
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jacopo Meldolesi | + 2221 word(s) | 2221 | 2020-10-07 05:27:55 | | | |
| 2 | Catherine Yang | Meta information modification | 2221 | 2020-10-16 05:07:04 | | | | |
| 3 | Lily Guo | Meta information modification | 2221 | 2021-04-06 03:20:18 | | |
Video Upload Options
Astrocytes are cerebral cells present in number close to that on neurons (50-60 mld). For decades they were considered only a glue, offering a mechanical and metaboli
1. Introduction
The discovery of astrocytes, the most numerous glial cells in the brain, occurred as soon as the study of brain cytology started. Astrocytes, different with respect to neurons, were already recognized in the exciting images produced by Cajal at the end of the nineteen century. At the time, however, for many decades, the complex of astrocytes was described as a glue, a continuous and structured multicellular complex providing adjacent neurons with molecular exchange and metabolic support. Only 25–30 years ago, an astrocyte “revolution” started with the recognition of various functional properties typical of these cells. Additional properties were identified and characterized by subsequent studies. In other words, astrocytes were recognized to operate in discrete territories of the brain tissue where they interact with specialized regions of the neuronal plasma membrane: at the cell body, the axon, and at hundreds of synapses [1][2].
The secretory activity of astrocytes, called gliotransmission, occurs by exocytosis, with various transmitters released by vesicles at synapses [1][3]. Two such transmitters (glutamate and ATP) are the same as those of neurons. Others (D-serine and eicosanoids) are more specific. Additional, highly active and trophic factors are also released by exocytosis. In addition, all mentioned factors are released also via channels of the plasma membrane. The released factors activate receptors expressed by neurons, various types of glia, and blood vessels. Within astrocytes and other cell targets, these factors induce transient increases and/or oscillations of cytosolic Ca2+; however, they are slower however with respect to those of neurons [4][5]. The feed-back and feed-forward signaling effects induced by astrocytes can ultimately tune the balance of neurons between excitation and inhibition [6]. In physiological conditions, astrocytes contribute to the state of the target cells, up to the protection of neurons.
The study of brain diseases has clarified the participation of astrocytes in various processes, from physiological events to strokes and neurodegenerative diseases, including the Alzheimer’s disease (AD). The role of astrocytes is known to vary during aging, with its contributions extending from the maintenance of health conditions to the development of diseases [7][8]. Aging, in fact, does not preclude the ability of astrocytes to maintain a positive environment in the central nervous system. The state of neurons depends on their interaction with neighboring cells and also on the inflammation often established in the brain. Some of these properties have already been presented in coordinated reviews, a few of which including recent presentations of diseases [9][10].
This initial interest was strengthened over the last few years, when intense studies have discovered several new properties, functions, and defects of astrocytes, which have attracted more and more interest also in the scientific community. At present, therefore, the “revolution” of astrocytes, developed before the 2000s [1], has been expanded in many directions. Many properties and functions typical of neurons or other glial cells have not been attributed to astrocytes. In other words, most properties and functions of astrocytes have remained the same; however, their relevance has become increasingly detailed and important.
The increased importance of astrocytes is generally recognized. Therefore, reviews written by others were conceived either as comprehensive reviews or focused on a single function. In view of my interest for the role of astrocytes in several directions, I decided to write my review according to a different strategy, focused on six areas, in which recent studies are presented separately: heterogeneity, inflammatory responses, strokes and repairs, senescence, tau and tauopathies, and AD. To provide information regarding the previous knowledge, each recent area is preceded by short presentations of relevant data published several years ago. To emphasize that the six areas are not fully separate from each other, the conclusion includes a few short comprehensive considerations that illustrate important findings obtained in various areas that are interactive with each other.
2. The Heterogeneity of Astrocytes
In the past, the structural homology of astrocytes was a common opinion. Such a property was considered critical, on one side, for the general and prolonged interaction of these cells with the vessels of the blood-brain-barrier (BBB); on the other side, for the connection of their branches to various neuronal components: bodies, axons, and thousands of synapses. At that time, astrocyte heterogeneity was limited to resting and stimulated conditions. Around 2010, however, astrocyte heterogeneity began to be recognized among brain regions and within single regions. These differences were due to gene dependence as well as to stimulation and disease responses [11]. At present, the research is clear that the properties of astrocytes, widespread in young age, are also maintained in mature mice and rats if in good health [12][13][14][15]. During aging, however, many changes and alterations have been reported [16][17][18][19]. The interactions of heterogeneous astrocytes with neurons are critical for many key events of brain development and function, including the formation and function of synapses, their release and reuptake of transmitters, the production of trophic and toxic factors, and the control of neuronal survival [12][13] (Figure 1). These interactions are due to the molecular properties of astrocytes, with differences in the expression of distinct genes (a) at various stages of their functions; (b) at various regions of the brain [20]; (c) in neuronal circuit regulation [21], and (d) also in the course of their pathologies [16][17][18][19].
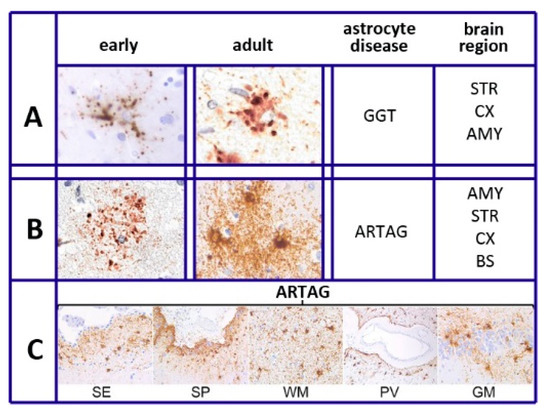
Figure 1. Tau heterogeneity and pathology of various astrocytes. (A,B) show the tau deposition of globular-glial tauopathies (GGT) and aging-related tau astrogliopathy (ARTAG), presented as early (left columns) and adult (right columns) morphologies. Brain regions of GGT and ARTAG distribution are listed on the right: AMY = amygdala; STR = striatum; CX = cortex; BS = brainstem. (C): thorn-shaped astrocytes of ARTAG type distributed at subependymal (SE), subpial (SP), white matter (WM), perivascular (PV), and rare gray matter (GM) location. Reproduced in part with permission from Figure 1 of [22]. Thanks to G.G. Kovacs and the Frontiers Production Office.
As summarized in [22], six different forms have been recognized in different diseases of astrocytes. This is also specified in our sixth section, Tau and Tauopathies: plaques, tufted, ramified, and globular astrocytes predominate in primary tauopathies and torn-shaped and granular fuzzy astrocytes predominate in aging-related tau (ARTAG) tauopathies (Figure 1). Many region-specific transcriptional properties change in a region-dependent manner. The traditional effects of astrocyte aging, induced by their interaction with neurons, vary depending on the state of the latter cells, healthy or sick. Changes are accompanied by forms of neuroinflammation sustained by cytokines, released by astrocytes and microglia [19]. In many such cases, the astrocyte interactions/communications with neighboring cells are reduced, while their expression of relevant factors, including brain-derived neural factor (BDNF) declines progressively [19][20][23].
3. The Role of Astrocytes in Alzheimer’s Disease
Astrocytes play critical roles in various neurodegenerative diseases. For decades, neurons were considered the cells playing the major role in the development and severity of AD, the alterations, and ensuing death. Astrocytes were known to be present, mostly surrounding the amyloid plaques; however, until about 10 years ago, their role and their interactions with neurons remained uncharacterized and poorly understood [24]. During the last few years, critical forms of the disease became more and more dependent on factors released by glial cells. Upon such insights, the focus of research, away from neurons, has largely moved toward glial cells, primarily astrocytes [25][26][27][28]. Consequently, knowledge regarding the role of astrocytes in AD has grown considerably. Here, I will report about the various developments now established.
A critical process sustained by astrocytes at various stages of AD is their reactivity. The latter process, established by astrocytes in response to various actions, results in the inhibition of neuronal resting activities, such as regulated homeostasis, synaptic plasticity, and neuroprotection. Concomitantly, astrocytes, structured in A1-like conditions [14], grow up in volume. For these developments, astrocytes are always complex and heterogeneous. Their identification is often based on the recognition of various markers, which are present only in a fraction of their population. This observation may explain why the effects of astrocytes in aging patients are variable. In many cases, astrocytes acquire aggressive properties, with a gain of toxic functions and loss of neurotrophic effects [29][30]. In other cases, however, astrocyte reactivity induces metabolic changes, including the JAK2-Stat3 cascade, contributing to reductions of amyloid deposition and restorations of defective synapses [31][32][33]. Thus, the final effects largely depend on the general state of participating astrocytes.
Another important process by astrocytes in the development of AD is the specific secretion of relevant agents. Apolipoprotein E (ApoE, active especially as ApoE4), carries lipids to neurons, plays an undisputed role in AD pathology. The secretion of this protein from astrocytes is positively controlled by Axl, a tyrosine kinase receptor. This mechanism supports the development of AD, while inhibition of the receptor results in the inhibition of ApoE release and, thus, in a slowing down of the AD process [34].
Analogous effects are induced by the protein phosphatase 2A (CIP2A), abundant in several cancer cells, which is also expressed by astrocytes. This expression stimulates the release of cytokines and the accumulation of Aβ, with the induction of synaptic degeneration and additional symptoms [35]. However, the astrocyte secretion of other agents, such as the protein thrombospondin-1 and the TGF-β1, can induce a reduction of various steps of AD pathology [36][37].
Recent studies revealed that, in AD and Parkinson’s, events reinforced by astrocytes are sustained by various processes of cell biology. The phagocytosis of apoptotic cell debris can induce various effects. In some cases, neuronal material is accumulated within lysosomes, with ensuing defects of the organelle with the extracellular release of toxic debris [38]. In other conditions, the uptake of Aβ within astrocytes results in digestion and, thus, in the control of its level [39]. An important event in AD is the progressive deletion of excitatory amino acid transporter 2 (EAAT2), a major glutamate transporter abundant in astrocytes but present also in neurons [40]. The lack of the same transporter in the two types of cells yields different defects: in astrocytes, an early defect of short-term memory, special reference learning, and also long-term memory; in neurons, of only long-term memory [40]. Neurons and astrocytes have been known for years to exchange extracellular vesicles [41]. The exchange effects induced by such types of intercellular communication can be variable [42]. In aged AD patients, most exosomes, released by reactive A1 astrocytes, activate various neurotoxic mechanisms, inducing fragmentation and death of neurons [42][43].
Interesting studies of the last few years have revealed the young precocity of long-term AD, whose patients begin expressing molecular alterations years before the appearance of clear symptoms. Among the relevant early data observed are the decreased astrocyte proliferation, the decreased expression of enzymes, such as glutamine synthetase, and the expression of a population of genes. Highly interesting are the RNA sequences, known to identify the disease-associated astrocytes. The latter properties, present already at early levels, increase with the progression of the disease [28][29][44].
The majority of the recent astrocyte data regarding AD were obtained by studies of mouse brain models [30][31][35][45]. Additional data were obtained from mouse astrocyte lines, in some cases, duplicated with human lines or cultures [24][34][38]. Thus, the validity of the data for the human disease has not yet been finally demonstrated. This problem is relevant as rodent astrocytes differ considerably and in many respects from human astrocytes, including the functionalities and gene expression, the recent development of research, including the use of human astrocytes derived from stem cells, and the analyses of single nucleus RNA sequencing, appear of interest for future in vivo studies, focused on the re-consideration of multiple aspects of the disease and its perspectives [24][28].
4. Conclusions
Compared to many other reviews on astrocytes, this presentation can be considered innovative. My strategy was neither comprehensive nor focused on a single area. Rather, it includes the distinct presentation of six areas intensely developed during the last few years. The six areas I chose are not the only ones with recent astrocyte development. For example, an important function previously attributed primarily to neurons—the circadian regulation governed by the brain clock—has been recently recognized as related to astrocytes [45][46]. However, circadian regulation is not easy to coordinate with other brain functions. Therefore, it was not chosen. Similar problems occurred with a few other activities. Thus, our choices remained limited to six.
As already mentioned in the introduction, a common property of the six astrocytic areas presented separately here, is their functional coordination within the cells. This is the case of astrocyte heterogeneity, useful for the development of inflammation, the restoration of structural lesions, and the distinction between aging of healthy humans from AD and tauopathy patients. Neuroinflammation is relevant for its unexpected participation in the discussed pathologies [24][28]. For some traumatic injuries, astrocyte cooperation exists with other areas: heterogeneity, inflammation, and neuronal diseases [37][39]. Senescence appears useful to support the dual role of astrocytes during aging, in healthy humans and diseased patients, protecting neurons or contributing to the toxicity.
Tau is an important component of AD and the key factor of tauopathies. In healthy humans, tau is also present, although at lower levels [15][18]. Finally, AD initial symptoms, which sometimes involve astrocytes, are present in young, apparently healthy humans, destined to become patients with aging. The astrocyte presentation of the disease is focused on the coordination with the other areas and in the development of new pathogenesis [35][36]. The relevance expected for future developments includes a general reconsideration based on the role of astrocytes, new innovative approaches to the understanding, and new therapies of diseases [28][44].
References
- Volterra, A.; Meldolesi, J. Astrocytes, from glue to communication elements: the revolution continues. Nature Rev. Neurosci. 2005, 6: 626-640.
- Allen, N.J.; Eroglu, C. Cell biology of astrocyte-synapse oscillations. Neuron 2017, 96:697-708.
- Bezzi, P.; Gundersen, V.; Galbete, J.S.; Seifert, G.; Steinhauser, C.; Pilati, E.; Volterra, A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nature Neurosci. 2004, 7: 613–620.
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997, 17: 7817–7830.
- Morita, M.; Higuchi, C.; Moto, T.; Kozuka, N.; Susuki, J.; Itofusa, R.; Yamashita, J.; Kudo, Y. Dual regulation of calcium oscillation in astrocytes by growth factors and pro-inflammatory cytokines via the mitogen-activated protein kinase cascade. J. Neurosci. 2003, 23: 10944-10952.
- Piet, R.; Vargova, L.; Sykova, E.; Poulain, D. A.; Oliet, S. H. Physiological contribution of the astrocytic environment of neurons to inter-synaptic crosstalk. Proc. Natl. Acad. Sci. USA 2004, 101, 2151–2155.
- Middleldorn, J.; Hol, E. M. GFAP in health and disease. Prog. Neurobiol. 2011, 93: 421-443.
- Lange, S.C.; Bak, L.K.; Waagenpetersen, H.S.; Schousboe, A.; Noremberg, M.D. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem. Res. 2012, 37: 2569-2588.
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: role and functions in brain pathologies. Front. Pharmacol. 2019, 10: 114. doi: 10.3389/fphar.2019.01114.
- Cragnolini, A.B.; Lampitella, G.; Virtuoso, A.; Viscovo, I.; Panetson, F.; Papa, M.; Cirillo, G. Regonal brain susceptibility to neurodegeneration: what is the role of glial cells? Neural Regen. Res. 2020, 15: 838-842.
- Zhang, Y.; Barres, B.A. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010, 20:588-594.
- Gomez-Gonzalo, M.; Martin-Fernandez, M.; Martinez-Murillo, R.; Maderos, S.; Hernandez-Vivanco, A.; Jamieson, S.; Fernandez, A.P.; Serrano, J.; Calero, P.; Futch, H.S.; Corpas, R.; Sanfeliu, C.; Perea, G.; Araque, A. Neuron-astrocyte signaling is preserved in the aging brain. Glia 2017, 65: 569-580.
- Khakh, B.S.; Deneen, B. The emerging nature of astrocyte diversity. Ann. Rev. Neurosci. 2019, 42: 187-207.
- Mathias, I; Morgado, J.; Gomes, F.C.A. Astrocyte heterogeneity: input to brain aging and disease. Front. Aging Neurosci. 2019, 11: 59. doi: 10.3389/fmagi.2019.
- Miki, T.; Yokota, O.; Haraguchi, T.; Ishizu, H.; Hasegawa, M.; Ishihara, T.; Ueno, S.I.; Takenoshita, S.; Terada, S.; Tamada, N. Factors associated with development and distribution of granular/fuzzy astrocytes in neurodegenerative diseases. Brain Pathol. 2020, 30: 811-830.
- Trias, E.; Barbeito, L.; Yamanaka, K. Phenotypic heterogeneity of astrocytes in motor neuron disease. Clin. Exp. Neuroimmunol. 2018, 9: 225-234.
- Pan, J.; Maa, N.; Yu, B.; Zhang, W., Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflammation 2020, 17: 97. doi: 10.1186/s12974-020-01774-9.
- Boisvert, M.M.; Erikson, G.A.; Shokhivert, M.N.; Allen, N.J. The aging astrocyte transcriptome: from multiple regions of the mouse brain. Cell Rep. 2018, 22: 269-285.
- Ben Haim, L.; Rowitch, D.H. Functional diversity of astrocytes in neural circuits. Nat. Rev. Neurosci. 2017, 18: 31-41.
- Clarke L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018. 115: E1896-E1905.
- Palmer, A.L.; Ousman, S.S. Astrocytes and aging. Front. Aging Neurosci. 2018. 10:337. doi 10.3389/fmagi.2018.00337.
- Kovacs, G.G. Astroglia and tau: new perspectives. Frontal Aging Neurosci. 2020, 12:96. doi 10.3389/fnagi.2020.00096.
- Bronzuoli, M.R.; Facchinetti, R.; Valenza, M.; Cassano, T.; Steardo, L.; Scuderi, C. Astrocyte function is affected by aging and not Alzheimer’s disease: a preliminary investigation in hippocampi of 3xTg-AD mice. Front. Pharmacol. 2019, 10: 644. doi: 10.3389/fphar.2019.00644.
- Simpson, J.E.; Ince, P.G.; Forster, L.G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B. Astrocyte phenotype in relation to Alzheimer-type pathology in the aging brain. Neurobiol. Aging 2010, 31: 578-590.
- Allaman, I.; Gavillet, M.; Bélanger, M.; Laroche, T.; Vertl, D.; Laschuel, H.A.; Magistretti,P.J. Amyloid-β aggregates cause alterations of astrocyte metabolic phenotype: impact on neuronal viability. J. Neurosci. 2010, 30: 3326-3338.
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Sternfeld, R.: Green, G.; Dionne, D. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23: 701-706.
- Zorec, R.; Parpura, V.; Vardjan, N.; Verkhratsky, A. Astrocytic face of Alzheimer’s disease. Behav. Brain. Res. 2017, 322: 250-257.
- Arranz, A.M.; De Strooper, B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 2019, 18: 406-414.
- Liang, Y; Raven, F.; Ward, J.F.; Zhen, S.; Zhang, S.; Sun, H. Miller, S.J.; Choi, S.H.; Tanzi, R.E.; Zhang, C. Up-regulation of Alzheimer’s disease amyloid- β protein precursor in astrocytes both in vitro and in vivo. J. Alzheimer’s Dis. 2020, 76: 1071-1082.
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the astrocyte reaction in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10: 114. doi: 10.3389/fnagi.2018.00114.
- Li, K.; Li, J.; Zheng, J.; Quin, S. Reactive astrocytes in neuro-degenerative diseases. Aging Dis. 2019, 10: 664-675.
- Habibi, N.; McCabe, C.; Medina, S.; Ceyzériat, K.; Haim, L.B.; Denizot, A.; Pommier, D.; Matos, M. et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Comm. 2018, 6: 104. doi: 10.1186/s40478-018-0606-1.
- Guillemaud, O.; Ceyzériat, K.; Saint-Georges, T.; Cambon, K.; Petit, F.; Haim, B.; Carillo-de Sauvage M.A.; Guillermier, M.; Bernier, S.; Herald, H.S.; Josephine, C.; Bemelmans, A.P.; Brouillet, E.; Hantraye, P.; Bonvento, G.; Escartin, C. Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 90: 135-146.
- Zhao, W.; Fan, J.; Kulic, I.; Koh, C.; Clark, A.; Mueller, J.; Engwist, O.; Barichievy, S.; Raynoschek, C.; Hicks, R.; Maresca, M.; Wang, O.; Brown, D.G.; Lok, A.; Parro, C.; Robert, J.; Chou, H.Y.; Zuhl, A.M.; Wood, M.W.; Brandon, N.J.; Wellington, C.L. Axl receptor tyrosine kinase is a regulator of apolipoprotein E. Mol. Brain, 2020, 13: 66. doi: 19.1186/s13041-020-00609-1.
- Shentu, Y.P.; Hu, W.T.; Zhang, Q.; Huo, Y.; Liang, J.W.; Liuyang, Z.Y.; Zhu, H; Wei, H.; Ke, D.; Wang, X.C.; Wang, J.Z.; Man, H.Y.; Westermarck, J.; Liu, R. CIP2A-promoted astrogliosis, induces AD-like synaptic degeneration and cognitive deficits. Neurobiol. Aging 2019, 75: 198-208.
- Son, S.M.; Nam, D.W.; Cha, M.Y.; Kim, K.H.; Byun, J.; Ryu, H.; Mook-Jung, I. Thrombospondin -1 prevents amyloid- β -mediated synaptic pathology in Alzheimer’s disease. Neurobiol. Aging 2015, 36: 3214-3227.
- Diniz, L.P.; Tortelli, V.; Mahias. I.; Morgado, J.; Bergamo Araujo, A.P.; Melo, H.M.; Seixas da Silva, G.S.; Alves-Leon, S.V.; de Souza J.M.; Ferreira, S.T.; De Felice, F.G.; Gomes F.C.A. Astrocyte transforming growth factor-β1 protects synapses against Aβ oligomers in Alzheimer’s disease model. J. Neurosci. 2017, 37: 6797-6809.
- Trenblay, M.E.M Cookson, M.R.; Civiero, L. Glial phagocytic clearance in Parkinson’s disease. Mol. Neurodegener. 2019, 14: 16. doi: 10.1186/s13024-019.0314-8.79.
- Ries, M.; Sastre, M. Mechanisms of clearance and degradation by glial cells. Front. Aging Neurosci. 2016, 8; 160. doi: 10.3389/fnagi.2016.00160.
- Sharma, A.; Kazim, S.F.; Larson, C.S.; Ramakrushnan, A.; Gray, J.D.; McEwen, B.S.; Rosemberg, B.A.; Shen, L.; Pereira, A.C. Divergent roles of astrocytic versus neuronal EAAT2 deficiency on cognitive and overlap with aging and Alzheimer’s molecular signatures. Proc. Natl. Acad. Sci. USA 2019, 116: 21800-21811.
- Fruhbeis, C.; Frohlich D.; Luo, W.P.; Kramer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell Neurosci. 2013, 7: 182. doi: 10.3389/fncel.2013.00182.
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83: 544-552.
- Elsharbini, A.; Kirov, A.S.; Dinkins, M.B.; Wang, G.; Quin, H.; Zhu, Z.; Tripathi, P.; Crivelli, S.M.; Bieberich, E. Association of Aβ with ceramide-enriched astrosomes mediates Aβ nerotoxicity. Acta Neuropathol. Commun. 2020, 8: 60. doi: 10.1186/s40478-020-00931.
- Li, K.Y.; Gong, P.F.; Li, J.T.; Xu, N.J.; Qin, S. Morphological and molecular alterations of reactive astrocytes without proliferation in cerebral cortex of an APP/PS1 transgenic mouse model and Alzheimer’s patients. Glia 2020, 68: 2361-2376.
- Brancaggio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Maywood, E.S.; Hasting, M.H. Cell autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363: 187.192.
- McKee, C.A.; Lananna B.V.; Musiel, E.S. Circadian regulation of astrocyte function: implications for Alzheimer’s disease. Cell Mol. Life Sci. 2020, 77: 1049-1058.





