The development of smart, efficient and multifunctional material systems for diseases treatment are imperative to meet current and future health challenges. Nanomaterials with theranostic properties have offered a cost effective and efficient solution for disease treatment, particularly, metal/oxide based nanotheranostic systems already offering therapeutic and imaging capabilities for cancer treatment. Nanoparticles can selectively generate/scavenge ROS through intrinsic or external stimuli to augment/diminish oxidative stress. An efficient treatment requires higher oxidative stress/toxicity in malignant disease, with a minimal level in surrounding normal cells. The size, shape and surface properties of nanoparticles are critical parameters for achieving a theranostic function in the microenvironment. The exhibition of therapeutics properties such as selective reactive oxygen species (ROS) scavenging, hyperthermia, antibacterial, antiviral, and imaging capabilities such as MRI, CT and fluorescence activity have been reported in a variety of developed nanosystems to combat cancer, neurodegenerative and emerging infectious diseases.
1. Introduction
Nanoparticles (NPs) offer a unique combination of physiochemical properties for the treatment of various disease. Their multifunctional use in therapeutics, imaging, drug delivery and diagnostics is increasing day by day
[1][2]. In the last decade, oxide-based nanotheranostic systems have rapidly expanded and emerged as a leading research field. The integration of therapeutic and imaging capabilities into a single nano-entity can form theranostic nanostructures
[3]. An efficient theranostic NPs drug system must have requisite biocompatibility, biodegradability and adequate clearance. Other properties, such as tumour accumulation, drug delivery, immune system escape and selective targeting are also matters of interest in disease therapy and diagnostics
[4]. In view of the theraputic aspects, NPs have excellently enhanced the efficiency of many cancer treatment methods, such as chemotherapy, radiation therapy, immunotherapy, targeted drug therapy and magnetic hyperthermia. Many in vitro and in vivo studies found an increase in reactive oxygen species (ROS) in most cancer treatment cases. New treatment strategies based on the augmentation of ROS levels to induce higher oxidative stress in cancer cells are being developed. Increased oxidative stress overwhelms the redox adaptation of cells, which is incompatible with the survival of cellular life and can eradicate tumour cells. ROS levels play a critical role in the development and progression of cancer, and are also considered to be vital for cancer treatment
[5].
ROS are produced from exogenous as well as endogenous sources by enzymatic or non-enzymatic reactions. Exogenous sources include smoking, certain drugs, pollutants and alcohol, whereas infection, stress, ischemia, immune cell activation and mitochondrion reactions are sources of endogenous ROS. The enzymatic reactions for ROS production include phagocytosis, cytochromes reactions, prostaglandin and the respiratory chain. Non-enzymatic free radicals are generated by the reaction of oxygen with organic compounds when cells are exposed to radiation. Non-enzymatic ROS can also be generated by mitochondrion respiration.
ROS accumulation affects the physiological signalling network to initiate the pathological conversion of normal cells into malignant cells and contributes towards malignant cell proliferation. Excessive ROS generation may have a damaging effect on cell organelles, including cell membranes, lipids, proteins and DNA, and can cause cell death. Therefore, the scavenging of ROS to prevent oxidative stress or generation of more ROS to kill the cancer cells by elevating oxidative stress levels are promising strategies for cancer treatment.
The neutralisation of excess ROS is achieved through well-known enzymes, including glutathione; flavonoids; vitamins A, C and E or through antioxidant compounds that specifically scavenge ROS. Superoxide dismutase (SOD) is a metalloenzyme that exists abundantly in eukaryotes and prokaryotes utilise metal ions, such as manganese (Mn
2+), iron (Fe
2+), zinc (Zn
2+) and copper (Cu
2+) for the dismutation of the superoxide anion (O
2•−) to oxygen (O
2) and hydrogen peroxide (H
2O
2)
[6]. The catalase (CAT) enzyme is involved in the decomposition of H
2O
2 into water (H
2O) and O
2 [7][8]. Similarly, many nanostructures or composite nanostructures based on Au, Ag, Mn, Mg Ce, Se, Fe, Ti, Zn, In, Bi, Ta, redox polymer and polyphenols are selectively employed in ROS scavenging or generation.
Various oxide NPs based non-invasive techniques, such as magnetic resonance imaging, optical imaging, computer tomography (CT), positron emission tomography, ultrasonic imaging and single photon emission computer tomography are being employed in disease treatment
[9][10][11]. Nanomaterial-based radiolabels find applications in nuclear medicine imaging
[12]. Silica-coated gold (Au) NPs are used as photoacoustic contrast agents for the imaging of sentinel lymph node. Supermagnetic theranostic NPs have been extensively studied for MRI and the local hyperthermia of tumour
[1][13].
Although nanotheranostics have great potential in the field of nanomedicine, the generation of unwanted ROS and oxidative stress in normal cells and retention inside the body present a hurdle in the translation to effective and viable treatment strategies
[14][15].
NP properties alter with the change in surface chemistry, size and shape, which ultimately depends upon synthesis conditions. The variations in pH, ROS level and other cellular and extracellular environments affect the biocompatibility and therapeutic and imaging properties of nanostructures. NPs can be specifically designed to optimise the desired performance under selective conditions. Furthermore, surface coating or encrustation and the formation of a composite or core shell structure have been adopted to increase the efficiency and biocompatibility of the nanostructures or nanomedicines. Combining the diagnostic and therapeutic properties into a single nano/nanocomposite entity presents great potential in the field of medicine.
There are a variety of conventional synthesis techniques, such as precipitation, gas condensation, sol–gel, laser ablation, sono-chemical, hydrothermal, spark discharge and microwave, which have been employed for the synthesis of the nanostructures of different metal oxides
[16]. The modifications in synthesis techniques or strategies, the use of non-toxic chemical reagents and controlled processing parameters can enhance biocompatibility and increase surface therapeutic efficiency and imaging capabilities. Surface property modifications enhance the chemical interaction with a site-specific target, such as in the case of tumour cells
[17][18]. In addition, they can be efficient carriers of drugs for the selective treatment of disease without harming the normal neighbouring tissues or cells. Enhanced permeability (EPR) and retention in the malignant tumour are also desired for efficient drug responses. In the case of NP-based immunotherapy, the system is designed for the controlled drug release, generation or scavenging of ROS in the complex tumour microenvironment by exploiting their enzymatic, pH, hypoxia, ultrasound, electricity and light-dependent response
[19][20][21]. Nanoparticles’ internalisation or delivery to specific sites is usually obtained by passive and active targeting. Passive targeting involves the passage of nanoparticles through the leaky vasculature and accumulation within a tumour. In active targeting, molecules/ligands are attached to the NP’s surface for specific receptor acceptance
[22]. Antioxidants, SOD conjugated polymers or metal-based NPs are employed as active and passive targeting simultaneously for intercellular and extracellular ROS management
[8][23][24]. Cell culture studies can provide detailed information about the biological process at the basic level in an organism. The cell culture models are very useful in evaluating the toxicology and physiology of NPs/drugs,
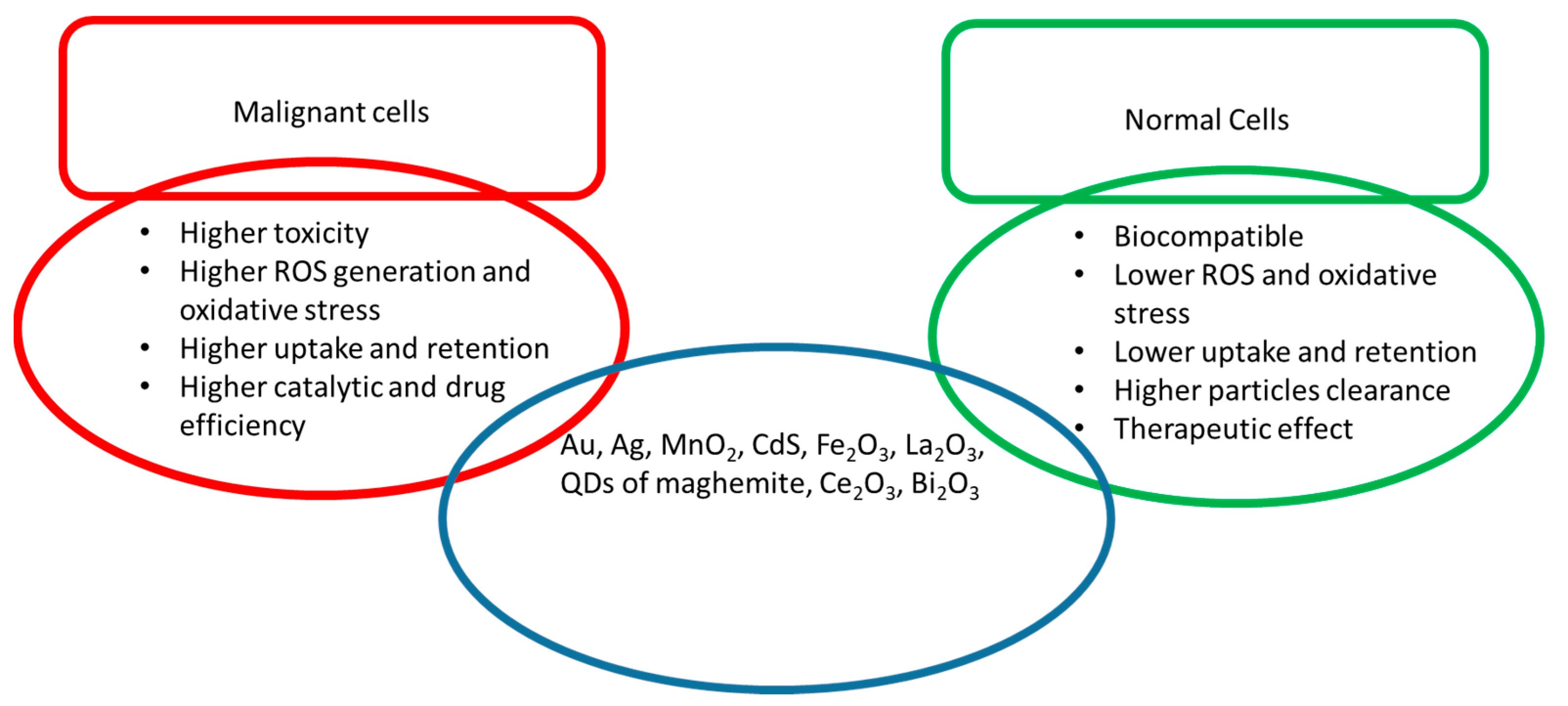
Figure 1. They play an important role in the development of vaccines, drugs, bioactive substances, diagnostic techniques, theranostic agents, food ingredients and cosmetics
[25]. This entry mainly focuses on the passive activity of NPs in disease (malignant) conditions with reference to normal cellular behaviour to record their effectiveness.
Figure 1. Functional properties of different metal and metal oxide NPs in malignant and normal cells.
2. Au-Based Nanostructures
Recently, Au NPs have received considerable interest in the field of medicine due to their theranaostics properties. The high specific surface area of NPs and excellent surface-dependent catalytic properties potentiate their use in a variety of biomedical and engineering applications. Au NPs are being used in solar cells, flash memory storage, pollution control, water and hydrogen purification and the catalytic oxidation of carbon monoxide. In the biomedical field, Au NPs are employed in genomics, cancer therapy, biosensors, drug delivery and cell monitoring and imaging
[26][27][28][29][30].
Recent studies have shown that Au NPs have anti-tumour properties against breast, colon, lungs and liver cancers
[31][32]. In addition to anti-tumour properties, the NPs’ effects on normal cells have also been studied. Similarly, Au NPs are also linked to cellular apoptosis through the generation of oxidative stress. J. Li et al. reported the induction of oxidative stress in Au NP-treated human lung fibroblast cells. The treatment with Au NPs caused lipid peroxidation, the upregulation of antioxidants, protein expression and stress-response genes
[33]. The chemically synthesised Au NPs exhibited higher toxicity in comparison to biosynthesised and surface-coated/core shell NPs
[31][34]. Fewer studies have reported that Au NPs can be synthesised for selective oxidative stress/toxicity in cancer cells and normal cells
[35][36]. The size, shape, surface charge and surface treatment of NPs influence their circulation, retention and toxicity in the body. The extent of toxicity of Au NPs also depends on the method of production and functionalisation of NPs with polymeric substances. Bare Au NPs usually exhibit greater toxicity than functionalised and biogenic NPs.
Au NPs absorb visible light and emit energy of a specific wavelength, which is used for diagnostic and light-mediated clinical treatments
[37]. Due to the unique surface plasmonic properties of Au NPs, they are extensively employed in optical imaging. They offer an excellent choice for cellular visualisation as the scattering signal from Au NPs is much stronger than the background scattering from cellular components and tissues. Even the interaction of a single Au NP with biological systems can be visualised using dark field (DF) and bright field (BF) microscopies, differential interference contrast (DIC) microscopy and photothermal and photoluminescence methods
[38]. Au NPs can offer multifunctional and theranostic treatment strategies with requisite biocompatibility, if developed by a suitable processing technique or with surface treatment using biocompatible materials. In recent studies, Au NPs expressed good biocompatibility, ease of synthesis, surface property modification, surface plasmonic properties and passive targeting for cancerous cells
[22]. Au NPs are considered as a better choice for passive targeting due to their excellent EPR effect. Recently, Au NPs systems have been developed for the active targeting of cancerous cells. Various molecular attachments based on proteins, peptides, polymers, carbohydrates and antibodies are attached to the surface of Au NPs for specific receptor targeting
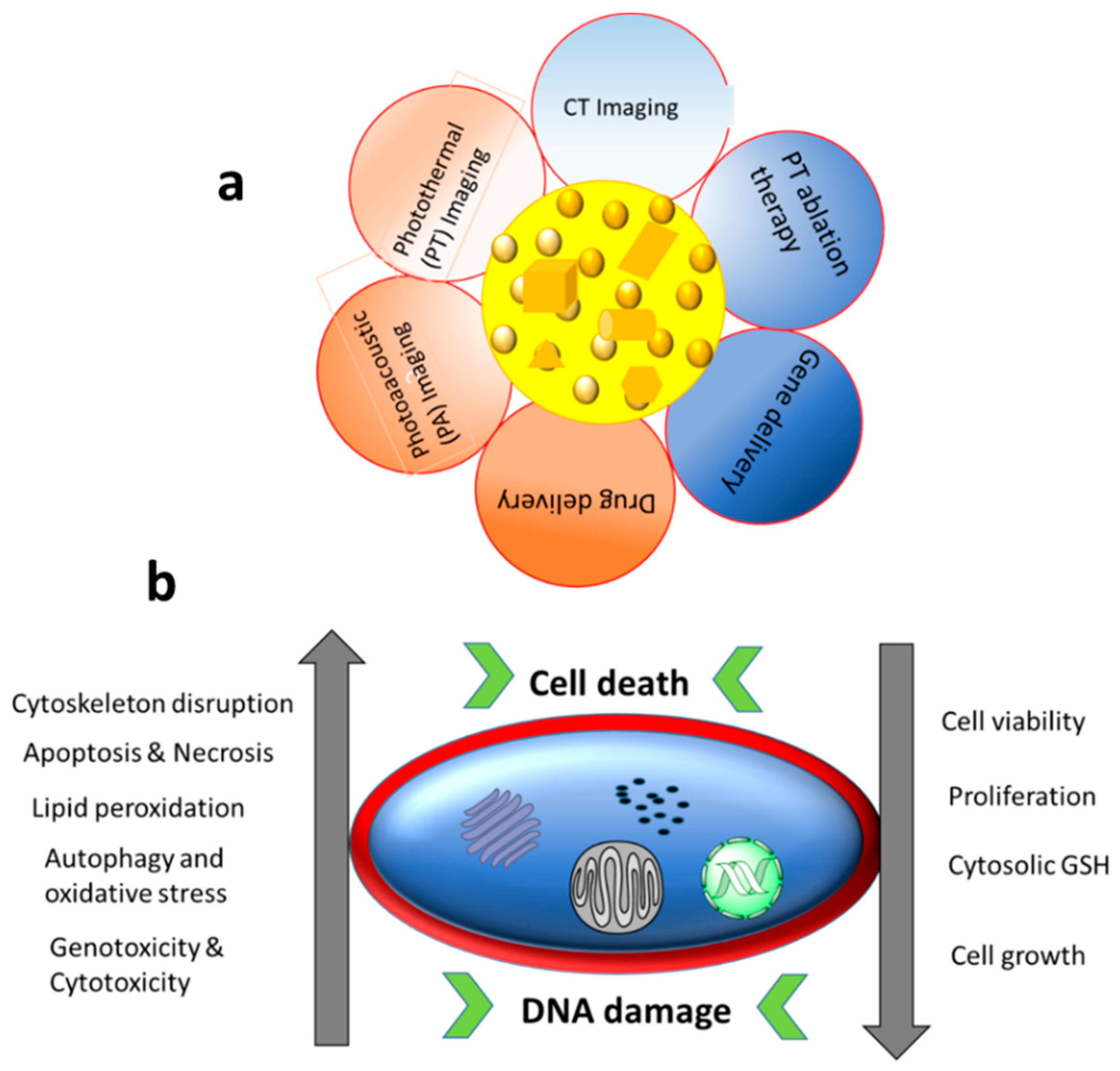
[22][39]. The major applications and common cellular effects of Au NPs are shown in
Figure 2.
Figure 2. (a) Biological applications of Au NPs and (b) different contributing factors (with increasing or decreasing trends) towards DNA damage and cell death caused by treatment with Au NPs.
3. Silver Nanoparticles
Due to well-established antimicrobial properties, Ag NPs are widely used in many consumer products, such as toothpaste, shampoos, washing powders, kitchen utensils, toys, filters and deodorants
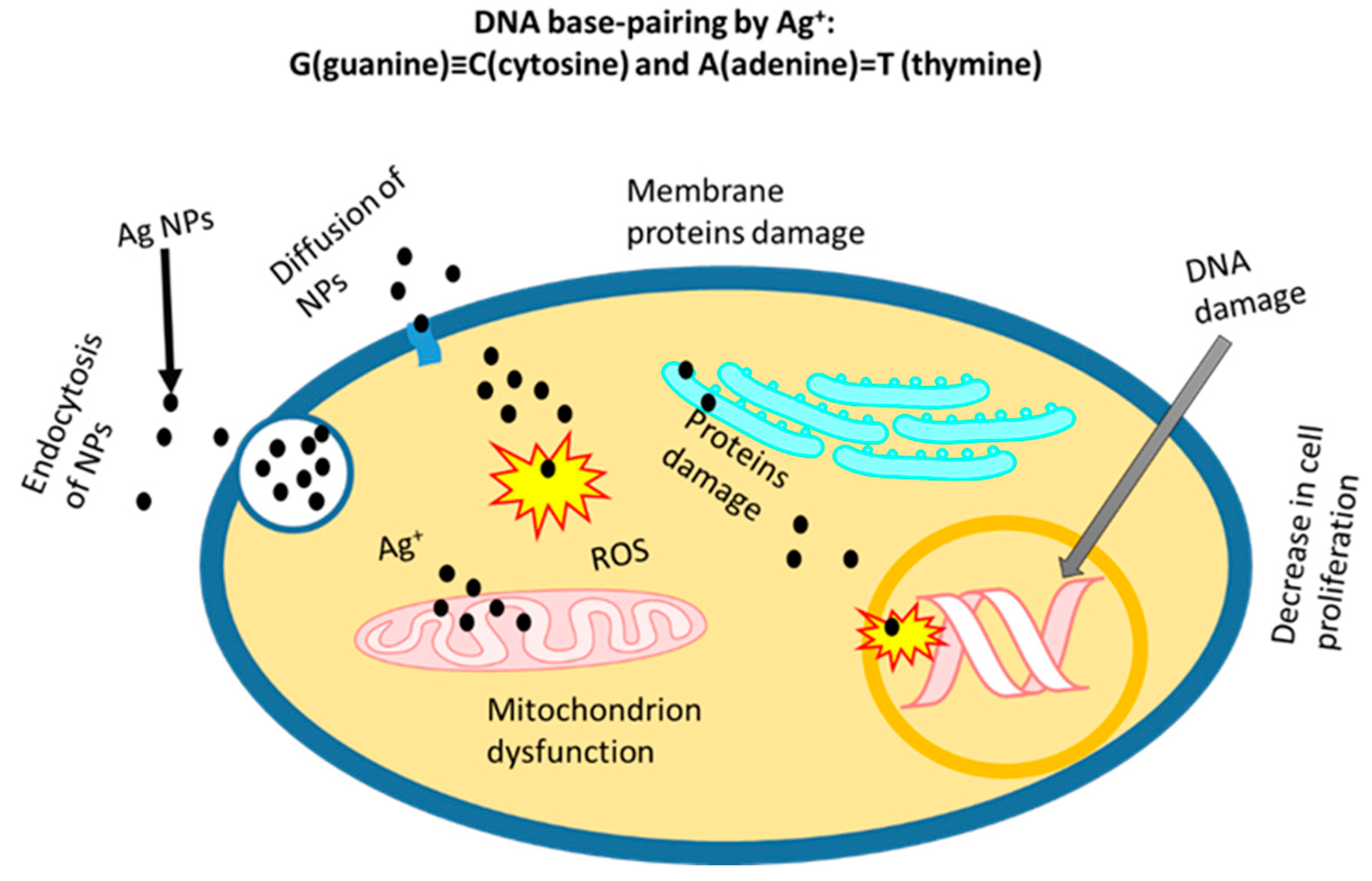
[40][41]. It is very important to evaluate the potential toxicity for safe and effective use as applications of Ag NPs are rapidly expanding. In vitro studies of normal and tumour cell culture in the presence of Ag NPs can be performed for the comparison of potential toxicity and therapy. The cytotoxicity of prepared NPs varies due to the different synthesis methods and functionalisation of NPs. The best approach is to compare the cytotoxic effects on cellular components (in vitro studies) using naked Ag NPs. It is highly desired that NPs only induce toxicity in tumour cells, such as human liver cancer cells (HepG2), human breast cancer cell lines (MDA-MB 231), human embryonic kidneys (HEK293T) cells, human neuroblastomas (SH-SY5Y) cell line and others, whereas therapeutic or neutral effects are required in normal cells (e.g., human macrophages and human keratinocyte (HaCaT) cell lines). Ag
+ addition results in modifications of DNA base pairs, deoxyribose fragmentations and DNA strand breakups. Due to the formation of two coordination complexes in DNA (high-energy and ground-energy states) by Ag
+, a modification in the base pairs of DNA by a reaction with double and triple hydrogen bonds can be the result
[42]. DNA base pairing and the changes in other organelles by Ag
+ is shown in
Figure 3. The Ag NPs can easily ionise to generate ROS to stimulate inflammatory responses through phagocytosis. Park et al. reported the generation of ROS in macrophage cells, and the activated macrophages increased the TNF-α secretion that led to cell membrane damage and apoptosis
[43]. The green synthesis and surface coating of Ag NPs can reduce the toxic effect on healthy cells. Chitosan-coated Ag NPs exhibited good biocompatibility and efficient cellular internalisation in human embryonic cells (HEKs), as reported by Boca et al.
[44]. The biogenic synthesis using different microorganisms where Ag
+ is reduced to Ag
0 in the presence of protein enzymes have been reported in several studies
[45][46][47]. The Ag NPs synthesised by these methods showed increased biocompatibility, cellular uptake, antimicrobial properties and secretion
[48]. The mechanisms of cellular internalisation and toxicity of Ag NPs are depicted in
Figure 3.
Figure 3. Cellular internalisation and cytotoxicity of Ag+ ion in the microenvironment.
4. Iron Oxide Nanostructures
Iron oxide is usually formed in two major forms (Fe II and Fe III) based on oxide structures, which include magnetite (Fe
3O
4), hematite (Fe
2O
3/α-Fe
2O
3) and maghemite (γ-Fe
2O
3). These oxides exist naturally in large quantities and are widely synthesised for their numerous applications. Fe
3O
4 and γ-Fe
2O
3 are extensively used in the field of biomedicine due to their paramagnetic/superparamagnetic nature and involvement in various biological processes. Iron oxide magnetic nanoparticles (IOMNPs) provide a theranostic platform where they can be exploited for diagnostic purposes, such as magnetic resonance imaging (MRI), and therapeutic purpose, such as drug delivery, magnetic cell separation, protein purification and bio-catalysts
[49][50][51]. IOMNPs can be synthesised in different sizes and shapes using a variety of synthesis methods. Due to their widespread application and varying morphologies and surface properties, the toxicity of IOMNPs must be evaluated in relation to different conditions and body components
[52]. The in vitro toxicity of IOMNPs varies with pH conditions and cell types. The Fenton reaction is a major ROS generating event leading to oxidative stress and cellular apoptosis. The Fenton reaction involves the reaction of Fe(II) with H
2O
2 to generate OH
− and OH
• radicals. The over production of ROS can cause damage to DNA and other cellular organelles (
Table 1).
In several studies, it has been reported that tumour cells have high levels of ROS
[53][54][55]. The major factors contributing to increased ROS might include oncogenic stimulation, mitochondrial malfunctions, increased metabolic activity and other dysregulated activities in the cells
[53]. It appears advantageous that a high level of ROS favours the growth of cancer cells by activating several stress kinase pathways
[56]. In addition, cancer cells can adopt high levels of ROS due to the presence of oncogene c-Myc, which increases the tolerance level by activating transcriptional genes for GSH biosynthesis in response to H
2O
2 [57]. It is apparent that increased levels of ROS and oncogenic transformation result in the increased sensitivity of the cells to generate ROS
[58]. Several chemicals and nanomaterials, such as phenylethyl isothiocyanate, titanium-based materials and piperlongumine increase ROS levels and selectively target tumour cells, and do not cause much damage to normal cells
[54][58]. On the other hand, for increased ROS levels, cells are treated with certain chemicals, such as N-acetyl-l-cysteine, to scavenge ROS
[59]. Thus, selective treatment with ROS generation/scavenging in malignant cells while protecting normal cells is an effective method for cancer therapy.
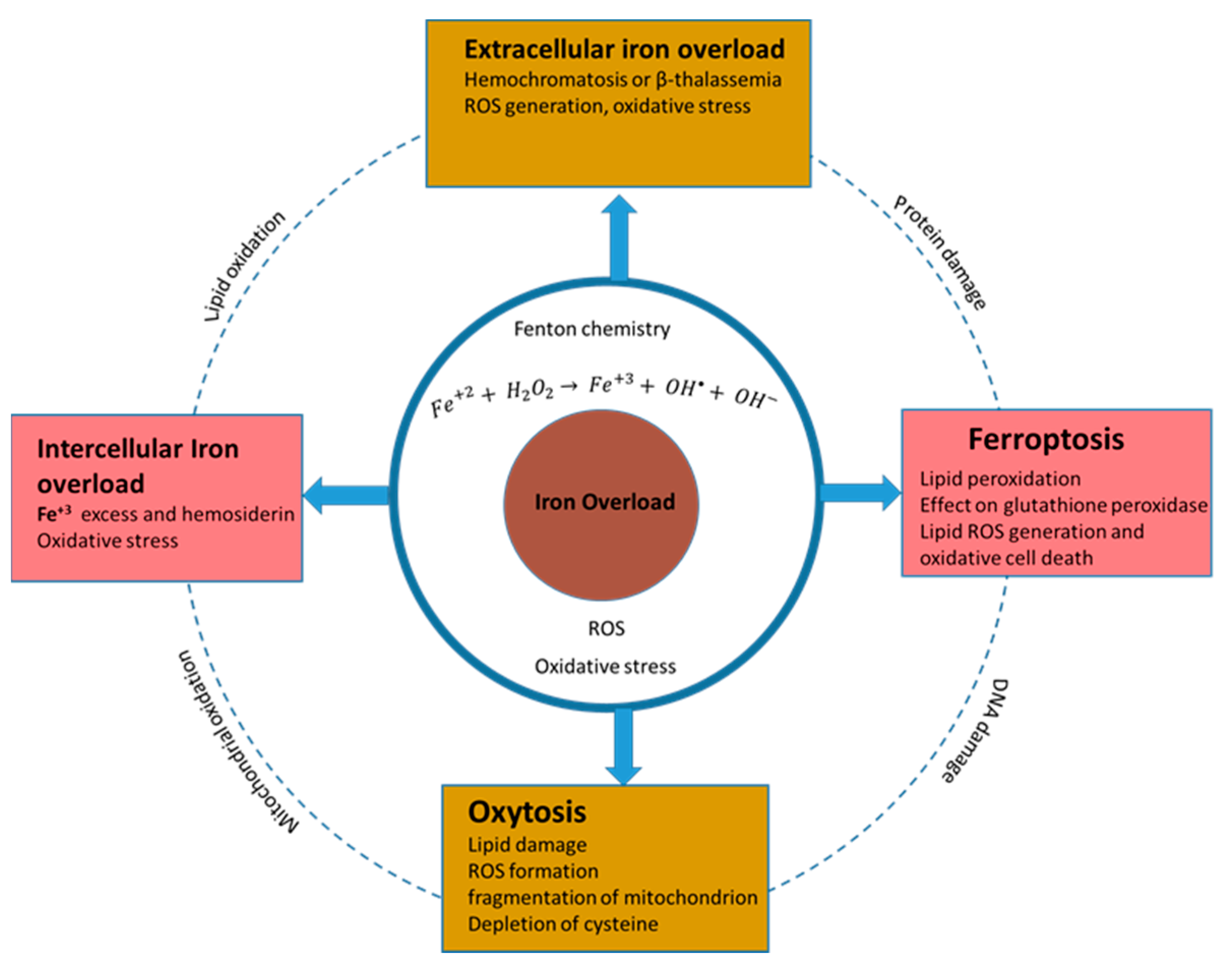
Iron is an important factor in the ROS-linked homeostasis of normal cells and can cause dysregulation to result in tumorigenesis. Iron regulates different functions in cells of different tissues in the body. The common Fe-related phenomena and their in vitro effects are presented in Figure 4, and are also described as follows:
Figure 4. Common Fe cytotoxicity mechanisms in the microenvironment.
Iron-dependent oxidative cell death is termed as ferroptosis. It is triggered by structurally different small molecules, such as erastin, RSL3 and sulfasalazine. Ferroptosis is different from apoptosis, autophagy and other forms of necrosis
[60][61]. The fundamental details of iron oxide involvement in ferroptosis are not clear. It is hypothesised that the inhibition of cysteine uptake causes the depletion of the endogenous antioxidant tripeptide glutathione, which causes the accumulation of iron-dependent ROS and leads to cell death
[60][62]. Ferroptosis can be prevented by using iron chelators (such as deferoxamine and lipophilic antioxidants, i.e., vitamin E and Trolox)
[60][63].
Oxytosis or oxidative glutamate toxicity is linked to iron and is observed in certain brain cells in the absence of cystine
[64]. Oxytosis in neural cells initially involves membrane lipid damage and results in cell death, including LOX activation, Ca
2+ influx into the cell, overproduction of mitochondrial ROS and fragmentation of mitochondrion
[61][62][64]. Iron-dependent oxidative cell death was observed in premature oligodendrocytes cells using high concentrations of glutamate or having the absence of cysteine. In the nervous system, the depletion of premyelinating cells causes periventricular leukomalacia (PVL) disease, which is characterised by white-matter lesions within the brain and acts as a precursor for cerebral palsy development
[65][66].
According to the research reports in recent years, liver toxicity is caused by iron overload and can be ameliorated by mitochondrially targeted oxidation
[67]. It is important to understand that high levels of iron are not always harmful for cell viability and proliferation because, sometimes, iron overload contribute to cell death in certain cell types and tissues, and it may increase cell proliferation and viability. Antiproliferative and proliferative functions can also be performed through cell-nonautonomous or cell-autonomous effects on cell mutation and tissue microenvironments, cell signalling and iron-dependent enzyme function. For example, Parkinson’s disease is linked to the depletion of the Tau protein (linked to iron export) by iron accumulation
[62][68].
Neurodegenerative disease are increasing rapidly; they are associated with iron accumulation within neurons
[69]. An accumulation of iron generates ROS through enhanced Fenton chemistry mediation. In Parkinson’s disease, dopaminergic neuronal populations are susceptible to degeneration and involve increased amounts of mitochondrion ROS
[70][71]. Iron chelators can be employed to prevent high levels of ROS production via the autophagy of ROS producing mitochondrion. Thus, iron chelators or ROS scavengers lower the damaging levels of ROS and, hence, oxidative stress
[70].
Iron dyshomeostasis is the common factor in different neurodegenerative diseases, such as AD, frontotemporal dementia (FTD) and Lewy body dementia. Iron promotes the aggregation and pathogenicity of the β-amyloid peptide, α-synuclein, TDP43 and tau protein
[72][73][74].
Iron acts as mediator for signal responses in the excitotoxic death of cortical neuronal populations of a mouse in response to N-methyl-d-aspartate (NMDA). The required iron is transported from the outside of the cell by an iron transporter divalent metal transporter 1 (DMT1)
[75]. An analysis of NDMA treatment results show that increased ROS production and oxidative stress induce cell death. It is quite obvious that iron import and NOX-derived ROS production enhance ROS-mediated neural cell deaths
[62][76]. The common phenomena of cellular toxicity due to iron overload and Fenton chemistry are depicted in
Figure 4.
Table 1. In vitro cellular effect with reference to size, shape and synthesis method of Fe2O3 and Fe3O4 nanostructures.
| Cell Line |
NP Size |
Synthesis Method |
Effect |
| Human hepatocyte (HL-7702) cell lines |
Fe3O4, unknown shape (50 nm) |
Commercial (Colorobbia Consulting-Cericol, Vinci, Italy) |
Induction of apoptosis and autophagy, nuclear condensation and chromosomal DNA fragmentation were observed [77]. |
| Human hepatoma HepG2 cells |
Fe2O3, spherical (50 nm) |
Commercial |
Mitochondrial apoptosis through activation of loop phosphorylation, release of cytochrome c from the mitochondria, decrease in Bcl-2 protein expression, PARP activation and caspase cascades, ROS generation and DNA damage [78]. |
| Human lung (BEAS-2B) cells |
Fe3O4, Fe2O3 irregular shape (˂100 nm) |
Commercial (Sigma-Aldrich, St. Louis, USA) |
Increased ROS generation and oxidative stress, mitochondrion and DNA damage [79]. |
| Human cerebral endothelial cells (HCECs) |
Fe3O4, unknown shape (9 nm) |
Commercial (PlasmaChem GmbH, Berlin, Germany) |
Overexpression of cathepsin D accelerated apoptosis, ROS generation transported into lysosomes interfering with the lysosomal hydrolases, cathepsins D and B, and induced oxidative stress and, hence, autophagy [80]. |
| Human lung cancer (A549) cell line |
Fe3O4, Fe2O3 irregular shape (˂100 nm) |
Commercial (Sigma-Aldrich, St. Louis, MO, USA) |
ROS generation, increased oxidative stress, cellular apoptosis and DNA damage [79]. |
| Lung cancer (HCC827) cell line |
Iron oxide, unknown shape (NPs (73 nm) |
Co-precipitation method and NP conjugation |
Reduced EGFR phosphorylation, increased γH2AX foci and induced apoptosis, which resulted in suppression of tumour growth [81]. |
| Chinese hamster ovary (H9T3) cell lines |
Fe2O3, hexagonal shape, (20–30 nm) |
Harvard Versatile Engineered Nanomaterial Generation System (VENGES) |
Cellular apoptosis and double-stranded DNA breaks [82]. |
| Human fibrosarcoma (HT-1080) cells |
Irregular and spherical shapes, Fe3O4 (10–150 nm) |
Massart’s method, and NP coating |
Increased ROS generation caused oxidative stress and lipid peroxidation. Oxidative damage induced DNA damage [83]. |