Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dabing Zhang | -- | 1716 | 2022-08-10 09:10:07 | | | |
| 2 | Camila Xu | -17 word(s) | 1699 | 2022-08-15 03:47:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, J.; Kim, Y.; Zhang, D. Source-To-Sink Transport of Sugar During Male Reproductive Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/26015 (accessed on 12 March 2026).
Li J, Kim Y, Zhang D. Source-To-Sink Transport of Sugar During Male Reproductive Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/26015. Accessed March 12, 2026.
Li, Jingbin, Yu-Jin Kim, Dabing Zhang. "Source-To-Sink Transport of Sugar During Male Reproductive Development" Encyclopedia, https://encyclopedia.pub/entry/26015 (accessed March 12, 2026).
Li, J., Kim, Y., & Zhang, D. (2022, August 10). Source-To-Sink Transport of Sugar During Male Reproductive Development. In Encyclopedia. https://encyclopedia.pub/entry/26015
Li, Jingbin, et al. "Source-To-Sink Transport of Sugar During Male Reproductive Development." Encyclopedia. Web. 10 August, 2022.
Copy Citation
Sucrose is produced in leaf mesophyll cells via photosynthesis and exported to non-photosynthetic sink tissues through the phloem. The molecular basis of source-to-sink long-distance transport in cereal crop plants is of importance due to its direct influence on grain yield—pollen grains, essential for male fertility, are filled with sugary starch, and rely on long-distance sugar transport from source leaves.
sugar partitioning
phloem
sink
source
sugar signaling
1. Introduction
Rice (Oryza sativa), the monocot model plant, is a major crop, meeting the food demands of more than 50% of the global population [1][2]. Reproductive development, which connects the dominant diploid sporophytic and short haploid gametophytic stages, is a critical element in grain production [3]. The male reproductive organ, the stamen, consists of a filament and an anther containing multiple specialized tissues that generate mature male gametophytes, the pollen grains, via a series of developmental events such as meristem specification, cell differentiation, meiosis, mitosis, and starch accumulation [4][5].
Sugars are the constituents of main anther, and play essential roles in cell structure formation, energy supply, and male fertility in response to environmental conditions [6]. In rice, the expression of Cell Wall Invertase 3 (OsCWIN3/OsINV4) correlates with sucrose accumulation and pollen sterility depending on temperature [7], while two MYB domain proteins, Carbon Starved Anther (CSA) and CSA2, regulate sugar partitioning and male fertility in response to photoperiod [8][9][10][11]. The sugar transporter OsXa13/OsSWEET11 plays essential roles in pollen development and disease resistance against bacterial blight [12][13].
2. Strategies of Source-to-Sink Sugar Partitioning
Carbon is fixed from carbon dioxide into carbohydrate in chloroplasts of leaf tissues, primarily mesophyll cells, and accumulated in the cytosol of the same cells. The energy demands of sink tissues, such as roots, flowers, and seeds, drive the export of sugars from the leaf, mainly in the form of sucrose, via long-distance transport in plant vasculature, the phloem [14]. Over half of the photo-assimilates (50–80%) are exported from source leaves to maintain non-photosynthetic sink tissues [15]. Carbohydrate partitioning from source-to-sink tissues comprises three elements [16]: phloem loading of sugars from source tissues; transportation in the sieve element of the phloem; phloem unloading of sugars to sink tissues [17].
Phloem is composed of several cell types, including parenchyma cells, sieve elements (SEs), and companion cells (CCs) [18][19]. Phloem loading is the first vital step in sugar’s long-distance transport—transferring the sugars from mesophyll cells to the SEs and CCs of the phloem [20][21][22]. Three different strategies are used for phloem loading by different plants according to the abundance of plasmodesmata, SUT activity, and the concentration gradient of photosynthates (Figure 1) [23].
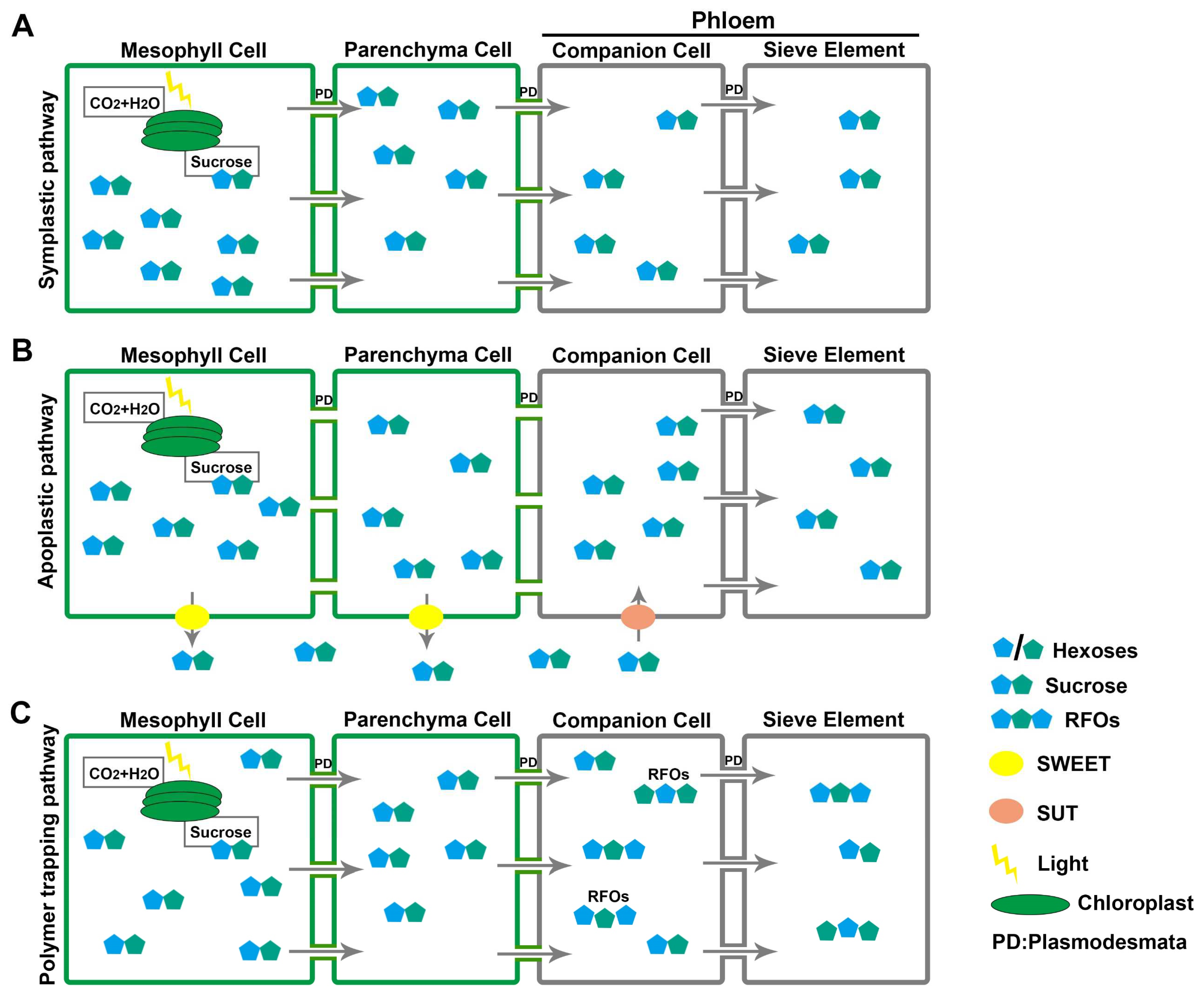
Figure 1. Three strategies for phloem loading. (A) Symplastic pathway: sucrose accumulates in mesophyll cells and is passively translocated to the phloem through plasmodesmata (PD) along the concentration gradient. (B) Apoplastic pathway: sucrose is exported to the apoplast by SWEETs and, after diffusion, imported into the phloem by SUTs. (C) Polymer trapping: sucrose is passively exported to phloem companion cells and synthesized into RFOs that can only move into sieve element cells due to their larger molecular mass.
The symplastic pathway is a passive loading process, driven by concentration gradients between mesophyll cells and phloem tissue (Figure 1A) [21][24], whereby the sucrose accumulated in mesophyll cells diffuses through plasmodesmata to reach phloem CCs [21][24]. Most tree species employ passive loading in the mesophyll cells, which meets the anatomical feature with high plasmodesmatal frequencies in the phloem of minor veins [23][25][26]. In most herbaceous plants, the apoplastic pathway is the main strategy for phloem loading [19]. Sucrose from mesophyll cells is actively exported to the apoplast by SWEET proteins (consuming energy), diffuses within the apoplast, and is actively loaded to phloem CCs via SUTs against a concentration gradient (Figure 1B) [19][25]. Polymer trapping, the third phloem-loading strategy, is an energy-consuming symplastic process adopted by a small number of specific plants (Figure 1C) [26].
3. Proteins Involved in Sugar Partitioning
3.1. Sucrose Transporters (SUTs)
Sucrose transporters (SUTs) act as symporters to import the sucrose from the apoplasm into phloem CCs against the concentration gradient, driven by the motive force generated by H+-ATPases (Figure 1B) [25][27]. The 12 transmembrane domains of the SUT protein forms a pore to transport sucrose across the plasma membrane [28].
The first sucrose transporter (SoSUT) was found in spinach (Spinacea oleracea) by an elegant yeast complementation strategy [29]. Nine and five SUTs have been found in Arabidopsis and rice, respectively [30][31]. Based on sequence, sub-cellular location, and activity, SUTs have been classified into three types: type I (specific to eudicots, plasma membrane–localized); type II (present in all plants, plasma membrane–localized); and type III (present in all plants, vacuolar membrane–localized) [32]. In rice, OsSUT1, OsSUT3, OsSUT4, and OsSUT5 are type II SUTs, and OsSUT2 is a type III tonoplast SUT (Table 1) [32].
Table 1. Proteins involved in sugar metabolism in rice.
| Gene Family | Number of Genes | Reported Genes/Reference |
|---|---|---|
| SUT | 5 | SUT1 [33][34]; SUT2 [35]; SUT3 [36]; SUT4, SUT5 [30] |
| SWEET | 21 | SWEET5 [37]; SWEET6a [38][39]; SWEET6b [39]; SWEET11 [12]; SWEET14 [13]; SWEET15 [40] |
| MST | 64 | |
| AZT subfamily | 6 | AZT3 [10] |
| ERD subfamily | 6 | |
| pGlcT subfamily | 4 | |
| Xylose subfamily | 2 | |
| STP subfamily | 15 | MST1, MST2, MST3 [41]; MST5 [42]; MST6 [43]; MST8 [8]; SPT17, SPT27 [38][39] |
| PLT subfamily | 28 | |
| INT subfamily | 3 | |
| Invertases | 18 | |
| VIN | 2 | VIN2 [44] |
| CIN | 8 | CIN8/Cyt-INV1 [45] |
| CWIN | 8 | GIF1 [46]; INV4 [7]; CWIN6 [39] |
The shaded areas represent subfamilies of these proteins.
3.2. Sugars Will Eventually Be Exported Transporters (SWEETs)
SWEETs are a group of evolutionally conserved genes expressed in eukaryotes, prokaryotes, and archaea [47][48]. These genes, encoding MtN3/saliva domain proteins, were initially found to encode glucose transporters [49], and have since been found to be capable of transporting a variety of mono- and di-saccharides [37][50][51][52]. According to their protein structures, SWEET proteins encode either one or two MtN3/saliva domains [53].
Rice encodes 21 SWEET proteins that are involved in multiple biological processes (Table 1) [53]. OsSWEET11, containing two MtN3/saliva domains, acts as a glucose uniporter in panicles and anthers [12]. Its knockdown mutant reveals defects in microspore development, suggesting a function in male development [12]. OsSWEET11 is also upregulated in response to bacterial infection by Xanthomonas oryzae pv. oryzae [12]. OsSWEET14 has a similar disease response, and its knockout mutant showed growth retardation, reduced plant size, and insensitivity to bacterial infection [13].
3.3. Invertases (INVs)
Invertases (INVs) encode proteins that hydrolyze sucrose into glucose and fructose [54], classified according to sub-cellular location into vacuolar (VIN), cell wall (CWIN), or cytoplasmic (CIN) invertases (Table 1) [55][56]. CINs prefer a neutral pH of 7.0–7.8 in the cytosol, while VINs and CWINs have an optimal pH of 4.5–5.5 [55]. Rice has 19 invertase genes, including nine CWINs, two VINs, and eight VINs (Table 1) [57]. CWIN proteins bind to the cell wall and play essential roles in sugar transmembrane transport during phloem unloading [55].
3.4. Monosaccharide Transporters (MSTs)
Monosaccharide transporters (MSTs) are membrane proteins involved in the transmembrane transport of hexoses, hydrolyzed from sucrose by INVs, in sink tissues in the apoplastic pathway (Figure 1B) [58]. An Arabidopsis phylogeny of 53 MST proteins suggests seven subfamilies—AZT, XTPH, ERD, pGlcT, PLT, INT, and STP (Table 1)—many of whose expression patterns or function have not yet been characterized [59]. Among the seven subfamilies of MST proteins, AZT and XTPH proteins localize on the tonoplast and play essential roles in sugar transport to the tonoplast [60][61][62]. AtERD6, a member of ERD proteins, was proved to be involved in the transport of monosaccharides, whose expression was induced by abiotic stress [63]. pGlcT proteins are transporters of glucose, and PLT proteins are symporters of polyols and monosaccharides [64][65]. AtINT4, the first identified member of the INT proteins, exhibits H+ symporter activities for myoinositol in yeast (Saccharomyces cerevisiae) and Xenopus laevis oocytes [66]. MST members of the STP sub-family are H+/hexose cotransporters locating on plasma membranes, which transport a series of hexoses, including glucose, fructose, galactose, xylose, mannose, pentose, and ribose [58].
4. Roles of Sugar Transporters in Phloem Loading and Unloading
Photosynthesis—“source activity”—and sink energy utilization—“sink strength”—combine to raise plant productivity [17][67]. Understanding the processes of phloem loading in source leaves and unloading in sink tissues can improve source activity and sink strength, leading to higher grain yields (Figure 2). After long-distance phloem transport from source tissues, sugar (mainly sucrose) is unloaded in sink organs; however, this process will lead to sucrose accumulation in sink tissues (reduced sink strength), resulting in reduced efficiency in sugar transport and source activity (Figure 2) [68].
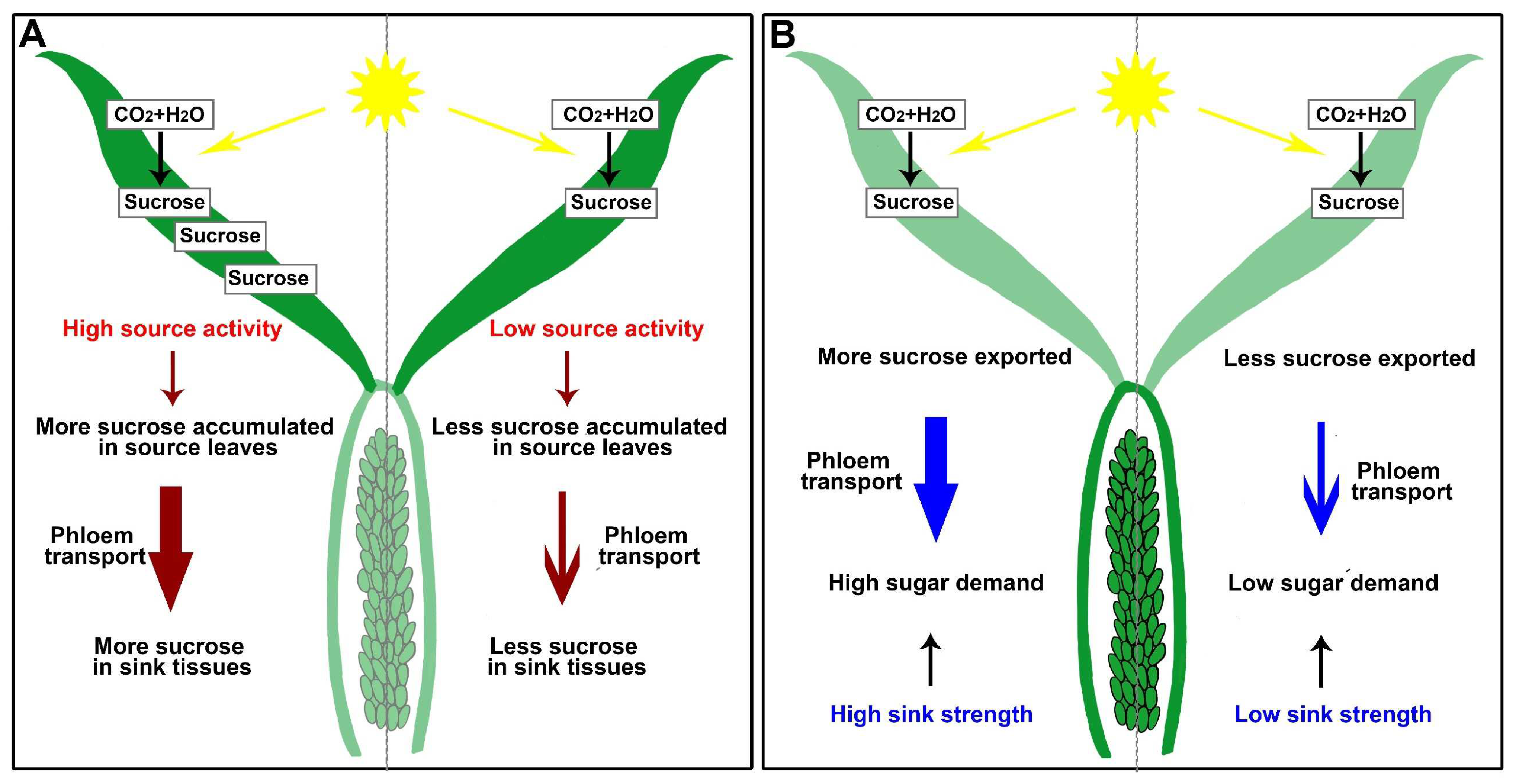
Figure 2. Schematic diagrams of sugar source-to-sink transport in rice. (A) High source activity in source leaves promotes phloem transport. (B) High sink strength results in high sugar demand, increasing the sugar transport.
In Arabidopsis, AtSUC2 is expressed in phloem CCs of minor leaf veins, which are supposed to be involved in the source-to-sink transition [69][70]. An AtSUT2 T-DNA insertion mutant line exhibits decreased sucrose exports from leaves, resulting in sucrose accumulation in leaves, and delayed root growth and flowering [71]. OsSUT1, a type II SUT like AtSUC2, is highly expressed in leaves, stems, and grains; however, knockdown lines of OsSUT1 do not show sucrose accumulation in source leaves [33][34]. OsSUT3, another type II SUT, is preferentially expressed in pollen, suggesting a function in pollen development and maturity rather than phloem loading in source leaves [36]. OsSUT2, a type III SUT, is involved in sucrose transfer across the tonoplast from the vacuole lumen to the cytosol in rice [35].
SWEETs transport mono- or di-saccharides across membranes for phloem transport [72]. In Arabidopsis, AtSWEET11/12 localizes in the plasma membrane of vascular tissues and participates in phloem transport [50]. Maize ZmSWEET4c functions in hexose transport during seed development, and its mutation demonstrates a lack of hexose transport and defect in seed filling [73]. In rice, OsSWEET11 and OsSWEET14, two response factors to bacterial infection, also show essential roles in grain filling, whose mutants reveal defective in grain filling, resulting in increased starch accumulation in the pericarp [13][74][75]. OsSWEET15, another symporter highly expressed in rice caryopses, is necessary for sucrose efflux from caryopses to grains during seed filling [40]. OsSWEET5 encodes a galactose transporter, whose overexpression causes growth retardation and precocious senescence in rice seedlings [37]. These SWEET proteins showed important roles in grain filling, demonstrating their biological function of sucrose transfer from caryopses (source) to grains (sink).
Cell wall invertases (CWINs) play important roles in apoplasmic unloading, decreasing the concentration of sucrose in sink tissues to improve sink strength [55][76]. In rice, grain yield significantly decreased when the expression of OsCWIN2 (GIF1) was suppressed [77], and a similar phenotype is observed in ZmCWIN2 (Incw2) mutants in maize (Zea mays) [74]. VfCWIN1 in Vicia faba, a dicot species, is also reported to impact seed size [75]. Moreover, OsCWIN3 (INV4) has high expression in rice anthers, and affects male fertility in response to temperature variations [7].
References
- Izawa, T.; Shimamoto, K. Becoming a model plant: The importance of rice to plant science. Trends Plant Sci. 1996, 1, 95–99.
- Delseny, M.; Salses, J.; Cooke, R.; Sallaud, C.; Regad, F.; Lagoda, P.; Guiderdoni, E.; Ventelon, M.; Brugidou, C.; Ghesquière, A. Rice genomics: Present and future. Plant Physiol. Biochem. 2001, 39, 323–334.
- Zhang, D.; Yang, L. Specification of tapetum and microsporocyte cells within the anther. Curr. Opin. Plant Biol. 2014, 17, 49–55.
- Zhang, D.; Luo, X.; Zhu, L. Cytological analysis and genetic control of rice anther development. J. Genet. Genom. 2011, 38, 379–390.
- Zhang, D.; Wilson, Z.A. Stamen specification and anther development in rice. Chin. Sci. Bull. 2009, 54, 2342–2353.
- Stanley, R.G. Pollen Chemistry and Tube Growth. Pollen 1971, 131–155.
- Oliver, S.N.; van Dongen, J.; Alfred, S.C.; Mamun, E.A.; Zhao, X.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.; Sutton, B.G.; Geigenberger, P.; et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell Environ. 2005, 28, 1534–1551.
- Zhang, H.; Liang, W.; Yang, X.; Luo, X.; Jiang, N.; Ma, H.; Zhang, D. Carbon Starved Anther Encodes a MYB Domain Protein That Regulates Sugar Partitioning Required for Rice Pollen Development. Plant Cell 2010, 22, 672–689.
- Zhang, H.; Xu, C.; He, Y.; Zong, J.; Yang, X.; Si, H.; Sun, Z.; Hu, J.; Liang, W.; Zhang, D. Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc. Natl. Acad. Sci. USA 2013, 110, 76–81.
- Wang, D.; Li, J.; Sun, L.; Hu, Y.; Yu, J.; Wang, C.; Zhang, F.; Hou, H.; Liang, W.; Zhang, D. Two rice MYB transcription factors maintain male fertility in response to photoperiod by modulating sugar partitioning. New Phytol. 2021, 231, 1612–1629.
- Sun, L.; Yuan, Z.; Wang, D.; Li, J.; Shi, J.; Hu, Y.; Yu, J.; Chen, X.; Chen, S.; Liang, W.; et al. Carbon Starved Anther modulates sugar and ABA metabolism to protect rice seed germination and seedling fitness. Plant Physiol. 2021, 187, 2405–2418.
- Chu, Z.; Yuan, M.; Yao, J.; Ge, X.; Yuan, B.; Xu, C.; Li, X.; Fu, B.; Li, Z.; Bennetzen, J.L.; et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006, 20, 1250–1255.
- Antony, G.; Zhou, J.; Huang, S.; Li, T.; Liu, B.; White, F.; Yang, B. Rice xa13 Recessive Resistance to Bacterial Blight Is Defeated by Induction of the Disease Susceptibility Gene Os-11N3. Plant Cell 2010, 22, 3864–3876.
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272.
- Kalt-Torres, W.; Kerr, P.S.; Usuda, H.; Huber, S.C. Diurnal changes in maize leaf photosynthesis: I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987, 83, 283–288.
- Turgeon, R.; Wolf, S. Phloem Transport: Cellular Pathways and Molecular Trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221.
- Osorio, S.; Ruan, Y.-L.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516.
- White, P.J. Long-distance transport in the xylem and phloem. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 49–70.
- Zhang, C.; Turgeon, R. Mechanisms of phloem loading. Curr. Opin. Plant Biol. 2018, 43, 71–75.
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant, Cell Environ. 2003, 26, 37–56.
- Turgeon, R.; Ayre, B.G. Pathways and Mechanisms of Phloem Loading. In Vascular Transport in Plants; Academic Press: Cambridge, MA, USA, 2005; pp. 45–67.
- McCaskill, A.; Turgeon, R. Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc. Natl. Acad. Sci. USA 2007, 104, 19619–19624.
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167.
- Turgeon, R.; Medville, R. The absence of phloem loading in willow leaves. Proc. Natl. Acad. Sci. USA 1998, 95, 12055–12060.
- Bush, D.R. Proton-coupled sugar and amino acid transporters in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 513–542.
- Turgeon, R. Phloem loading and plasmodesmata. Trends Plant Sci. 1996, 1, 418–423.
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67.
- Braun, D.M.; Slewinski, T.L. Genetic Control of Carbon Partitioning in Grasses: Roles of Sucrose Transporters and Tie-dyed Loci in Phloem Loading. Plant Physiol. 2009, 149, 71–81.
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992, 11, 4705–4713.
- Aoki, N.; Hirose, T.; Scofield, G.N.; Whitfeld, P.R.; Furbank, R.T. The Sucrose Transporter Gene Family in Rice. Plant Cell Physiol. 2003, 44, 223–232.
- Kühn, C. A Comparison of the Sucrose Transporter Systems of Different Plant Species. Plant Biol. 2003, 5, 215–232.
- Lim, J.D.; Cho, J.-I.; Park, Y.-I.; Hahn, T.-R.; Choi, S.-B.; Jeon, J.-S. Sucrose transport from source to sink seeds in rice. Physiol. Plant. 2006, 126, 572–584.
- Ishimaru, K.; Hirose, T.; Aoki, N.; Takahashi, S.; Ono, K.; Yamamoto, S.; Wu, J.; Saji, S.; Baba, T.; Ugaki, M.; et al. Antisense Expression of a Rice Sucrose Transporter OsSUT1 in Rice (Oryza sativa L.). Plant Cell Physiol. 2001, 42, 1181–1185.
- Scofield, G.N.; Hirose, T.; Aoki, N.; Furbank, R.T. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J. Exp. Bot. 2007, 58, 3155–3169.
- Eom, J.S.; Cho, J.I.; Reinders, A.; Lee, S.W.; Yoo, Y.; Tuan, P.Q.; Choi, S.B.; Bang, G.; Park, Y.I.; Cho, M.H.; et al. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 2011, 157, 109–119.
- Eom, J.-S.; Choi, S.-B.; Ward, J.; Jeon, J.-S. The mechanism of phloem loading in rice (Oryza sativa). Mol. Cells 2012, 33, 431–438.
- Zhou, Y.; Liu, L.; Huang, W.; Yuan, M.; Zhou, F.; Li, X.; Lin, Y. Overexpression of OsSWEET5 in Rice Causes Growth Retardation and Precocious Senescence. PLoS ONE 2014, 9, e94210.
- Wang, G.; Wu, Y.; Ma, L.; Lin, Y.; Hu, Y.; Li, M.; Li, W.; Ding, Y.; Chen, L. Phloem loading in rice leaves depends strongly on the apoplastic pathway. J. Exp. Bot. 2021, 72, 3723–3738.
- Li, J.; Wang, D.; Sun, S.; Sun, L.; Zong, J.; Lei, Y.; Yu, J.; Liang, W.; Zhang, D. The regulatory role of CARBON STARVED ANTHER-mediated photoperiod-dependent male fertility in rice. Plant Physiol. 2022, 189, 955–971.
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J. SWEET 11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615.
- Toyofuku, K.; Kasahara, M.; Yamaguchi, J. Characterization and Expression of Monosaccharide Transporters (OsMSTs) in Rice. Plant Cell Physiol. 2000, 41, 940–947.
- Ngampanya, B.; Sobolewska, A.; Takeda, T.; Toyofuku, K.; Narangajavana, J.; Ikeda, A.; Yamaguchi, J. Characterization of rice functional monosaccharide transporter, OsMST5. Biosci. Biotechnol. Biochem. 2003, 67, 556–562.
- Monfared, H.H.; Chew, J.K.; Azizi, P.; Xue, G.-P.; Ee, S.-F.; Kadkhodaei, S.; Hedayati, P.; Ismail, I.; Zainal, Z. Overexpression of a Rice Monosaccharide Transporter Gene (OsMST6) Confers Enhanced Tolerance to Drought and Salinity Stress in Arabidopsis thaliana. Plant Mol. Biol. Rep. 2020, 38, 151–164.
- Xu, X.; Ren, Y.; Wang, C.; Zhang, H.; Wang, F.; Chen, J.; Liu, X.; Zheng, T.; Cai, M.; Zeng, Z.; et al. OsVIN2 encodes a vacuolar acid invertase that affects grain size by altering sugar metabolism in rice. Plant Cell Rep. 2019, 38, 1273–1290.
- Jia, L.; Zhang, B.; Mao, C.; Li, J.; Wu, Y.; Wu, P.; Wu, Z. OsCYT-INV1 for alkaline/neutral invertase is involved in root cell development and reproductivity in rice (Oryza sativa L.). Planta 2008, 228, 51–59.
- Wang, E.; Xu, X.; Zhang, L.; Zhang, H.; Lin, L.; Wang, Q.; Li, Q.; Ge, S.; Lu, B.R.; Wang, W.; et al. Research article Duplication and independent selection of cell-wall invertase genes GIF1 and OsCIN1 during rice evolution and domestication. BMC Evol. Biol. 2010, 10, 108.
- Saier, M.H. TCDB: The Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006, 34, D181–D186.
- Hamada, M.; Wada, S.; Kobayashi, K.; Satoh, N. Ci-Rga, a gene encoding an MtN3/saliva family transmembrane protein, is essential for tissue differentiation during embryogenesis of the ascidian Ciona intestinalis. Differentiation 2005, 73, 364–376.
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532.
- Chen, L.-Q.; Qu, X.-Q.; Hou, B.-H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science 2012, 335, 207–211.
- Lin, I.W.; Sosso, D.; Chen, L.-Q.; Gase, K.; Kim, S.-G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.-H.; Qu, X.-Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549.
- Chen, L.-Q.; Lin, I.W.; Qu, X.-Q.; Sosso, D.; McFarlane, H.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A Cascade of Sequentially Expressed Sucrose Transporters in the Seed Coat and Endosperm Provides Nutrition for the Arabidopsis Embryo. Plant Cell 2015, 27, 607–619.
- Yuan, M.; Zhao, J.; Huang, R.; Li, X.; Xiao, J.; Wang, S. Rice MtN3/saliva/SWEET gene family: Evolution, expression profiling, and sugar transport. J. Integr. Plant Biol. 2014, 56, 559–570.
- Ruan, Y.L.; Jin, Y.; Huang, J. Capping invertase activity by its inhibitor: Roles and implications in sugar signaling, carbon allocation, senescence and evolution. Plant Signal. Behav. 2009, 4, 983–985.
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955.
- Shen, S.; Ma, S.; Liu, Y.; Liao, S.; Li, J.; Wu, L.; Kartika, D.; Mock, H.-P.; Ruan, Y.-L. Cell Wall Invertase and Sugar Transporters Are Differentially Activated in Tomato Styles and Ovaries During Pollination and Fertilization. Front. Plant Sci. 2019, 10, 506.
- Ji, X.; Ende, W.V.D.; Van Laere, A.; Cheng, S.; Bennett, J. Structure, Evolution, and Expression of the Two Invertase Gene Families of Rice. J. Mol. Evol. 2005, 60, 615–634.
- Deng, X.; An, B.; Zhong, H.; Yang, J.; Kong, W.; Li, Y. A Novel Insight into Functional Divergence of the MST Gene Family in Rice Based on Comprehensive Expression Patterns. Genes 2019, 10, 239.
- Johnson, D.A.; Hill, J.P.; Thomas, M.A. The monosaccharide transporter gene family in land plants is ancient and shows differential subfamily expression and expansion across lineages. BMC Evol. Biol. 2006, 6, 64.
- Schulz, A.; Beyhl, D.; Marten, I.; Wormit, A.; Neuhaus, E.; Poschet, G.; Büttner, M.; Schneider, S.; Sauer, N.; Hedrich, R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011, 68, 129–136.
- Wormit, A.; Trentmann, O.; Feifer, I.; Lohr, C.; Tjaden, J.; Meyer, S.; Schmidt, U.; Martinoia, E.; Neuhaus, H.E. Molecular Identification and Physiological Characterization of a Novel Monosaccharide Transporter from Arabidopsis Involved in Vacuolar Sugar Transport. Plant Cell 2006, 18, 3476–3490.
- Aluri, S.; Buttner, M. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc. Natl. Acad. Sci. USA 2007, 104, 2537–2542.
- Yamada, K.; Osakabe, Y.; Mizoi, J.; Nakashima, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of an Arabidopsis thaliana Abiotic Stress-inducible Facilitated Diffusion Transporter for Monosaccharides. J. Biol. Chem. 2010, 285, 1138–1146.
- Cho, M.-H.; Lim, H.; Shin, D.H.; Jeon, J.-S.; Bhoo, S.H.; Park, Y.-I.; Hahn, T. Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana. New Phytol. 2011, 190, 101–112.
- Klepek, Y.-S.; Volke, M.; Konrad, K.R.; Wippel, K.; Hoth, S.; Hedrich, R.; Sauer, N. Arabidopsis thaliana POLYOL/MONOSACCHARIDE TRANSPORTERS 1 and 2: Fructose and xylitol/H+ symporters in pollen and young xylem cells. J. Exp. Bot. 2010, 61, 537–550.
- Schneider, S.; Schneidereit, A.; Konrad, K.; Hajirezaei, M.-R.; Gramann, M.; Hedrich, R.; Sauer, N. Arabidopsis INOSITOL TRANSPORTER4 Mediates High-Affinity H+ Symport of Myoinositol across the Plasma Membrane. Plant Physiol. 2006, 141, 565–577.
- Kaschuk, G.; Hungria, M.; Leffelaar, P.A.; Giller, K.E.; Kuyper, T.W. Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max Merrill) dependent on N2 fixation or nitrate supply. Plant Biol. 2010, 12, 60–69.
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547.
- Wright, K.M.; Roberts, A.G.; Martens, H.J.; Sauer, N.; Oparka, K.J. Structural and Functional Vein Maturation in Developing Tobacco Leaves in Relation to AtSUC2 Promoter Activity. Plant Physiol. 2003, 131, 1555–1565.
- Truernit, E.; Sauer, N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: Evidence for phloem loading and unloading by SUC2. Planta 1995, 196, 564–570.
- Gottwald, J.R.; Krysan, P.J.; Young, J.C.; Evert, R.F.; Sussman, M.R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13979–13984.
- Han, L.; Zhu, Y.; Liu, M.; Zhou, Y.; Lu, G.; Lan, L.; Wang, X.; Zhao, Y.; Zhang, X.C. Molecular mechanism of substrate recognition and transport by the AtSWEET13 sugar transporter. Proc. Natl. Acad. Sci. USA 2017, 114, 10089–10094.
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; Mccarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493.
- Cheng, W.H.; Taliercio, E.W.; Chourey, P.S. The Miniature1 Seed Locus of Maize Encodes a Cell Wall Invertase Required for Normal Development of Endosperm and Maternal Cells in the Pedicel. Plant Cell 1996, 8, 971–983.
- Weber, H.; Borisjuk, L.; Wobus, U. Weber-Controlling seed development and seed size in Vicia faba a role for seed. Plant J. 1996, 10, 823–834.
- Ruan, Y.L.; Chourey, P.S.; Delmer, D.P.; Perez-Grau, L. The Differential Expression of Sucrose Synthase in Relation to Diverse Patterns of Carbon Partitioning in Developing Cotton Seed. Plant Physiol. 1997, 115, 375–385.
- Wang, E.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
15 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





