Astrocytes are cerebral cells present in number close to that on neurons (50-60 mld). For decades they were considered only a glue, offering a mechanical and metaboli
- glial cells, tau/tauopathies, Alzheimer's disease
1. Introduction
The discovery of astrocytes, the most numerous glial cells in the brain, occurred as soon as the study of brain cytology started. Astrocytes, different with respect to neurons, were already recognized in the exciting images produced by Cajal at the end of the nineteen century. At the time, however, for many decades, the complex of astrocytes was described as a glue, a continuous and structured multicellular complex providing adjacent neurons with molecular exchange and metabolic support. Only 25–30 years ago, an astrocyte “revolution” started with the recognition of various functional properties typical of these cells. Additional properties were identified and characterized by subsequent studies. In other words, astrocytes were recognized to operate in discrete territories of the brain tissue where they interact with specialized regions of the neuronal plasma membrane: at the cell body, the axon, and at hundreds of synapses [1,2].
The secretory activity of astrocytes, called gliotransmission, occurs by exocytosis, with various transmitters released by vesicles at synapses [1,3]. Two such transmitters (glutamate and ATP) are the same as those of neurons. Others (D-serine and eicosanoids) are more specific. Additional, highly active and trophic factors are also released by exocytosis. In addition, all mentioned factors are released also via channels of the plasma membrane. The released factors activate receptors expressed by neurons, various types of glia, and blood vessels. Within astrocytes and other cell targets, these factors induce transient increases and/or oscillations of cytosolic Ca2+; however, they are slower however with respect to those of neurons [4,5]. The feed-back and feed-forward signaling effects induced by astrocytes can ultimately tune the balance of neurons between excitation and inhibition [6]. In physiological conditions, astrocytes contribute to the state of the target cells, up to the protection of neurons.
The study of brain diseases has clarified the participation of astrocytes in various processes, from physiological events to strokes and neurodegenerative diseases, including the Alzheimer’s disease (AD). The role of astrocytes is known to vary during aging, with its contributions extending from the maintenance of health conditions to the development of diseases [7,8]. Aging, in fact, does not preclude the ability of astrocytes to maintain a positive environment in the central nervous system. The state of neurons depends on their interaction with neighboring cells and also on the inflammation often established in the brain. Some of these properties have already been presented in coordinated reviews, a few of which including recent presentations of diseases [9,10].
This initial interest was strengthened over the last few years, when intense studies have discovered several new properties, functions, and defects of astrocytes, which have attracted more and more interest also in the scientific community. At present, therefore, the “revolution” of astrocytes, developed before the 2000s [1], has been expanded in many directions. Many properties and functions typical of neurons or other glial cells have not been attributed to astrocytes. In other words, most properties and functions of astrocytes have remained the same; however, their relevance has become increasingly detailed and important.
The increased importance of astrocytes is generally recognized. Therefore, reviews written by others were conceived either as comprehensive reviews or focused on a single function. In view of my interest for the role of astrocytes in several directions, I decided to write my review according to a different strategy, focused on six areas, in which recent studies are presented separately: heterogeneity, inflammatory responses, strokes and repairs, senescence, tau and tauopathies, and AD. To provide information regarding the previous knowledge, each recent area is preceded by short presentations of relevant data published several years ago. To emphasize that the six areas are not fully separate from each other, the conclusion includes a few short comprehensive considerations that illustrate important findings obtained in various areas that are interactive with each other.
2. The Heterogeneity of Astrocytes
In the past, the structural homology of astrocytes was a common opinion. Such a property was considered critical, on one side, for the general and prolonged interaction of these cells with the vessels of the blood-brain-barrier (BBB); on the other side, for the connection of their branches to various neuronal components: bodies, axons, and thousands of synapses. At that time, astrocyte heterogeneity was limited to resting and stimulated conditions. Around 2010, however, astrocyte heterogeneity began to be recognized among brain regions and within single regions. These differences were due to gene dependence as well as to stimulation and disease responses [11]. At present, the research is clear that the properties of astrocytes, widespread in young age, are also maintained in mature mice and rats if in good health [12,13,14,15]. During aging, however, many changes and alterations have been reported [16,17,18,19]. The interactions of heterogeneous astrocytes with neurons are critical for many key events of brain development and function, including the formation and function of synapses, their release and reuptake of transmitters, the production of trophic and toxic factors, and the control of neuronal survival [12,13] (Figure 1). These interactions are due to the molecular properties of astrocytes, with differences in the expression of distinct genes (a) at various stages of their functions; (b) at various regions of the brain [20]; (c) in neuronal circuit regulation [21], and (d) also in the course of their pathologies [16,17,18,19].
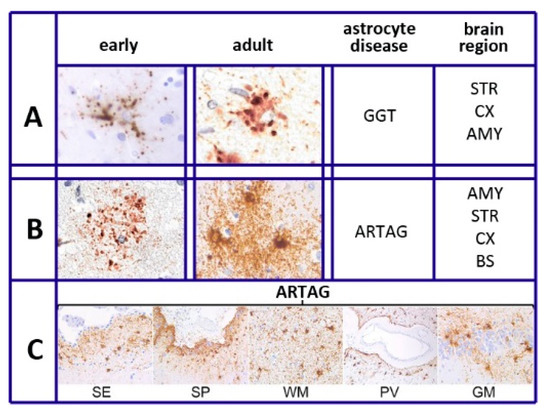
As summarized in [22], six different forms have been recognized in different diseases of astrocytes. This is also specified in our sixth section, Tau and Tauopathies: plaques, tufted, ramified, and globular astrocytes predominate in primary tauopathies and torn-shaped and granular fuzzy astrocytes predominate in aging-related tau (ARTAG) tauopathies (Figure 1). Many region-specific transcriptional properties change in a region-dependent manner. The traditional effects of astrocyte aging, induced by their interaction with neurons, vary depending on the state of the latter cells, healthy or sick. Changes are accompanied by forms of neuroinflammation sustained by cytokines, released by astrocytes and microglia [19]. In many such cases, the astrocyte interactions/communications with neighboring cells are reduced, while their expression of relevant factors, including brain-derived neural factor (BDNF) declines progressively [19,20,23].
3. The Role of Astrocytes in Alzheimer’s Disease
4. Conclusions
Compared to many other reviews on astrocytes, this presentation can be considered innovative. My strategy was neither comprehensive nor focused on a single area. Rather, it includes the distinct presentation of six areas intensely developed during the last few years. The six areas I chose are not the only ones with recent astrocyte development. For example, an important function previously attributed primarily to neurons—the circadian regulation governed by the brain clock—has been recently recognized as related to astrocytes [45,46]. However, circadian regulation is not easy to coordinate with other brain functions. Therefore, it was not chosen. Similar problems occurred with a few other activities. Thus, our choices remained limited to six.
As already mentioned in the introduction, a common property of the six astrocytic areas presented separately here, is their functional coordination within the cells. This is the case of astrocyte heterogeneity, useful for the development of inflammation, the restoration of structural lesions, and the distinction between aging of healthy humans from AD and tauopathy patients. Neuroinflammation is relevant for its unexpected participation in the discussed pathologies [24,28]. For some traumatic injuries, astrocyte cooperation exists with other areas: heterogeneity, inflammation, and neuronal diseases [37,39]. Senescence appears useful to support the dual role of astrocytes during aging, in healthy humans and diseased patients, protecting neurons or contributing to the toxicity [56,57].
Tau is an important component of AD and the key factor of tauopathies. In healthy humans, tau is also present, although at lower levels [15, 18]. Finally, AD initial symptoms, which sometimes involve astrocytes, are present in young, apparently healthy humans, destined to become patients with aging. The astrocyte presentation of the disease is focused on the coordination with the other areas and in the development of new pathogenesis [35, 36]. The relevance expected for future developments includes a general reconsideration based on the role of astrocytes, new innovative approaches to the understanding, and new therapies of diseases [28, 44].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines8100394
