
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samar Elkhalifa | -- | 835 | 2022-08-10 13:04:07 | | | |
| 2 | Lindsay Dong | -35 word(s) | 800 | 2022-08-11 02:52:45 | | | | |
| 3 | Samar Elkhalifa | + 1532 word(s) | 2332 | 2022-09-05 14:46:57 | | | | |
| 4 | Lindsay Dong | -291 word(s) | 2041 | 2022-09-06 04:15:32 | | | | |
| 5 | Lindsay Dong | + 79 word(s) | 2120 | 2022-09-14 08:49:22 | | |
Video Upload Options
Biochar is a solid, charcoal material that can be used in different applications such as its use in agriculture as a soil enhancer in which moisture content is sustained for long periods of times. Biosolids or biosludges will refer to any form of sludge that has undergone some form of treatment (e.g., chemical, biological, heat treatment, etc.) in order to transform it into a less hazardous and organic form. More importantly, there seems to be an inconsistency in defining what classifies as biosolids, hence, a unified definition is established and provided. The aim is to enable the scientific community as a whole to use a standard definition that allows for synergies to take place. Specifically, the pyrolysis process is selected and is known as a thermochemical technique in which heat and an oxygen-free environment facilitate the decomposition of the feedstock into different products, depending on the operating parameters.
1. Biosolids/Biosludge
The world is experiencing an accelerated level of advancement in which people aim at enhancing their living standards, while competing for food, water, and energy. This threatens the availability of enough resources for future generations, hence hindering the achievement of sustainability goals. As a result of inequalities and mindless consumption, wastes are generated in abundance impacting the environment and transgressing the Earth into undesired shifts from its equilibrium (i.e., its balance). Waste management, hence, plays a pivotal role in alleviating the suffering of the environment, and contributes to the prosperity of societies. Examples of typical waste management techniques include landfilling and incineration, both are associated with technical and environmental difficulties. There are other techniques including the application of methods that implement the concept of circular economy, where wastes are reused as input for the production of various commodities thus creating a market for waste.
Biosolids or biosludges will refer to any form of sludge that has undergone some form of treatment (e.g., chemical, biological, heat treatment, etc.) in order to transform it into a less hazardous and organic form. Additional forms of SS resulting from industrial activities involving the above are also considered (e.g., due to the use of a bioreactor or from the pharmaceuticals industry). Figure 1 is an illustration of the different classes of biosolids.
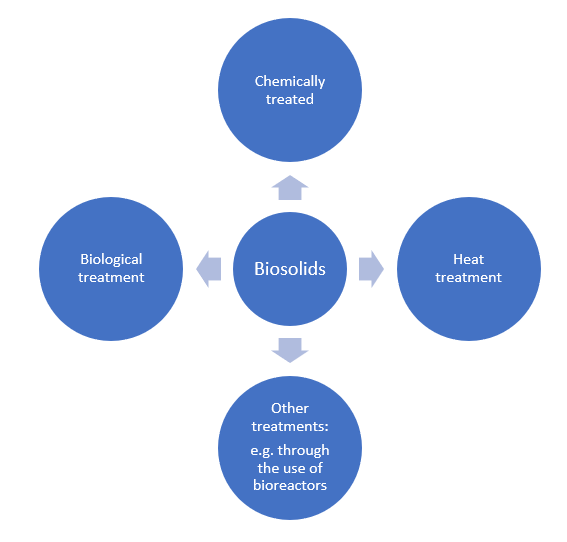
Figure 1. Different classifications of what constitutes biosolids
The pyrolysis process holds great potential as a thermal treatment method [1][2], that is capable of transforming biomass into value-added products. Typically, a biomass feedstock will undergo thermal treatment to an elevated temperature that goes beyond 400 °C in the pyrolysis process in an inert environment, that involves partial or total removal of oxygen. In addition to containing the gas produced resulting from the thermal degradation of the organic components [3][4], the pyrolysis process results in the production of three major products, namely, biochar, bio-oil, and biogas.
2. Management of Biosolids and the Use of the Pyrolysis Process
Solids resulting from wastewater are either applied to land as soil amendment and nutrient source, incinerated, or landfilled [5]. These solids are full of micropollutants that include organic chemicals originating from consumer-based products, which end up in the sewer drains during disposal (e.g., pharmaceuticals, antimicrobial compounds, personal care products, medicines and hormones, etc.) [6][7]. Therefore, the sewage sludges and wastewater treatment sludges and their by-products from these locations require new and innovative handling methods that alleviate environmental burdens, while producing value-added products, e.g., capacitors [8]. The thermal process of pyrolysis to biochars has been reported to combat some of these issues, for instance, the destruction of organic molecules, the destruction of microorganisms and the fixation of heavy metals, preventing them from leaching out into the soil.
3. The Pyrolysis Process
Pyrolysis is a relatively uncomplicated technology requiring no complex process plant compared with incineration and gasification and it is quite environmentally friendly [9]. Additional benefits include its ability to handle many waste types [10], while directly producing several product materials (for example, biochars, bio-oils and syngas) [11][12]. In terms of the limitations of the sustainability of the pyrolysis process for a treatment alternative, the issue for wet biosolids feedstock is a problem but it has been stated recently that the introduction of low-cost pre-drying solutions can improve the outcomes, prior to pyrolysis [13]. For example, solar drying methods are promising techniques as a low-cost drying pretreatment in arid environmental conditions, having plentiful sunlight and a low humidity environment. Alternatively, flue gases, available from the combustion of the bio-oils/biogas, in the temperature range 140–180 °C could achieve the same objective and even indirect heat exchange with the hot outlet pyrolysis gases. The pyrolysis process is classified into different types with the slow and fast pyrolysis being among the most commonly applied types (Figure 2).
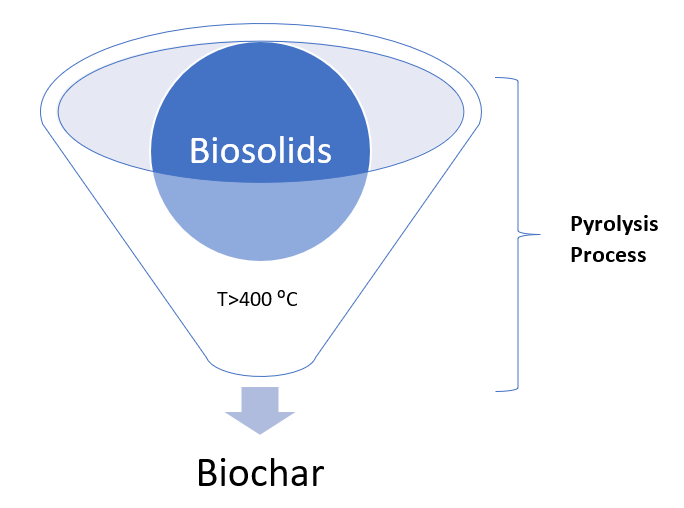
Figure 2. Depiction of the pyrolysis of biosolids
4. Benefits of Pyrolysis
Pyrolysis is a relatively uncomplicated technology requiring no complex process plant compared with incineration and gasification and it is quite environmentally friendly [14]. Additional benefits include its ability to handle many waste types [15], while directly producing several product materials (for example, biochars, bio-oils and syngas) [16][17]. In terms of the limitations of the sustainability of the pyrolysis process for a treatment alternative, the issue for wet biosolids feedstock is a problem but it has been stated recently that the introduction of low-cost pre-drying solutions can improve the outcomes, prior to pyrolysis [18]. For example, solar drying methods are promising techniques as a low-cost drying pretreatment in arid environmental conditions, having plentiful sunlight and a low humidity environment. Alternatively, flue gases, available from the combustion of the bio-oils/biogas, in the temperature range 140–180 °C could achieve the same objective and even indirect heat exchange with the hot outlet pyrolysis gases.
5. Limitations of the Pyrolysis Process
The pyrolysis process, whether fast or slow, is associated with a number of challenges which have been recently discussed in the work of [19] who has explored the use of wet biomass for bioenergy production. Briefly, the use of fast pyrolysis is typically associated with the requirements for dewatering or drying [18] stages, in addition to the resulting low yield and poor quality bio-oil. It also places significant pressure on vapor condensation and the gas cleaning system. Slow pyrolysis is also associated with a number of challenges that need remediation steps that are also outlined in the work of [19]. Such limitations include high energy requirements of the process in addition to the requirements for vapors separation and gas cleaning systems.
6. Treatment of Sludge Prior to Pyrolysis
Table 1. Previous studies on the pyrolysis of treated sewage sludge.
|
Pyrolysis Method |
Feedstock Type |
Process Conditions |
Products |
References |
||||
|
Temperature |
Time |
Pressure |
Char |
Oil |
Gas |
|||
|
Slow pyrolysis |
Air-dried biosolids |
300 °C and 500 °C |
Residence time of 30 min |
- |
86 ± 8 and 65 ± 4% |
- |
- |
[64] |
|
Slow pyrolysis |
Three different biowastes including biosolids |
550 °C |
Held for 1.5 h |
- |
18.6% |
- |
- |
[65] |
|
Microwave assisted pyrolysis in a customised single-mode microwave chamber connected to a 1.2 kW microwave source |
Biosolids |
600 °C |
Holding time of 10 min |
- |
|
- |
- |
[66] |
|
Fast microwave-assisted |
Continuous biomass (SS) |
450–600 °C |
- |
- |
63–34% |
17–26% |
20–40% |
[67] |
|
Microwave pyrolysis technology |
Biosolids |
The quartz crucible outer temperature was reported in the range 300 °C to 350 °C immediately after each experimental run; but the sample temperatures were in the range 600 °C to 650 °C |
- |
- |
59.93% |
2.37% |
37.7% |
[68] |
|
Microwave assisted pyrolysis in a customised single-mode microwave chamber connected to a 1.2 kW microwave source |
Biosolids |
300–800 °C |
Mean residence time of 6.38 s for the nitrogen in the pyrolysis chamber |
The control valve was manually adjusted to maintain the pressure within −15 kPa gauge pressure |
0.91–0.77 g |
- |
- |
[69] |
|
Slow pyrolysis |
Biosolids |
300–750 °C |
- |
- |
67.5 ± 1.2 to 48.1 ± 0.4% and 70.1 ± 1.2 to 44.4 ± 0.2 |
- |
- |
[70] |
|
Co-pyrolysis |
Anaerobically digested and thermally dried SS |
525 °C |
- |
1.01 × 105 Pa |
28% |
58% |
14% |
[71] |
|
Co-pyrolysis |
A triple oxidation ditch process was used for wastewater treatment and the SS feed samples were taken from the dewatering stage |
400–600 °C |
Pyrolysis for 1 h |
- |
44.5–44.1% |
- |
- |
[61] |
|
Flash pyrolysis |
Anaerobically digested and thermally dried sewage sludge |
450–600 °C |
1 s vapor residence time |
- |
24–10% |
70–73% |
5–17% |
[72] |
|
Slow pyrolysis |
Four different anaerobically digested sewage sludges |
500–700 °C |
Pyrolysis time of 5 hr |
- |
Ranged from 54.5 to 40.2% |
- |
- |
[73] |
|
Fast pyrolysis |
Biophysical dried sludge |
500–900 °C |
- |
- |
63.10 ± 0.50 to 53.31 ± 0.48% |
- |
- |
[74] |
|
Slow pyrolysis |
Digested wastewater sludge/biosolids |
300–700 °C |
- |
- |
72.3 ± 2.5 to 52.4 ± 2.6% |
- |
- |
[75] |
7. The Potential for Energy Recovery from the Pyrolysis Bio-Oil and Biogas
It is important to take into account the two major by-products, biogas and bio-oil, because their effective utilization will have a tremendous benefit on the economic attractiveness of the pyrolysis process package [27]. Besides biochars, the pyrolysis products from biosolids include bio-oil and biogas, which are associated with the energy content that can be potentially recovered and utilized. Table 2 presents a summary of high calorific values or higher heating values (HHV) reported by several studies.
Table 2. HHV of bio-oil and biogas from the literature.
|
Reference |
Bio-oil HHV |
Biogas HHV |
|
[107] |
27.8–31.4 kJ/kg in nitrogen and hydrogen atmosphere |
- |
|
[108] |
12.19–22.32 MJ/kg |
- |
|
[109] |
Energy yield of 1042–7762 kJ/kg-fuel |
Energy yield of 22–3745 kJ/kg-fuel |
|
[110] |
25.1 MJ/kg |
- |
|
[111] |
23.9–27.9 MJ/kg |
13–17.5 MJ/kg |
|
[112] |
36–39 MJ/kg-oil |
- |
The study conducted by [28] report the HHV of bio-oil and char only, where HHV of 36–39 MJ/kg-oil was observed for the process. The HHVs were not influenced by the operating temperatures nor the sludge type as per the study. Another promising results were demonstrated by the work of [29] in which the energy content of biogas and bio-oil was always greater than the energy required for the pyrolysis study.
Woody and non-woody biomass materials were used in the production of bio-oil and biochar, with the investigation of HHV of both products [30]. The HHV of bio-oil was comparable to other studies mentioned in Table 2, with values in the range 12.19–22.32 MJ/kg. It is evident that the slight variations in the reported values for HHV are due to the type of biomass used, its composition and the type of pyrolysis used. Moreover, bio-oil HHV was influenced by the inert environment used with heating value increasing from 27.75 MJ/kg to 31.40 MJ/kg when using nitrogen and then hydrogen in the work of [31]. The results imply the importance of the type of gases in the subsequent values of HHV of bio-oil products.
The energy recovery from both streams comes with some challenges. For instance, the liquid oil is heterogenous with fractions that contain dissimilar HHV [32], in addition to being a corrosive material. It is, however, a very attractive source of energy when compared to both the gas and char products’ energy content. The inclusion of the by-products into the economic analysis can improve the economic effectiveness by 2 or 3 fold [27].
8. Benefits of Biosolids-Derived Biochars
It is crucial to distinguish between biochars produced from biosolids and those obtained from other types of biomass materials. There are commonalities in the uses of all types of biochar and as such the same benefits are expected from biosolids-derived biochars. From the results of previous work on biochars produced from other types of biomasses, the different biochar products were found successful for use in amending soils in commercial potting soil mixes, green roofs, and commercial agriculture [5]. However, the same benefits from biochars derived from biosolids require further research to determine its applicability, opening room for more research opportunities in the applied side of this type of research.
Furthermore, the addition of different biochars to soils promotes plant growth. This addition is associated with interesting biological and chemical changes such that a shift in rhizosphere microbial and fungal communities to more favorable compositions for plant growth is attained. The same changes can be achieved through the contribution of some chemicals to the soil-system that lead to similar increases in nutrient concentrations [5][33][34].
References
- Panwar, N.L.; Kothari, R.; Tyagi, V.V. Thermo Chemical Conversion of Biomass-Eco Friendly Energy Routes. Renew. Sustain. Energy Rev. 2012, 16, 1801–1816.
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass Pyrolysis Kinetics: A Comparative Critical Review with Relevant Agricultural Residue Case Studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33.
- Lehmann, J. A Handful of Carbon. Nature 2007, 447, 143–144.
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the Pyrolysis Platform for Coproducing Bio-Oil and Biochar. Biofuels Bioprod. Biorefin. 2009, 3, 547–562.
- Liu, Z.; Singer, S.; Tong, Y.; Kimbell, L.; Anderson, E.; Hughes, M.; Zitomer, D.; McNamara, P. Characteristics and Applications of Biochars Derived from Wastewater Solids. Renew. Sustain. Energy Rev. 2018, 90, 650–664.
- McClellan, K.; Halden, R.U. Pharmaceuticals and Personal Care Products in Archived U.S. Biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010, 44, 658–668.
- Farzaneh, H.; Loganathan, K.; Saththasivam, J.; McKay, G. Ozone and Ozone/Hydrogen Peroxide Treatment to Remove Gemfibrozil and Ibuprofen from Treated Sewage Effluent: Factors Influencing Bromate Formation. Emerg. Contam. 2020, 6, 225–234.
- Xu, Z.X.; Deng, X.Q.; Zhang, S.; Shen, Y.F.; Shan, Y.Q.; Zhang, Z.M.; Luque, R.; Luque, R.; Duan, P.G.; Hu, X. Benign-by-Design N-Doped Carbonaceous Materials Obtained from the Hydrothermal Carbonization of Sewage Sludge for Supercapacitor Applications. Green Chem. 2020, 22, 3885–3895.
- Jo, J.H.; Kim, S.S.; Shim, J.W.; Lee, Y.E.; Yoo, Y.S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191.
- Delmiro, T.M.; Wilson, R.R.Y.O.V.; Melo, D.M.A.; Viana, G.A.C.M.; Mendes, L.B.B.; Braga, R.M. Catalytic Flash Pyrolysis of Monoraphidium Sp. before and after Lipid Extraction. Algal Res. 2021, 54, 102199.
- Foust, T.D.; Aden, A.; Dutta, A.; Phillips, S. An Economic and Environmental Comparison of a Biochemical and a Thermochemical Lignocellulosic Ethanol Conversion Processes. Cellulose 2009, 16, 547–565.
- Vakalis, S.; Sotiropoulos, A.; Moustakas, K.; Malamis, D.; Vekkos, K.; Baratieri, M. Thermochemical Valorization and Characterization of Household Biowaste. J. Environ. Manag. 2017, 203, 648–654.
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food Waste to Biochars through Pyrolysis: A Review. Resour. Conserv. Recycl. 2019, 144, 310–320.
- Jo, J.H.; Kim, S.S.; Shim, J.W.; Lee, Y.E.; Yoo, Y.S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191.
- Delmiro, T.M.; Wilson, R.R.Y.O.V.; Melo, D.M.A.; Viana, G.A.C.M.; Mendes, L.B.B.; Braga, R.M. Catalytic Flash Pyrolysis of Monoraphidium Sp. before and after Lipid Extraction. Algal Res. 2021, 54, 102199.
- Foust, T.D.; Aden, A.; Dutta, A.; Phillips, S. An Economic and Environmental Comparison of a Biochemical and a Thermochemical Lignocellulosic Ethanol Conversion Processes. Cellulose 2009, 16, 547–565.
- Vakalis, S.; Sotiropoulos, A.; Moustakas, K.; Malamis, D.; Vekkos, K.; Baratieri, M. Thermochemical Valorization and Characterization of Household Biowaste. J. Environ. Manag. 2017, 203, 648–654.
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food Waste to Biochars through Pyrolysis: A Review. Resour. Conserv. Recycl. 2019, 144, 310–320.
- Li, J.; Li, L.; Suvarna, M.; Pan, L.; Tabatabaei, M.; Ok, Y.S.; Wang, X. Wet Wastes to Bioenergy and Biochar: A Critical Review with Future Perspectives. Sci. Total Environ. 2022, 817, 152921.
- Jin, Z.; Chang, F.; Meng, F.; Wang, C.; Meng, Y.; Liu, X.; Wu, J.; Zuo, J.; Wang, K. Sustainable Pyrolytic Sludge-Char Preparation on Improvement of Closed-Loop Sewage Sludge Treatment: Characterization and Combined in-Situ Application. Chemosphere 2017, 184, 1043–1053.
- Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D.; Seifert, H. Screw Pyrolysis Technology for Sewage Sludge Treatment. Waste Manag. 2018, 73, 487–495.
- Zhang, H.; Gao, Z.; Ao, W.; Li, J.; Liu, G.; Fu, J.; Ran, C.; Mao, X.; Kang, Q.; Liu, Y.; et al. Microwave Pyrolysis of Textile Dyeing Sludge in a Continuously Operated Auger Reactor: Char Characterization and Analysis. J. Hazard. Mater. 2017, 334, 112–120.
- Zielińska, A.; Oleszczuk, P. Evaluation of Sewage Sludge and Slow Pyrolyzed Sewage Sludge-Derived Biochar for Adsorption of Phenanthrene and Pyrene. Bioresour. Technol. 2015, 192, 618–626.
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative Effects of Bamboo Sawdust Addition on Pyrolysis of Sewage Sludge: Biochar Properties and Environmental Risk from Metals. Bioresour. Technol. 2017, 228, 218–226.
- Li, W.; Lin, H.; Yang, Y.; Shang, Z.; Li, Q.; Ma, Y.; Liu, A.; Jiang, M. Enhanced Separation of Oil and Solids in Oily Sludge by Froth Flotation at Normal Temperature. Processes 2021, 9, 2163.
- Shie, J.L.; Chang, C.Y.; Lin, J.P.; Lee, D.J.; Wu, C.H. Use of Inexpensive Additives in Pyrolysis of Oil Sludge. Energy Fuels 2002, 16, 102–108.
- Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies 2021, 14, 1092.
- Méndez, A.; Terradillos, M.; Gascó, G. Physicochemical and Agronomic Properties of Biochar from Sewage Sludge Pyrolysed at Different Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130.
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of Bio-Oils and Solids from Flash Pyrolysis of Sewage Sludges. Fuel 2009, 88, 1344–1350.
- Trinh, T.N.; Jensen, P.A.; Kim, D.J.; Knudsen, N.O.; Sørensen, H.R. Influence of the Pyrolysis Temperature on Sewage Sludge Product Distribution, Bio-Oil, and Char Properties. Energy Fuels 2013, 27, 1419–1427.
- McNamara, P.J.; Koch, J.D.; Liu, Z.; Zitomer, D.H. Pyrolysis of Dried Wastewater Biosolids Can Be Energy Positive. Water Environ. Res. 2016, 88, 804–810.
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil-Concepts and Mechanisms. Plant Soil 2007, 300, 9–20.
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631.
- Altland, J.E.; Locke, J.C. Biochar Affects Macronutrient Leaching from a Soilless Substrate. HortScience 2012, 47, 1136–1140.





