M.; Dianat, O. The Effect of Calcium Hydroxide and Nano–calcium Hydroxide on Microhardness and Superficial Chemical Structure of Root Canal Dentin: An Ex Vivo Study. J. Endod. 2019, 45, 1148–1154.
- Yang, S.Y.; Liu, Y.; Mao, J.; Wu, Y.B.; Deng, Y.L.; Qi, S.C.; Zhou, Y.C.; Gong, S.-Q. The antibiofilm and collagen-stabilizing effects of proanthocyanidin as an auxiliary endodontic irrigant. Int. Endod. J. 2020, 53, 824–833.
- Meto, A.; Droboniku, E.; Blasi, E.; Colombari, B.; Tragaj, E.; Cervino, G.; Fiorillo, L.; Meto, A. Copper–Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis. Materials 2021, 14, 678.
- Patel, S.; Mavridou, A.M.; Lambrechts, P.; Saberi, N. External cervical resorption-part 1: Histopathology, distribution and presentation. Int. Endod. J. 2018, 51, 1205–1223.
- Zinelis, S.; Akhtar, R.; Tsakiridis, P.; Watts, D.C.; Silikas, N. In-depth hardness profiles of Stainless Steel and Ni-Ti endodontic instrument cross-sections by nano-indentation. Int. Endod. J. 2008, 41, 747–754.
- Jamleh, A.; Sadr, A.; Nomura, N.; Yahata, Y.; Ebihara, A.; Hanawa, T.; Tagami, J.; Suda, H. Nano-indentation testing of new and fractured nickel-titanium endodontic instruments. Int. Endod. J. 2011, 45, 462–468.
- Mavridou, A.M.; Pyka, G.; Kerckhofs, G.; Wevers, M.; Bergmans, L.; Gunst, V.; Huybrechts, B.; Schepers, E.; Hauben, E.; Lambrechts, P. A novel multimodular methodology to investigate external cervical tooth resorption. Int. Endod. J. 2015, 49, 287–300.
- Mavridou, A.M.; Hauben, E.; Wevers, M.; Schepers, E.; Bergmans, L.; Lambrechts, P. Understanding external cervical resorption patterns in endodontically treated teeth. Int. Endod. J. 2017, 50, 1116–1133.
- Iacono, F.; Pirani, C.; Generali, L.; Bolelli, G.; Sassatelli, P.; Lusvarghi, L.; Gandolfi, M.G.; Giorgini, L.; Prati, C. Structural analysis of HyFlex EDM instruments. Int. Endod. J. 2017, 50, 303–313.
- Petitjean, E.; Mavridou, A.; Li, X.; Hauben, E.; Cotti, E.; Lambrechts, P. Multimodular assessment of a calcified extraradicular deposit on the root surfaces of a mandibular molar. Int. Endod. J. 2017, 51, 375–385.
- Hildebrand, T.; Nogueira, L.; Sunde, P.T.; Ørstavik, D.; Glasmacher, B.; Haugen, H.J. Contrast-enhanced nano-CT reveals soft dental tissues and cellular layers. Int. Endod. J. 2021, 54, 1275–1288.
- Holmes, S.; Gibson, R.; Butler, J.; Pacheco, R.; Askar, M.S.; Paurazas, S. Volumetric Evaluation of 5 Root Canal Obturation Methods in TrueTooth 3-dimensional–Printed Tooth Replicas Using Nano–computed Tomography. J. Endod. 2020, 47, 485–491.e4.
- Gazzaneo, I.; Amoroso-Silva, P.; Pacheco-Yanes, J.; Alves, F.R.; Marceliano-Alves, M.; Olivares, P.; Meto, A.; Mdala, I.; Siqueira, J.F.; Rôças, I.N. Disinfecting and Shaping Type I C-shaped Root Canals: A Correlative Micro–computed Tomographic and Molecular Microbiology Study. J. Endod. 2020, 47, 621–630.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
3 times
(View History)
Update Date:
11 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




