You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Celia C. G. Silva | -- | 4909 | 2022-08-04 18:24:25 | | | |
| 2 | Beatrix Zheng | + 3 word(s) | 4912 | 2022-08-05 07:52:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses. Encyclopedia. Available online: https://encyclopedia.pub/entry/25867 (accessed on 29 December 2025).
Coelho MC, Malcata FX, Silva CCG. Lactic Acid Bacteria in Raw-Milk Cheeses. Encyclopedia. Available at: https://encyclopedia.pub/entry/25867. Accessed December 29, 2025.
Coelho, Márcia C., Francisco Xavier Malcata, Célia C. G. Silva. "Lactic Acid Bacteria in Raw-Milk Cheeses" Encyclopedia, https://encyclopedia.pub/entry/25867 (accessed December 29, 2025).
Coelho, M.C., Malcata, F.X., & Silva, C.C.G. (2022, August 04). Lactic Acid Bacteria in Raw-Milk Cheeses. In Encyclopedia. https://encyclopedia.pub/entry/25867
Coelho, Márcia C., et al. "Lactic Acid Bacteria in Raw-Milk Cheeses." Encyclopedia. Web. 04 August, 2022.
Copy Citation
Lactic acid bacteria (LAB) are of great economic importance because they play an important role throughout the fermentation process of traditional cheeses when added accidentally or intentionally. Their metabolic features not only contribute to the development of desirable sensory characteristics of food products but also allow the nutritional value of the raw material to be maintained or even enhanced.
cheese
LAB
bacteria
lactobacilli
bacteriocins
probiotics
health-promoting effects
1. LAB as Starter Cultures
The bacteria most commonly used as starter cultures in cheeses are Lactic acid bacteria (LAB) [1]. The chief role of these cultures is to acidify the milk, and thereby inhibit the growth of other (undesired) bacteria [2][3][4][5]. The starter bacteria must produce enough acid to lower the pH of the milk to below 5.3 within 6 h at 30–37 °C, depending on the type of cheese [3][6]. The production of acid in the right amount and at the right time is a crucial factor to obtain high-quality cheeses [7][8][9]. Therefore, the ability of LAB to produce acid rapidly is one of their most important technological features [10]. The temperature during production, salt levels, and humidity should be controlled to ensure that the activity of starter cultures is sufficient to rapidly reach the targeted pH [3]. Starter cultures should also promote a sustainable environment in the cheese in terms of redox potential, salinity, and moisture that allows suitable rennet enzyme activity and the growth of the secondary microbiota [3][11][12]. Starter bacteria are undoubtedly the main players in the first hours of cheese production. However, from the 18th day to the 25th day of ripening, the number of these bacteria decreases drastically as a consequence of the decrease of lactose as a nutrient and their own autolytic behavior [7].
In addition to acid production during the fermentation process, starter cultures also contribute to cheese ripening since their enzymes are involved in the proteolysis, lipolysis, and conversion of amino acids into compounds that directly contribute to the flavor of the final product [3][6][9][13]. In addition, the use of starter cultures ensures microbiologically safe products, because these cultures inhibit the development of undesirable microorganisms by producing compounds that prevent their growth, such as organic acids, bacteriocins, and hydrogen peroxide [14][15][16].
The most commonly used starter cultures are members of the genera Lactococcus, Lactobacillus, Streptococcus, Leuconostoc, and Enterococcus [3]. Currently, Enterococcus is not granted this qualification due to regulations related to the qualified presumption of safety (QPS). However, some well-characterized strains continue to be used as starter cultures, co-cultures, or protective cultures in the food industry owing to their beneficial properties [17]. The most commonly used species in cheese production are Lc. lactis, S. salivarius subsp. thermophilus, L. helveticus, and L. delbrueckii [12].
At the beginning of production, LAB may be present as a native component of the milk, as happens with many artisanal raw milk cheeses [3]. In these cheeses, the spontaneous fermentation of the milk is driven by the development of the aforementioned microbiota. However, the outcome of such processes is unpredictable, as the physiological stage and extent of inoculum are beyond operator’s control [18].
Conversely, starter cultures are intentionally added and previously selected based on their effect upon fermentation and the desired properties of the product. The selection criteria vary, but the dominant criterion is usually the acidification rate at a given temperature, and the insensitivity to phages [11]. Handling characteristics and stability during production are also criteria for starter culture selection [19].
The proper selection of starter cultures and the characterization of each strain is very important to obtain products with reproducible organoleptic and structural properties by the end of cheese production [2][4][11]. By controlling the fermentation process, the said cultures reduce the variations in organoleptic quality and microbiological stability observed in cheeses without them.
1.1. Type of Starter Cultures
Starter cultures can be categorized as mesophilic or thermophilic, depending on the incubation and manufacturing temperatures at which they are used [4]. Mesophilic starter cultures have an optimal growth temperature of ca. 30 °C, while thermophilic starter cultures grow best between 40 and 45 °C [14]. Mesophilic and thermophilic cultures can be divided into defined and undefined cultures [3].
1.1.1. Mesophilic and Thermophilic Starter Cultures
The starter cultures most commonly used in the production of fermented dairy products belong to the genera Lactobacillus and Streptococcus, namely the species S. salivarius subsp. thermophilus, Lb. helveticus, Lb. delbrueckii subsp. Lactis, and L. delbrueckii subsp. bulgaricus [14][15][20][21].
Mesophilic starter cultures include mainly the genera Lactococcus and Leuconostoc [15][21]. The LAB most commonly used as mesophilic starter cultures are Lc. lactis, including subspecies lactis and cremoris for being good acid producers [20][21]. Other mesophilic starter cultures include the species Ln. lactis and Ln. cremoris [20]. Mixed mesophilic cultures are usually 90% acid producers and 10% aroma producers [21].
In the production of hard cheeses, mesophilic starter cultures are predominantly used (e.g., Lactococcus spp.), although thermophilic cultures may also be used (e.g., S. salivarius subsp. thermophilus) [12].
1.1.2. Defined and Undefined Starter Cultures
Starter cultures are usually composed of different species, or multiple strains of one species. Starter cultures can be divided into defined and undefined cultures [11][15][20]. The former usually consist of one or more strains with known characteristics [18]. They have usually been isolated from mixed cultures and selected based on important characteristics such as phage resistance, acid production, citrate utilization, and aroma and flavor formation [10][22]. Undefined starter cultures have partially known or all unknown species and strains in their composition [21].
Starter cultures used in the production of cheese can be divided into: (1) defined cultures with multiple strains (e.g., Lc. lactis subsp. lactis and Lc. lactis subsp. cremoris in Camembert and Brie cheeses); (2) defined cultures with a single strain (e.g., S. thermophilus in Mozzarella cheese); (3) defined mixed cultures (e.g., S. thermophilus, Lb. helveticus, Lb. delbrueckii subsp. lactis, Lb. delbrueckii subsp. bulgaricus and Propionibacterium shermanii in Emmental and Gruyere cheeses); and (4) undefined mixed cultures (e.g., whey starter in Italian cheeses such as Parmigiano Reggiano) [7][11][21][23].
For instance, the cultures used for the production of Gouda cheese were isolated from an undefined starter culture traditionally used for the production of this cheese, consisting of L. lactis subsp. cremoris, L. lactis subsp. lactis biovar diacetylactis, and Ln. mesenteroides [18][23].
1.1.3. Natural Whey Starter (NWS)
Natural whey starter cultures (NWS) consist of an undefined culture of LAB and are mostly acid producers [24]. This type of starter is commonly used in the production of traditional artisanal cheeses using the back-slopping technique, which requires the inoculation of milk with whey or fermented milk from the previous day [10].
2. LAB as Adjunct Cultures
2.1. Selected Adjunct Cultures
Adjunct cultures can be defined as those added to cheese for purposes other than acid production, even though they often consist of microorganisms derived from ingredients (raw milk) or the cheese-making environment [3]. Adjunct cultures, selected from adventitious LAB, also called non-starter LAB (NSLAB), can therefore be added with starter to accelerate the ripening process and produce the desired flavor [21]. These cultures are selected to survive cheese curd cooking temperatures and participate in flavor development at a later stage of cheese ripening. Mesophilic cultures such as L. casei and L. paracasei are traditionally added with the starter to improve the flavor of dairy products [21]. They can also mitigate defects caused by contaminating adventitious LAB by inhibiting their development [2].
2.2. Natural Adjunct Cultures
Natural adjunct cultures are often adventitious cultures LAB, which are not part of the added starter culture [25][26]. Such adventitious LAB are usually difficult to grow in milk and do not contribute to acid production [3], but are critical for the final flavor and texture of the cheese [12][15]. These bacteria can grow with energy sources other than lactose, and are more resistant to environmental stress [7][27]. Adventitious LAB are present at very low concentrations in the curd but their populations start to increase during the first months of ripening and eventually become the dominant microbiota of longer ripened cheeses [7][10][27][28].
The composition of adventitious LAB varies depending on cheese type, the mode of processing, and ripening time [2][25][29][30]. The development of adventitious LAB during cheese ripening can be attributed in part to their ability to utilize available nutrient sources [10]. As lactose is metabolized during the first weeks of ripening, adventitious LAB can obtain energy from compounds such as lactic acid, citric acid, ribose, fatty acids, glycerol, and amino acids [10][31]. Because LAB possess a variety of hydrolytic enzymes convenient for cheese proteolysis and lipolysis, they are able to grow and act during cheese ripening [2][27][29][32].
2.3. Charaterization of Adventitious NSLAB
The adventitious NSLAB are a particularly heterogeneous group, and include mesophilic lactobacilli, enterococci, pediococci, and Leuconostoc [6][25][26]. Mesophilic lactobacilli are the predominant and most important group in the microbiota of NSLAB [33]. Among the mesophilic lactobacilli, facultative heterofermenters are the most abundant in NSLAB [25], mainly L. casei subsp. casei, L. casei subsp. pseudoplantarum, L. paracasei subsp. paracasei, L. plantarum, L. rhamnosus, L. curvatus [3][27], and L. pentosus [12]. The obligate heterofermentative species commonly found in cheese are: L. fermentum, L. buchneri, L. parabuchneri, and L. brevis [12], although other species of facultative or obligate heterofermentative lactobacilli also occur [27].
The most common pediococci found in cheese are Pediococcus acidilactici and P. pentosaceus [3]. Among enterococci, Enterococcus durans, E. faecalis, and E. faecium are most abundant in cheese [12][34]. Within the genus Leuconostoc, the species Ln. mesenteroides, Ln. peseudomesenteroides, and Ln. citreum have been detected in artisanal cheeses produced from raw milk [34][35][36][37].
The origin of NSBAL can vary, but the main source is raw milk [25][26][33] and, to a lesser extent, whey used as starter—NWS [33]. The microbial diversity of raw milk cheeses depends on the microbiota of the milk, the ingredients utilized, and the processes used in cheese production [25][26][27][33]. The cheese processing environment can also be a potential source of NSBAL, especially in the case of mesophilic bacteria, which can survive the processing environment and on the equipment itself, even after cleaning and disinfection, due to their ability to form biofilms [10].
NSBALs are generally associated with raw milk but are also present in cheese produced from pasteurized milk. The presence of NSBAL in cheese produced from pasteurized milk is due to airborne contamination, contact with equipment and/or ingredients used in cheese making, or thermoduric strains that survive pasteurization [32].
When artisanal cheese is produced without direct inoculation with starter cultures, the microorganisms involved in fermentation are derived from starting material and environmental sources [38]. Therefore, the inherent and unique flavors known in cheeses produced from raw milk are the result of a diverse indigenous microbiota [10]. These NSBALs dominate the microbiota of many aged cheeses and play a key role in the development of flavor and aroma throughout ripening. For instance, they contribute to the release of small peptides and amino acids, which in turn can be converted into alcohols, aldehydes, esters, and sulfur compounds that are associated with specific flavors and aromas of the ripened cheese [2].
3. Antimicrobial Activity of LAB
LAB can be used to inhibit or destroy undesirable microorganisms in foods, increase their safety, and extend their shelf life [39]. The use of LAB as bioprotective agents also ensures food quality and safety without the need to resort to chemical preservatives [40].
3.1. Antibacterial Activity
In the dairy industry, the main bacterial pathogens that need to be controlled are those that can survive and multiply in products produced from raw milk, or that arise from contamination after pasteurization, such as Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, and Salmonella spp. [40][41][42].
The use of LAB as starter cultures in food fermentation promotes food preservation through rapid acid production [43]. In addition to lowering pH, some LAB species/strains possess antibacterial properties resulting from a combination of factors, including competitive growth and the production of a variety of antibacterial compounds [44]. Antibacterial compounds produced by LAB include various organic acids, such as lactic acid, acetic acid, formic acid, and propionic acid, as well as such other compounds as diacetyl, acetoin, hydrogen peroxide, reuterin, and bacteriocins [45][46][47][48][49][50][51].
The efficacy of LAB to inhibit various bacterial pathogens has been demonstrated in several food matrices, including cheese, meat, and vegetables [52]. In fermented milk, the application of a bacteriocin-producing strain of Lc. lactis ssp. lactis reduced L. monocytogenes contamination to undetectable levels [53]. Several other studies have also shown a reduction in L. monocytogenes in various cheeses by using Lc. lactis strains that produce bacteriocins [54][55]. Such bacteriocin-producing LAB species as L. plantarum, Streptococcus spp. and Enterococcus spp. have been shown to reduce L. monocytogenes and S. aureus contamination in various dairy products [40][54][56][57][58][59].
3.2. Antifungal Activity
Molds and yeasts are ubiquitous contaminants of dairy products, especially under conditions that favor their growth [42][48]. In the case of cheese, fungal contamination occurs in all types of cheese, although more readily in soft and unripened cheeses [60][61].
Fungal spoilage causes visible or invisible sensory defects in cheese, such as the visible growth of the fungus on the surface, and the production of metabolites that lead to noticeable and unpleasant changes in aroma, flavor, and texture, thus resulting in a loss of product quality [42][47][61][62][63].
In addition to the major economic losses associated with spoilage, some fungi pose a threat to food safety due to their ability to produce mycotoxins [51][61][64][65][66][67][68]. Therefore, the risk of mycotoxins in cheese increases when toxigenic fungi are allowed to grow during production and storage [61]. Filamentous fungi belonging to the genera Aspergillus, Fusarium, and Penicillium can grow on the cheese surface and produce mycotoxins that are highly toxic [45][50][63][69][70][71]. Some mycotoxins are present only in the fungus, while most are excreted in food [72]. Aflatoxins are considered one of the most important and well-known classes of mycotoxins in food [72][73]. These compounds have numerous and diverse toxic properties, including carcinogenic, teratogenic, mutagenic, nephrotoxic, hepatotoxic, neurotoxic, immunosuppressive, and estrogenic effects, even when ingested at low concentrations [45][47][61][73].
Some LAB species/strains have shown activity against common cheese spoilage molds [46]. The antifungal activity of LAB is attributed to multiple compounds acting individually or in synergy to provide multiple barriers against spoilage molds [46][65]. Some LAB species are also able to reduce mycotoxins produced by molds [71].
The LAB best known for their ability to prevent or retard the growth of toxinogenic fungi belong to the genera Lactococcus and Lactobacillus and, to a lesser extent, Pediococcus and Leuconostoc [45]. The antifungal activity of the genus Lactobacillus has been extensively studied, with particular emphasis on the species L. plantarum [45][63]. Different strains of L. plantarum and its metabolites have been tested in a variety of foods, where they were able to inhibit various fungal species belonging to the genera Aspergillus, Penicillium, Rhizopus, and Rhodotorula [63][66]. In addition to L. plantarum, other species such as L. casei, L. paracasei, and L. brevis have also shown antifungal activity against a broad spectrum of spoilage molds [62].
3.3. Antimicrobial Metabolites Produced by LAB
3.3.1. Organic Acids
The antimicrobial activity of LAB is associated with the production of organic acids, mainly lactic and acetic acids, but also formic, propionic, butyric, phenyllactic, hydroxy-phenyllactic, and indole-3-lactic acids, among others [73]. The most extensively studied acids are lactic, acetic, propionic, and phenyllactic acids [65].
Organic acids lower pH and create unfavorable conditions for the growth of many potentially pathogenic microorganisms [73]. In addition to their effects on pH, the undissociated form can diffuse across the cell membrane of the target organism, dissociate within the cell, and lower the cytoplasmic pH. Therefore, the most important parameter that determines the antimicrobial activity of an organic acid is pKa, because when pH < pKa, the undissociated form enters the cell and consequently neutralizes the electrochemical proton gradient, leading to the death of the susceptible organisms [45][47][74].
Similar to lactic acid, acetic and propionic acids interact with cell membranes to neutralize the electrochemical proton gradient; however, the effect of these acids is often dependent on the pH reduction achieved [74].
Phenyllactic acid has been described as an antimicrobial compound that exhibits a broad spectrum of antibacterial and antifungal activities [75]. This acid contributes to microbial inhibition in synergy with other compounds produced by LAB [74][75]. Phenyllactic acid can retard the growth of many fungi, including species belonging to the genera Aspergillus, Fusarium, and Penicillium. However, many studies have reported that very high concentrations of this acid are required to inhibit fungal growth, thus making it less suitable as antifungal agent in foods [76].
3.3.2. Hydrogen Peroxide
Hydrogen peroxide (H2O2) is produced by most LAB in the presence of oxygen [45][47]. Since LAB are unable to produce catalase, they cannot degrade hydrogen peroxide, so it accumulates in the medium, where it exerts a strong oxidizing effect on the lipid membrane, while destroying the basic molecular structures of the cell proteins of the target organisms [45][47][74].
The bactericidal action of hydrogen peroxide has been shown to be effective in reducing spoilage bacteria and pathogens such as E. coli, L. ivanovii, and S. aureus [77].
3.3.3. Diacetyl
Diacetyl (also known as 2,3-butanedione) is an aromatic compound, characterized by its buttery taste when associated with dairy products [78]. Diacetyl is produced by some LAB strains during citrate fermentation and is present in many dairy products such as cheese [34][79]. Diacetyl has been shown to exert antifungal and antibacterial effects at low pH [80][81]. However, the amounts of diacetyl required to exert antimicrobial activity significantly alter the taste and flavor of the final product [74].
3.3.4. Reuterin
Reuterin was first described as produced by L. reuteri, and is an antimicrobial compound with a broad spectrum of activity [65][74]. It consists of acrolein and 3-hydroxypropionaldehyde (3-HPA), which can be further metabolized to 1,3-propanediol and 3-hydroxypropionic acid (3-HP) [82]. This low molecular weight compound is capable of inhibiting the growth of a wide range of microorganisms, and is one of the most intensively studied antifungal compounds [74][75].
Reuterin is produced by several LAB under anaerobic conditions via the fermentation of glycerol [50][65][74]. The main LAB producers of reuterin are lactobacilli, including the species L. reuteri, L. brevis, L. buchneri, L. collinoids, and L. coryniformis [45].
Gram-positive bacteria are generally more resistant to reuterin than Gram-negative strains, including common food pathogens such as E. coli, Salmonella, and L. monocytogenes [82]. In target organisms, reuterin can suppress ribonuclease activity [45][50] or induce oxidative stress by modifying thiol groups in proteins and glutathione [83]. In fungi, reuterin inhibits the growth of species belonging to the genera Fusarium, Aspergillus, and Penicillium [45][50][84].
3.3.5. Fatty Acids
Fatty acids may also possess antibacterial and antifungal activity. The length of the fatty acid chain appears to play an important role in antimicrobial activity, with lauric (C12) and capric (C10) acids showing the best antimicrobial results [85].
LAB can produce several types of fatty acids that improve the sensory quality of fermented products. Caproic acid is one of these fatty acids and it has strong antifungal activity. It can act synergistically with propionic, butyric, or valeric acid [50].
According to Crowley et al. [75], antifungal fatty acids cleave the lipid bilayers of fungal membranes, thus causing a loss of membrane integrity. The increase in fluidity increases membrane permeability, leading to the uncontrolled release of electrolytes and intracellular proteins, as well as the cytoplasmic disintegration of fungal cells.
Some strains of lactobacilli can produce hydroxylated fatty acids from linoleic acid [86]. Sjogren et al. [87] found that hydroxylated fatty acids possess strong antifungal activity against a broad spectrum of yeasts and molds.
3.3.6. Cyclic Dipeptides
Cyclic dipeptides include several types of diketopiperazines such as the 2,5-diketopiperazines, which are among the most abundant peptide derivatives in nature [75]. They can be formed in foods by chemical reactions during thermal processing, or by yeast and LAB during fermentation [88].
Several bioactive properties are attributed to these dipeptides, including antimicrobial and antitumor activities [75]. The broad spectrum of antimicrobial effects of cyclic dipeptides produced by LAB has been demonstrated in several studies [46][89][90].
3.3.7. Bacteriocins
In recent years, bacteriocins have attracted considerable interest as a safe alternative to chemical preservatives for being rapidly hydrolyzed in the human gastrointestinal tract [91][92][93][94][95].
Bacteriocins are peptides with antimicrobial activity, synthesized by bacteria in ribosomes. These peptides often exhibit a narrow inhibitory spectrum and inhibit taxonomically-close bacteria [56][96][97][98]. The most common mechanisms used by bacteriocins to kill other microorganisms include the formation of pores in the cell membrane or the inhibition of cell wall synthesis [99]. Most bacteriocins produced by LAB, especially those that inhibit Gram-positive bacteria, exert their antimicrobial effects by forming pores in the membrane of target cells, thereby depleting the transmembrane potential and/or pH gradient, which eventually leads to loss of cell contents [100][101].
Bacteriocins are produced by only a few strains of different bacterial species, including LAB [102]. Some of these bacteriocins are effective against important foodborne pathogens, such as L. monocytogenes, S. aureus, Pseudomonas aeruginosa, and Salmonella enterica, as well as other spoilage microorganisms [91][102][103][104][105]. Some studies have shown that LAB can also produce bacteriocins with antifungal activity. Although Lactococcus, Streptococcus, and Pediococcus have been reported to produce bacteriocin-like peptides against a variety of fungi, Lactobacillus strains have been most commonly associated with the production of antifungal peptides/proteins [75]. However, the mode of action of protein compounds in inhibiting fungal growth by LAB is not completely clear [45][65][75].
The only bacteriocins commercially available at present are nisin A, produced by Lc. lactis, and pediocin, produced by P. acidilactici [98][106]. Nisin has a broad spectrum of antimicrobial inhibition, and inhibits the growth of most Gram-positive bacteria that contaminate food, such as L. monocytogenes, S. aureus, and Clostridium perfringens [107]. However, the efficacy of nisin has some limitations, since it cannot be used in foods with neutral or alkaline pH, or in foods that require LAB for fermentation [108]. Other bacteriocins, such as enterocins, have been shown to be more effective than nisin in inhibiting L. monocytogenes [108]. Bacteriocins that are effective against this bacterium are important for use in foods, especially cheeses produced from raw milk, as they may be contaminated with this pathogen [109].
Bacteriocins produced by LAB are often active over a wide pH range, resist high temperatures, and inhibit the growth of a variety of food spoilage and pathogenic bacteria. In addition, bacteriocins are sensitive to digestive proteases such as pancreatin, trypsin, and chymotrypsin, and therefore have no negative effects on the gut microbiota [108]. Since they are not toxic to eukaryotic cells and become inactive toward proteolytic enzymes (e.g., digestive proteases), bacteriocins are generally considered safe substances [110][111].
4. Probiotic Potential of LAB
Consumers are becoming increasingly aware of the beneficial effects of probiotics, and this has led to greater demand for probiotic products worldwide [112][113]. Most microorganisms residing in the gastrointestinal tract are harmless or otherwise beneficial to the host, thus resulting in a generally harmonious and symbiotic relationship [114]. The potential benefits of consuming probiotics are primarily due to positive changes in the gut microbiota, known to play a key role upon the immune system [115].
In 2002, the Food and Agriculture Organization of the United Nations (OAA) and the World Health Organization (WHO) defined probiotics as “live microorganisms that, when ingested and administered in sufficient quantities, have health benefits for the host” [116]. Therefore, probiotics are preparations of viable and non-pathogenic microorganisms included in foods or dietary supplements that interact directly with the gastrointestinal microbiota and immune system, so as to produce health-promoting effects [117][118]. According to these definitions, a large number of LAB strains have been proposed as probiotics [116][118].
In addition to modulating the immune system, the positive health effects of taking probiotics include: the improvement of lactose tolerance and digestion [113][119], the prevention and treatment of gastrointestinal infections [120], the prevention of colorectal cancer [117][121], reduction in blood cholesterol levels [122][123][124], and the improvement of mental health via the gut-brain axis [125].
Probiotic LAB strains used in the production of fermented foods or pharmaceuticals must be recognized as safe for human use and possess GRAS or QPS status [115][126]. Probiotic microorganisms must not only fulfill safety aspects, but also have functional and technological properties that are of interest. These include ease of propagation and incorporation in food, long-term survival, and clinically valid and documented beneficial health effects [115]. The safety and efficacy of probiotics must be scientifically proven in advance for each strain and product [127].
4.1. LAB Used as Probiotics
Most microorganisms currently recognized as probiotics belong to the LAB group [115][116][128]. This is not at all surprising, because LAB are part of the natural microbiota of the healthy gastrointestinal tract of humans and animals [129][130].
A large amount of LAB, which can be classified as probiotics, are also present in milk and fermented dairy products, such as cheese, yogurt, and fermented milk [131][132][133]. As mentioned earlier, LAB can ferment various sugars and produce organic acids such as lactate and acetate, as well as other antimicrobial metabolites such as hydrogen peroxide and bacteriocins, all of which can effectively inhibit the growth of pathogenic organisms in the gut [119][134].
Species belonging to the genera Lactobacillus and Bifidobacterium are most commonly used as probiotics because they play a very important role in maintaining proper intestinal function and stimulating the host immune system [126][135][136]. Other genera with species that exhibit probiotic properties include Pediococcus, Lactococcus, and Enterococcus [137][138][139].
The most commonly used probiotic lactobacilli species in the food industry are L. acidophilus, L. plantarum, L. rhamnosus, L. paracasei, L. casei, L. gasseri, L. johnsonii, L. reuteri [130][135], L. fermentum, L. salivarius [126], and L. delbrueckii subsp. bulgaricus [115]. As for the genus Bifidobacteria, the most common species in food applications are: B. adolescentis, B. animalis subsp. lactis, B. bifidum, B. breve, B. longum subsp. longum, and B. longum subsp. children [126].
4.2. Mechanisms of Action of Probiotics
The mechanisms of action may vary from one probiotic strain to another, but in most cases a combination of activities is likely, making the research of the responsible mechanisms a difficult and complex task [140]. Furthermore, the response to probiotic treatment may be specific to each individual. Several studies have shown that the gut microbiota can influence the expected effect of treatments, as it may vary greatly from person to person [141].
Several mechanisms of action have been proposed for the therapeutic effect of probiotics, as shown in Figure 1. Probiotics may be active in preventing gastrointestinal infections by making it more difficult for pathogens to colonize the gastrointestinal tract, either by competing for nutrients or by competing for receptors. In this case, probiotics compete for a limited number of receptors on the surface of the intestinal epithelium [141][142]. The release of antimicrobial compounds such as organic acids, hydrogen peroxide, and bacteriocins may also exert antagonistic effects against pathogenic organisms [143][144].
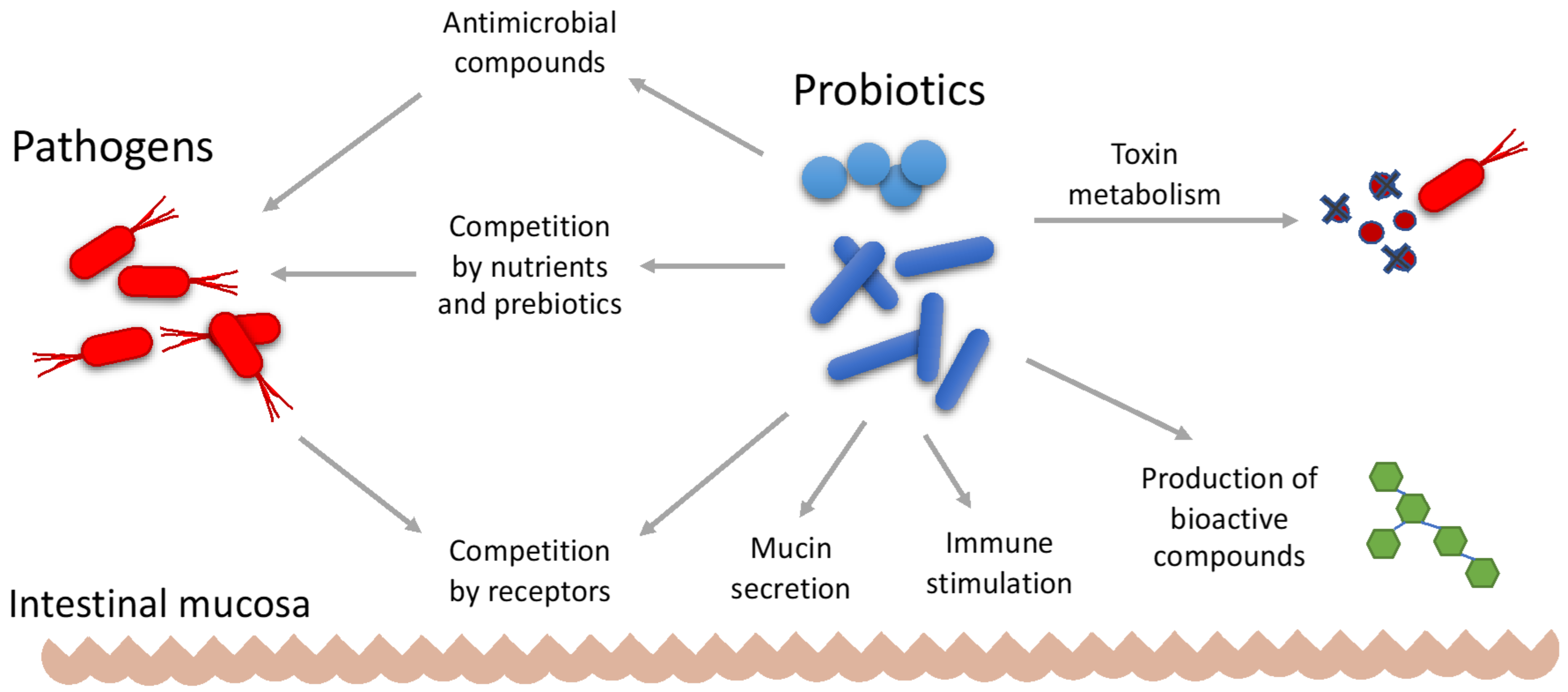
Figure 1. Mechanisms of action of probiotics.
Probiotics may also act by strengthening and increasing the intestinal mucosal barrier. Increased mucin secretion enhances the binding of probiotics to the intestinal mucosa. This effect competitively prevents the binding of enteropathogens to the receptors of the epithelium. The stabilization of the intestinal barrier permeability limits pathogen colonization, eliminates foreign antigens that have invaded the mucosa, and regulates antigen-specific immune responses [141][142]. The use of appropriate strains of probiotics may be helpful in eliminating bacteria associated with colorectal cancer, thereby reducing the risk of developing this disease. Some studies have shown promising results regarding the use of probiotics as a prevention strategy for colorectal cancer; however, clinical trials are still needed to demonstrate this therapeutic effect [145].
The modulation of the host immune system represents another form of probiotic effect. Some LAB strains can modulate innate and acquired immune responses by binding to specific receptors on immune cells and other tissues such as intestinal epithelial tissue, and by stimulating the production of cytokines, T cells, the activation of dendritic cells and macrophages, and the production of specific antibodies [146][147][148].
With the increasing recognition of the importance of healthy gut microbiota in the development of autoimmune diseases, many studies have focused on the immunomodulatory effects of some probiotic strains [147][149][150]. In one of these studies, a Ln. citreum strain isolated from an artisanal cheese was shown to have an immunomodulatory effect due to its ability to decrease the production of proinflammatory cytokines (IL-8) by intestinal cells [148]. In animal studies, oral ingestion of this bacterium in an asthma model (nasal administration of an allergen) resulted in immune tolerance to the allergen [148].
Probiotic LAB may also be involved in the synthesis of neurotransmitters and neuromodulators. For example, certain species of Lactobacillus and Bifidobacterium produce γ-aminobutyric acid (GABA), Streptococcus spp. and Enterococcus spp. produce serotonin, and Lactobacillus spp. produce acetylcholine [151][152]. The gut microbiota is also involved in modulating the expression of neurochemical receptors and modulating the brain-gut axis, leading to psychotropic, antidepressant, and anxiolytic effects [153]. Several studies in animal models have unfolded the therapeutic effect associated to the administration of probiotic LAB strains upon cognitive processes and a reduction in psychophysiological markers of anxiety and depression [154]. As a result of the potential effect of probiotics on improving mental health, the term “psychobiotics” has been proposed [155]. Psychobiotics refer to a group of probiotics that are able to produce and release neuroactive substances such as GABA and serotonin. These act through the brain-gut axis, and exert antidepressant effects by altering emotional, cognitive, and neuronal indices [153][156].
4.3. Bioactive Compounds Produced by Probiotic LAB
Probiotics can increase the availability of nutrients and produce bioactive soluble factors (byproducts of metabolism) that are beneficial to the host and are referred to as postbiotics [157].
Fermented dairy products, especially cheese, may contain substances that have beneficial effects on human health [158][159]. In the last decade, fundamental studies have opened a new field of research dealing with bioactive compounds from food. Bioactive compounds are components of ready-to-eat foods that can exert a regulatory effect in the human body, regardless of their nutritional function [160].
The proteolysis of milk proteins by LAB during milk fermentation and cheese ripening can result in peptides with bioactive properties that confer immunostimulatory, opioid, or angiotensin I-converting enzyme (ACE) inhibitory activity [161]. Numerous studies have shown that milk fermented by Lactobacillus spp. can exert beneficial effects in controlling cardiovascular disease caused by hypertension via the production of ACE-inhibitory peptides [162].
Bioactive compounds produced by probiotic microorganisms also include vitamins (thiamine, riboflavin, cobalamin, folic acid, and vitamin K), enzymes (lactase or β-galactosidase), bioactive peptides (from the hydrolysis of proteins), conjugated linoleic acid (CLA), short-chain fatty acids (SCFA), gamma-aminobutyric acid (GABA), exopolysaccharides (EPS), and antimicrobial compounds such as bacteriocins (Figure 2) [159][163]. Some of these compounds stand out for their potential, yet poorly studied effects on human health.
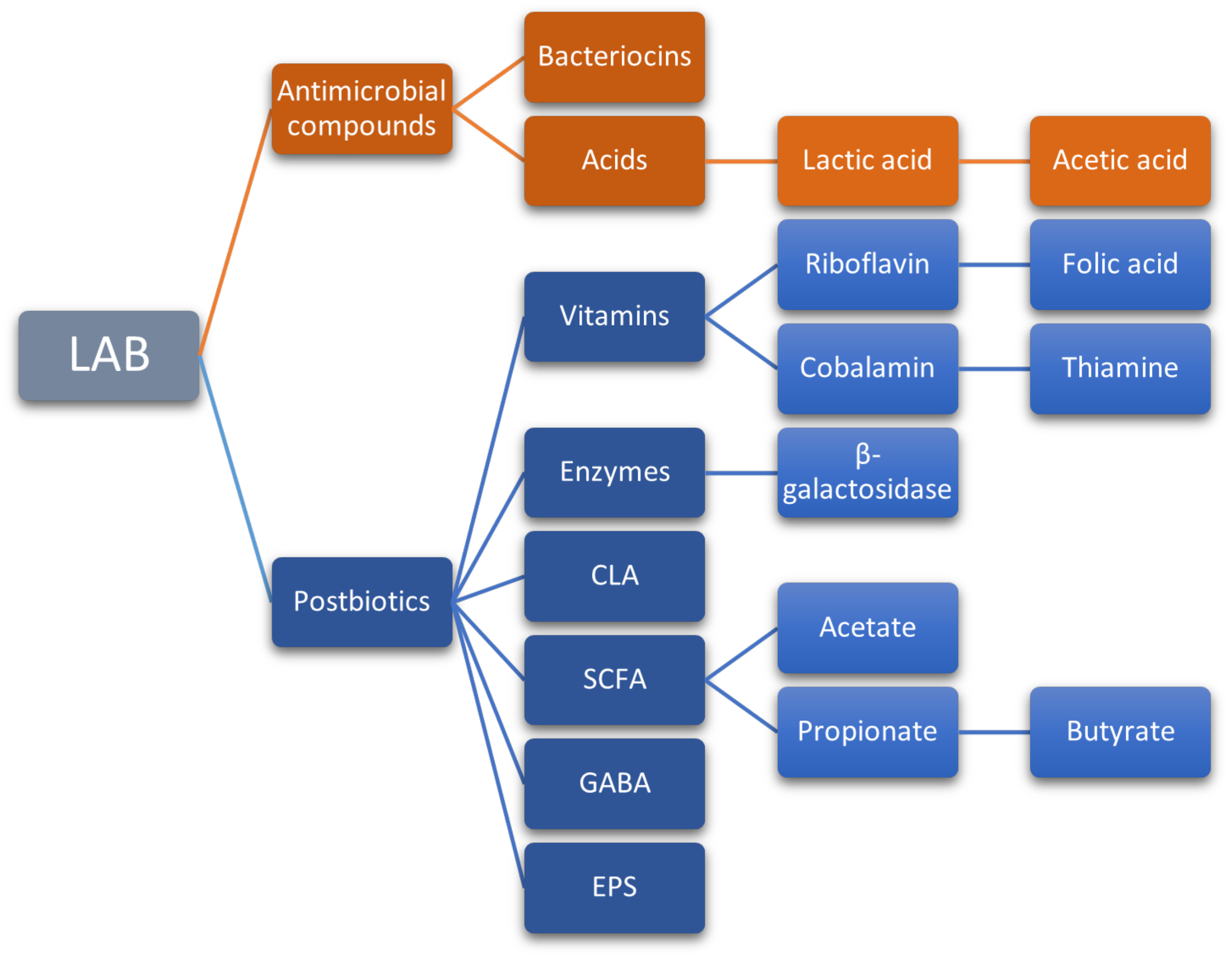
Figure 2. Main bioactive compounds produced by probiotic LAB.
References
- Rzepkowska, A.; Zielińska, D.; Ołdak, A.; Kołożyn-Krajewska, D. Organic whey as a source of Lactobacillus strains with selected technological and antimicrobial properties. Int. J. Food Sci. Technol. 2017, 52, 1983–1994.
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109.
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274.
- Carminati, D.; Giraffa, G.; Quiberoni, A.; Binetti, A.; Suárez, V.; Reinheimer, J. Advances and trends in starter cultures for dairy fermentations. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2010; Volume 177, pp. 177–192.
- Law, B. Cheese-ripening and cheese flavour technology. In Technology of Cheesemaking. Blackwell, MA, USA; Law, B.A., Tamime, A.Y., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 231–359.
- Beresford, T.; Williams, A. The microbiology of cheese ripening. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; Volume 1, pp. 287–318.
- Gatti, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Mucchetti, G. Invited review: Microbial evolution in raw-milk, long-ripened cheeses produced using undefined natural whey starters. J. Dairy Sci. 2014, 97, 573–591.
- Fox, P.; Guinee, T.; Cogan, T.; McSweeney, P. Fundamentals of Cheese Science; Aspen Publishers Inc.: Gaithersburg, MD, USA, 2000; pp. 98–137.
- Fox, P.F.; Cogan, T.M.; Guinee, T.P. Factors that affect the quality of cheese. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 617–641.
- Blaya, J.; Barzideh, Z.; LaPointe, G. Symposium review: Interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. J. Dairy Sci. 2018, 101, 3611–3629.
- Høier, E.; Janzen, T.; Rattray, F.; Sørensen, K.; Børsting, M.; Brockmann, E.; Johansen, E. The production, application and action of lactic cheese starter cultures. In Technology of Cheesemaking, 2nd ed.; Law, B.A., Tamime, A.Y., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 166–192.
- Pintado, M.; Da Cruz, A.G.; de Sá, P. Cheese microbiology. In Dairy Microbiology and Biochemistry: Recent Developments; Özer, B.H., Akdemir-Evrendilek, G., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 113–133.
- Câmara, S.; Dapkevicius, A.; Riquelme, C.; Elias, R.B.; Silva, C.; Malcata, F.X.; Dapkevicius, M. Potential of lactic acid bacteria from Pico cheese for starter culture development. Food Sci. Technol. Int. 2019, 25, 303–317.
- Callanan, M.; Ross, R. Starter cultures: Genetics. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; Volume 4, pp. 149–161.
- Parente, E.; Cogan, T. Starter cultures: General aspects. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., McSweeney, P.L.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; Volume 1, pp. 123–148.
- Vázquez-Velázquez, R.; Salvador-Figueroa, M.; Adriano-Anaya, L.; DeGyves–Córdova, G.; Vázquez-Ovando, A. Use of starter culture of native lactic acid bacteria for producing an artisanal Mexican cheese safe and sensory acceptable. CyTA-J. Food 2018, 16, 460–468.
- Ben Braiek, O.; Smaoui, S. Enterococci: Between emerging pathogens and potential probiotics. BioMed Res. Int. 2019, 2019, 5938210.
- Smid, E.J.; Erkus, O.; Spus, M.; Wolkers-Rooijackers, J.; Alexeeva, S.; Kleerebezem, M. Functional implications of the microbial community structure of undefined mesophilic starter cultures. Microb. Cell Factories 2014, 13, 1–9.
- Marshall, V.M. Inoculated ecosystems in a milk environment. J. Appl. Bacteriol. 1992, 73, 127s–135s.
- Mayra-Makinen, A.; Bigret, M. Industrial use and production of lactic acid bacteria. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Salminen, S., von Wright, A., Ouwehand, A., Eds.; Marcel Dekker: New York, NY, USA, 2004; Volume 139, pp. 175–198.
- Ustunol, Z.; Özer, B.; Akdemir-Evrendilek, G. Dairy starter cultures. In Dairy Microbiology and Biochemistry: Recent Developments; Özer, B.H., Akdemir-Evrendilek, G., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 39.
- Kelleher, P.; Murphy, J.; Mahony, J.; van Sinderen, D. Next-generation sequencing as an approach to dairy starter selection. Dairy Sci. Technol. 2015, 95, 545–568.
- Johnson, M.; Law, B. The origins, development and basic operations of cheesemaking technology. In Technology of Cheesemaking, 2nd ed.; Law, B.A., Tamime, A.Y., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 68–97.
- Coppola, R.; Nanni, M.; Iorizzo, M.; Sorrentino, A.; Sorrentino, E.; Grazia, L. Survey of lactic acid bacteria isolated during the advanced stages of the ripening of Parmigiano Reggiano cheese. J. Dairy Res. 1997, 64, 305–310.
- Casey, M.G.; Häni, J.P.; Gruskovnjak, J.; Schaeren, W.; Wechsler, D. Characterisation of the non-starter lactic acid bacteria (NSLAB) of Gruyère PDO cheese. Le Lait 2006, 86, 407–414.
- Cogan, T.; Beresford, T.; Steele, J.; Broadbent, J.; Shah, N.; Ustunol, Z. Invited review: Advances in starter cultures and cultured foods. J. Dairy Sci. 2007, 90, 4005–4021.
- De Angelis, M.; Corsetti, A.; Tosti, N.; Rossi, J.; Corbo, M.R.; Gobbetti, M. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 2001, 67, 2011–2020.
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697.
- Williams, A.G.; Banks, J.M. Proteolytic and other hydrolytic enzyme activities in non-starter lactic acid bacteria (NSLAB) isolated from Cheddar cheese manufactured in the United Kingdom. Int. Dairy J. 1997, 7, 763–774.
- Fitzsimons, N.A.; Cogan, T.M.; Condon, S.; Beresford, T. Spatial and temporal distribution of non-starter lactic acid bacteria in Cheddar cheese. J. Appl. Microbiol. 2001, 90, 600–608.
- Broadbent, J.R.; Houck, K.; Johnson, M.; Oberg, C. Influence of adjunct use and cheese microenvironment on nonstarter bacteria in reduced-fat Cheddar-type cheese. J. Dairy Sci. 2003, 86, 2773–2782.
- Fitzsimons, N.; Cogan, T.; Condon, S.; Beresford, T. Phenotypic and genotypic characterization of non-starter lactic acid bacteria in mature cheddar cheese. Appl. Environ. Microbiol. 1999, 65, 3418–3426.
- Gobbetti, M.; de Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178.
- Domingos-Lopes, M.; Stanton, C.; Ross, P.; Dapkevicius, M.; Silva, C. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190.
- Terzić-Vidojević, A.; Mihajlović, S.; Uzelac, G.; Golić, N.; Fira, Đ.; Kojić, M.; Topisirović, L. Identification and characterization of lactic acid bacteria isolated from artisanal white brined Golija cows’ milk cheeses. Arch. Biol. Sci. 2014, 66, 179–192.
- Sánchez-Juanes, F.; Teixeira-Martín, V.; González-Buitrago, J.M.; Velázquez, E.; Flores-Félix, J.D. Identification of species and subspecies of lactic acid bacteria present in spanish cheeses type “Torta” by MALDI-TOF MS and pheS gene analyses. Microorganisms 2020, 8, 301.
- Biolcati, F.; Andrighetto, C.; Bottero, M.T.; Dalmasso, A. Microbial characterization of an artisanal production of Robiola di Roccaverano cheese. J. Dairy Sci. 2020, 103, 4056–4067.
- Bokulich, N.A.; Mills, D.A. Facility-specific “house” microbiome drives microbial landscapes of artisan cheesemaking plants. Appl. Environ. Microbiol. 2013, 79, 5214–5223.
- Chen, H.; Hoover, D. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100.
- Galvez, A.; Lopez, R.L.; Abriouel, H.; Valdivia, E.; Omar, N.B. Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit. Rev. Biotechnol. 2008, 28, 125–152.
- Pinto, M.S.; de Carvalho, A.F.; dos Santos Pires, A.C.; de Paula, J.C.J.; Sobral, D.; Magalhães, F.A.R. Survival of Listeria innocua in Minas Traditional Serro cheese during ripening. Food Control 2009, 20, 1167–1170.
- Al-Gamal, M.S.; Ibrahim, G.A.; Sharaf, O.M.; Radwan, A.A.; Dabiza, N.M.; Youssef, A.M.; El-Ssayad, M.F. The protective potential of selected lactic acid bacteria against the most common contaminants in various types of cheese in Egypt. Heliyon 2019, 5, e01362.
- Gobbetti, M.; Di Cagno, R.; Calasso, M.; Neviani, E.; Fox, P.F.; de Angelis, M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci. Technol. 2018, 78, 244–254.
- O’Connor, P.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Antimicrobial antagonists against food pathogens: A bacteriocin perspective. Curr. Opin. Food Sci. 2015, 2, 51–57.
- Dalié, D.; Deschamps, A.; Richard-Forget, F. Lactic acid bacteria–Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380.
- Ryan, L.A.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011, 146, 276–283.
- Blagojev, N.; Škrinjar, M.; Vesković-Moračanin, S.; Šošo, V. Control of mould growth and mycotoxin production by lactic acid bacteria metabolites. Rom. Biotechnol. Lett. 2012, 17, 7219–7226.
- Sevgi, E.; Tsveteslava, I.-I. Antifungal activity of lactic acid bacteria, isolated from Bulgarian wheat and rye flour. J. Life Sci. 2015, 9, 1–6.
- Sedaghat, H.; Eskandari, M.H.; Moosavi-Nasab, M.; Shekarforoush, S.S. Application of non-starter lactic acid bacteria as biopreservative agents to control fungal spoilage of fresh cheese. Int. Dairy J. 2016, 56, 87–91.
- Perczak, A.; Goliński, P.; Bryła, M.; Waśkiewicz, A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh. Za Hig. Rada I Toksikol. 2018, 69, 32–44.
- Ouiddir, M.; Bettache, G.; Salas, M.L.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian lactic acid bacteria for use as antifungal bioprotective cultures and application in dairy and bakery products. Food Microbiol. 2019, 82, 160–170.
- Hammami, R.; Fliss, I.; Corsetti, A. Application of protective cultures and bacteriocins for food biopreservation. Front. Microbiol. 2019, 10, 1561.
- Benkerroum, N.; Ghouati, Y.; Ghalfi, H.; Elmejdoub, T.; Roblain, D.; Jacques, P.; Thonart, P. Biocontrol of Listeria monocytogenes in a model cultured milk (lben) by in situ bacteriocin production from Lactococcus lactis ssp. lactis. Int. J. Dairy Technol. 2002, 55, 145–151.
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.; Rosa, H.J.D. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int. J. Food Microbiol. 2014, 191, 53–59.
- Ribeiro, S.C.; O’Connor, P.M.; Ross, R.P.; Stanton, C.; Silva, C.C.G. An anti-listerial Lactococcus lactis strain isolated from Azorean Pico cheese produces lacticin 481. Int. Dairy J. 2016, 63, 18–28.
- Mills, S.; Serrano, L.M.; Griffin, C.; O’Connor, P.M.; Schaad, G.; Bruining, C.; Hill, C.; Ross, R.P.; Meijer, W.C. Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Factories 2011, 10, S7.
- Pingitore, E.V.; Todorov, S.D.; Sesma, F.; de Melo Franco, B.D.G. Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenes in fresh Minas cheese. Food Microbiol. 2012, 32, 38–47.
- Vandera, E.; Lianou, A.; Kakouri, A.; Feng, J.; Koukkou, A.-I.; Samelis, J. Enhanced control of Listeria monocytogenes by Enterococcus faecium KE82, a multiple enterocin–producing strain, in different milk environments. J. Food Prot. 2017, 80, 74–85.
- Ribeiro, S.C.; Ross, R.P.; Stanton, C.; Silva, C.C. Characterization and application of antilisterial enterocins on model fresh cheese. J. Food Prot. 2017, 80, 1303–1316.
- Barrios, M.J.; Medina, L.; Lopez, M.C.; Jordano, R. Fungal biota isolated from Spanish cheeses. J. Food Saf. 1998, 18, 151–157.
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19.
- Garnier, L.; Valence, F.; Pawtowski, A.; Auhustsinava-Galerne, L.; Frotté, N.; Baroncelli, R.; Deniel, F.; Coton, E.; Mounier, J. Diversity of spoilage fungi associated with various French dairy products. Int. J. Food Microbiol. 2017, 241, 191–197.
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37.
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090.
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, É.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control 2017, 78, 350–356.
- Nayyeri, N.; Edalatian Dovom, M.R.; Habibi Najafi, M.B.; Bahreini, M. A Preliminary study on antifungal activity of lactic acid bacteria isolated from different production stages of Lighvan cheese on Penicillium expansum and Rhodotorula mucilaginosa. J. Food Meas. Charact. 2017, 11, 1734–1744.
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54.
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A. Novel antifungal bacteriocin from Lactobacillus paracasei KC39 with anti-mycotoxigenic properties. Biosci. Res. 2018, 15, 4171–4183.
- Kim, J.-D. Antifungal activity of lactic acid bacteria isolated from Kimchi against Aspergillus fumigatus. Mycobiology 2005, 33, 210–214.
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.L.; Barbier, G.; Coton, E. Filamentous fungi and mycotoxins in cheese: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456.
- Varsha, K.K.; Nampoothiri, K.M. Appraisal of lactic acid bacteria as protective cultures. Food Control 2016, 69, 61–64.
- Filtenborg, O.; Frisvad, J.C.; Thrane, U. Moulds in food spoilage. Int. J. Food Microbiol. 1996, 33, 85–102.
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38.
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78.
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109.
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436.
- Ito, A.; Sato, Y.; Kudo, S.; Sato, S.; Nakajima, H.; Toba, T. The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr. Microbiol. 2003, 47, 0231–0236.
- Hernandez-Valdes, J.A.; Solopova, A.; Kuipers, O.P. Development of Lactococcus lactis Biosensors for Detection of Diacetyl. Front. Microbiol. 2020, 11, 1032.
- Terzic-Vidojevic, A.; Tolinacki, M.; Nikolic, M.; Veljovic, K.; Jovanovic, S.; Macej, O.; Topisirovic, L. Artisanal Vlasina raw goat’s milk cheese: Evaluation and selection of autochthonous lactic acid bacteria as starter cultures. Food Technol. Biotechnol. 2013, 51, 554–563.
- Aunsbjerg, S.; Honoré, A.; Marcussen, J.; Ebrahimi, P.; Vogensen, F.; Benfeldt, C.; Skov, T.; Knøchel, S. Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 2015, 194, 46–53.
- Olasupo, N.; Fitzgerald, D.; Gasson, M.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451.
- Liang, N.; Neužil-Bunešová, V.; Tejnecký, V.; Gänzle, M.; Schwab, C. 3-Hydroxypropionic acid contributes to the antibacterial activity of glycerol metabolism by the food microbe Limosilactobacillus reuteri. Food Microbiol. 2021, 98, 103720.
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589.
- Hernández-Carrillo, J.G.; Orta-Zavalza, E.; González-Rodríguez, S.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.; Ortiz-Rivera, Y. Evaluation of the effectivity of reuterin in pectin edible coatings to extend the shelf-life of strawberries during cold storage. Food Packag. Shelf Life 2021, 30, 100760.
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.l.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212.
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gänzle, M.G. Antifungal hydroxy fatty acids produced during sourdough fermentation: Microbial and enzymatic pathways, and antifungal activity in bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873.
- Sjögren, J.r.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557.
- Borthwick, A.D.; Da Costa, N.C. 2, 5-diketopiperazines in food and beverages: Taste and bioactivity. Crit. Rev. Food Sci. Nutr. 2017, 57, 718–742.
- Kwak, M.-K.; Liu, R.; Kang, S.-O. Antimicrobial activity of cyclic dipeptides produced by Lactobacillus plantarum LBP-K10 against multidrug-resistant bacteria, pathogenic fungi, and influenza A virus. Food Control 2018, 85, 223–234.
- Dal Bello, F.; Clarke, C.; Ryan, L.; Ulmer, H.; Schober, T.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318.
- O’sullivan, L.; Ross, R.; Hill, C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 2002, 84, 593–604.
- Soomro, A.; Masud, T.; Anwaar, K. Role of lactic acid bacteria (LAB) in food preservation and human health—A review. Pak. J. Nutr. 2002, 1, 20–24.
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138.
- Mills, S.; Stanton, C.; Hill, C.; Ross, R. New developments and applications of bacteriocins and peptides in foods. Annu. Rev. Food Sci. Technol. 2011, 2, 299–329.
- Ha, T.M.; Shakur, S.; Do, K.H.P. Consumer concern about food safety in Hanoi, Vietnam. Food Control 2019, 98, 238–244.
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788.
- Mirhosseini, M.; Nahvi, I.; Emtiazi, G.; Tavassoli, M. Characterisation of anti-Listeria monocytogenes bacteriocins from Enterococcus faecium strains isolated from dairy products. Int. J. Dairy Technol. 2010, 63, 55–61.
- Kaškonienė, V.; Stankevičius, M.; Bimbiraitė-Survilienė, K.; Naujokaitytė, G.; Šernienė, L.; Mulkytė, K.; Malakauskas, M.; Maruška, A. Current state of purification, isolation and analysis of bacteriocins produced by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2017, 101, 1323–1335.
- Hassan, M.; Kjos, M.; Nes, I.; Diep, D.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20.
- Leite, J.A.; Tulini, F.L.; dos Reis-Teixeira, F.B.; Rabinovitch, L.; Chaves, J.Q.; Rosa, N.G.; Cabral, H.; de Martinis, E.C.P. Bacteriocin-like inhibitory substances (BLIS) produced by Bacillus cereus: Preliminary characterization and application of partially purified extract containing BLIS for inhibiting Listeria monocytogenes in pineapple pulp. LWT Food Sci. Technol. 2016, 72, 261–266.
- Piard, J.; Desmazeaud, M. Inhibiting factors produced by lactic acid bacteria. 2. Bacteriocins and other antibacterial substances. Le Lait 1992, 72, 113–142.
- Lozo, J.; Vukasinovic, M.; Strahinic, I.; Topisirovic, L. Characterization and antimicrobial activity of bacteriocin 217 produced by natural isolate Lactobacillus paracasei subsp. paracasei BGBUK2-16. J. Food Prot. 2004, 67, 2727–2734.
- Sobrino-López, A.; Martín-Belloso, O. Use of nisin and other bacteriocins for preservation of dairy products. Int. Dairy J. 2008, 18, 329–343.
- Balciunas, E.M.; Martinez, F.A.C.; Todorov, S.D.; de Melo Franco, B.D.G.; Converti, A.; de Souza Oliveira, R.P. Novel biotechnological applications of bacteriocins: A review. Food Control 2013, 32, 134–142.
- Bali, V.; Panesar, P.S.; Bera, M.B.; Kennedy, J.F. Bacteriocins: Recent trends and potential applications. Crit. Rev. Food Sci. Nutr. 2016, 56, 817–834.
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594.
- Silva, S.P.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486.
- Malini, M.; Savitha, J. Heat stable bacteriocin from Lactobacillus paracasei subsp. tolerans isolated from locally available cheese: An in vitro study. E3 J. Biotechnol. Pharm. Res. 2012, 3, 28–41.
- Lozo, J.; Topisirovic, L.; Kojic, M. Natural bacterial isolates as an inexhaustible source of new bacteriocins. Appl. Microbiol. Biotechnol. 2021, 105, 477–492.
- Jena, P.K.; Trivedi, D.; Thakore, K.; Chaudhary, H.; Giri, S.S.; Seshadri, S. Isolation and characterization of probiotic properties of lactobacilli isolated from rat fecal microbiota. Microbiol. Immunol. 2013, 57, 407–416.
- Colombo, M.; Castilho, N.; Todorov, S.D.; Nero, L.A. Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol. 2018, 18, 219.
- Celiberto, L.S.; Pinto, R.A.; Rossi, E.A.; Vallance, B.A.; Cavallini, D.C. Isolation and characterization of potentially probiotic bacterial strains from mice: Proof of concept for personalized probiotics. Nutrients 2018, 10, 1684.
- Żukiewicz-Sobczak, W.; Wróblewska, P.; Adamczuk, P.; Silny, W. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent.-Eur. J. Immunol. 2014, 39, 104.
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; de Paula, J.A. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J. Clin. Gastroenterol. 2012, 46, 468–481.
- Kook, S.-Y.; Chung, E.-C.; Lee, Y.; Lee, D.W.; Kim, S. Isolation and characterization of five novel probiotic strains from Korean infant and children faeces. PLoS ONE 2019, 14, e0223913.
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365s–373s.
- Azat, R.; Liu, Y.; Li, W.; Kayir, A.; Lin, D.-b.; Zhou, W.-w.; Zheng, X.-d. Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J. Zhejiang Univ. Sci. B 2016, 17, 597–609.
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the prevention of antibiotic-associated diarrhea in outpatients—A systematic review and meta-analysis. Antibiotics 2017, 6, 21.
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019, 234, 17127–17143.
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A mini-review of human studies on cholesterol-lowering properties of probiotics. Sci. Pharm. 2019, 87, 26.
- Sivamaruthi, B.S.; Bharathi, M.; Kesika, P.; Suganthy, N.; Chaiyasut, C. The Administration of Probiotics against Hypercholesterolemia: A Systematic Review. Appl. Sci. 2021, 11, 6913.
- Domingos-Lopes, M.; Stanton, C.; Ross, R.; Silva, C. Histamine and cholesterol lowering abilities of lactic acid bacteria isolated from artisanal Pico cheese. J. Appl. Microbiol. 2020, 129, 1428–1440.
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Probiotics in human mental health and diseases—A minireview. Trop. J. Pharm. Res. 2019, 18, 889–895.
- Zielińska, D.; Kolożyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties. BioMed Res. Int. 2018, 2018, 1784.
- Salminen, S.; Gueimonde, M. Human studies on probiotics: What is scientifically proven. J. Food Sci. 2004, 69, M137–M140.
- Abdullah-Al-Mamun, M.; Rahman, S. Assessment of Probiotic Properties of Isolated Lactic Acid Bacteria from Human Milk Sample. Adv. Biores. 2017, 8, 140–146.
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253.
- Padmavathi, T.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018, 16, 357–362.
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982.
- Doğan, M.; Ay, M. Evaluation of the probiotic potential of Pediococcus strains from fermented dairy product kefir. Czech J. Food Sci. 2021, 39, 376–383.
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian dairy products: Ethnic foods with health benefits. Microorganisms 2021, 9, 480.
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.; Sreenivasa, M. Probiotic properties of lactic acid bacteria isolated from neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019, 10, 1382.
- Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019, 2019, 7179514.
- Gill, H.S.; Grover, S.; Batish, V.K.; Gill, P. Immunological effects of probiotics and their significance to human health. In Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; p. 901.
- Powthong, P.; Suntornthiticharoen, P. Isolation, identification and analysis of probiotic properties of lactic acid bacteria from selective various traditional thai fermented food and kefir. Pak. J. Nutr. 2015, 14, 67.
- Lavanya, K.; Dayakar, Y. Isolation and characterization of probiotic bacteria from the soil samples of the coastal areas of (Gudur division, Nellore Dt.) for utilization in Shrimp farming. Int. J. Fish. Aquafic Stud. 2017, 5, 371–376.
- Felis, G.E.; Dellaglio, F.; Torriani, S. Taxonomy of probiotic microorganisms. In Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; pp. 591–637.
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846.
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472.
- Gupta, R.; Jeevaratnam, K.; Fatima, A. Lactic Acid Bacteria: Probiotic Characteristic, Selection Criteria, and its Role in Human Health (A Review). Int. J. Emerg. Technol. Innov. Res. 2018, 5, 10.
- Siró, I. Challenges of Beneficial Health Claims. In Probiotics: Biology, Genetics and Health Aspects, Microbioloy Monographs; Liong, M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 243–268.
- Harzallah, D.; Belhadj, H. Lactic acid bacteria as probiotics: Characteristics, selection criteria and role in immunomodulation of human GI muccosal barrier. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; InTech: Zagreb, Croatia, 2013; pp. 197–216.
- Lawrence, G.W.; Begley, M.; Cotter, P.D.; Guinane, C.M. Potential use of biotherapeutic bacteria to target colorectal cancer-associated taxa. Int. J. Mol. Sci. 2020, 21, 924.
- Erickson, K.L.; Hubbard, N.E. Probiotic immunomodulation in health and disease. J. Nutr. 2000, 130, 403S–409S.
- Kanmani, P.; Kim, H. Immunobiotic strains modulate Toll-like receptor 3 agonist induced innate antiviral immune response in human intestinal epithelial cells by modulating IFN regulatory factor 3 and NF-κB signaling. Front. Immunol. 2019, 10, 1536.
- Domingos-Lopes, M.; Nagy, A.; Stanton, C.; Ross, P.; Gelencsér, E.; Silva, C. Immunomodulatory activity of exopolysaccharide producing Leuconostoc citreum strain isolated from Pico cheese. J. Funct. Foods 2017, 33, 235–243.
- Ramalho, J.B.; Spiazzi, C.C.; Bicca, D.F.; Rodrigues, J.F.; Sehn, C.P.; da Silva, W.P.; Cibin, F.W.S. Beneficial effects of Lactococcus lactis subsp. cremoris LL95 treatment in an LPS-induced depression-like model in mice. Behav. Brain Res. 2022, 426, 113847.
- Saliganti, V.; Kapila, R.; Kapila, S.; Bhat, M.I. Probiotics in the modulation of maternal–infant immunity: Implications for allergic diseases. Food Rev. Int. 2017, 33, 516–537.
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9.
- O’Mahony, S.M.; Clarke, G.; Borre, Y.; Dinan, T.G.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48.
- Misra, S.; Mohanty, D. Psychobiotics: A new approach for treating mental illness? Crit. Rev. Food Sci. Nutr. 2019, 59, 1230–1236.
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781.
- Oleskin, A.V.; Shenderov, B.A. Probiotics and psychobiotics: The role of microbial neurochemicals. Probiot. Antimicrob. Proteins 2019, 11, 1071–1085.
- Tyagi, P.; Tasleem, M.; Prakash, S.; Chouhan, G. Intermingling of gut microbiota with brain: Exploring the role of probiotics in battle against depressive disorders. Food Res. Int. 2020, 137, 109489.
- Barros, C.P.; Guimaraes, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8.
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154.
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757.
- Siragusa, S.; de Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290.
- Alhaj, O.; Kanekanian, A. Milk-derived bioactive components from fermentation. In Milk and Dairy Products as Functional Foods, First Edition; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 237–288.
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527.
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
05 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




