You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Celia C. G. Silva and Version 2 by Beatrix Zheng.
Lactic acid bacteria (LAB) are of great economic importance because they play an important role throughout the fermentation process of traditional cheeses when added accidentally or intentionally. Their metabolic features not only contribute to the development of desirable sensory characteristics of food products but also allow the nutritional value of the raw material to be maintained or even enhanced.
- cheese
- LAB
- bacteria
- lactobacilli
- bacteriocins
- probiotics
- health-promoting effects
1. LAB as Starter Cultures
The bacteria most commonly used as starter cultures in cheeses are Lactic acid bacteria (LAB) [1][96]. The chief role of these cultures is to acidify the milk, and thereby inhibit the growth of other (undesired) bacteria [2][3][4][5][84,97,98,99]. The starter bacteria must produce enough acid to lower the pH of the milk to below 5.3 within 6 h at 30–37 °C, depending on the type of cheese [3][6][97,100]. The production of acid in the right amount and at the right time is a crucial factor to obtain high-quality cheeses [7][8][9][27,66,101]. Therefore, the ability of LAB to produce acid rapidly is one of their most important technological features [10][26]. The temperature during production, salt levels, and humidity should be controlled to ensure that the activity of starter cultures is sufficient to rapidly reach the targeted pH [3][97]. Starter cultures should also promote a sustainable environment in the cheese in terms of redox potential, salinity, and moisture that allows suitable rennet enzyme activity and the growth of the secondary microbiota [3][11][12][23,97,102]. Starter bacteria are undoubtedly the main players in the first hours of cheese production. However, from the 18th day to the 25th day of ripening, the number of these bacteria decreases drastically as a consequence of the decrease of lactose as a nutrient and their own autolytic behavior [7][27].
In addition to acid production during the fermentation process, starter cultures also contribute to cheese ripening since their enzymes are involved in the proteolysis, lipolysis, and conversion of amino acids into compounds that directly contribute to the flavor of the final product [3][6][9][13][97,100,101,103]. In addition, the use of starter cultures ensures microbiologically safe products, because these cultures inhibit the development of undesirable microorganisms by producing compounds that prevent their growth, such as organic acids, bacteriocins, and hydrogen peroxide [14][15][16][104,105,106].
The most commonly used starter cultures are members of the genera Lactococcus, Lactobacillus, Streptococcus, Leuconostoc, and Enterococcus [3][97]. Currently, Enterococcus is not granted this qualification due to regulations related to the qualified presumption of safety (QPS). However, some well-characterized strains continue to be used as starter cultures, co-cultures, or protective cultures in the food industry owing to their beneficial properties [17][107]. The most commonly used species in cheese production are Lc. lactis, S. salivarius subsp. thermophilus, L. helveticus, and L. delbrueckii [12][102].
At the beginning of production, LAB may be present as a native component of the milk, as happens with many artisanal raw milk cheeses [3][97]. In these cheeses, the spontaneous fermentation of the milk is driven by the development of the aforementioned microbiota. However, the outcome of such processes is unpredictable, as the physiological stage and extent of inoculum are beyond operator’s control [18][95].
Conversely, starter cultures are intentionally added and previously selected based on their effect upon fermentation and the desired properties of the product. The selection criteria vary, but the dominant criterion is usually the acidification rate at a given temperature, and the insensitivity to phages [11][23]. Handling characteristics and stability during production are also criteria for starter culture selection [19][108].
The proper selection of starter cultures and the characterization of each strain is very important to obtain products with reproducible organoleptic and structural properties by the end of cheese production [2][4][11][23,84,98]. By controlling the fermentation process, the said cultures reduce the variations in organoleptic quality and microbiological stability observed in cheeses without them.
1.1. Type of Starter Cultures
Starter cultures can be categorized as mesophilic or thermophilic, depending on the incubation and manufacturing temperatures at which they are used [4][98]. Mesophilic starter cultures have an optimal growth temperature of ca. 30 °C, while thermophilic starter cultures grow best between 40 and 45 °C [14][104]. Mesophilic and thermophilic cultures can be divided into defined and undefined cultures [3][97].
1.1.1. Mesophilic and Thermophilic Starter Cultures
The starter cultures most commonly used in the production of fermented dairy products belong to the genera Lactobacillus and Streptococcus, namely the species S. salivarius subsp. thermophilus, Lb. helveticus, Lb. delbrueckii subsp. Lactis, and L. delbrueckii subsp. bulgaricus [14][15][20][21][104,105,109,110].
Mesophilic starter cultures include mainly the genera Lactococcus and Leuconostoc [15][21][105,110]. The LAB most commonly used as mesophilic starter cultures are Lc. lactis, including subspecies lactis and cremoris for being good acid producers [20][21][109,110]. Other mesophilic starter cultures include the species Ln. lactis and Ln. cremoris [20][109]. Mixed mesophilic cultures are usually 90% acid producers and 10% aroma producers [21][110].
In the production of hard cheeses, mesophilic starter cultures are predominantly used (e.g., Lactococcus spp.), although thermophilic cultures may also be used (e.g., S. salivarius subsp. thermophilus) [12][102].
1.1.2. Defined and Undefined Starter Cultures
Starter cultures are usually composed of different species, or multiple strains of one species. Starter cultures can be divided into defined and undefined cultures [11][15][20][23,105,109]. The former usually consist of one or more strains with known characteristics [18][95]. They have usually been isolated from mixed cultures and selected based on important characteristics such as phage resistance, acid production, citrate utilization, and aroma and flavor formation [10][22][26,111]. Undefined starter cultures have partially known or all unknown species and strains in their composition [21][110].
Starter cultures used in the production of cheese can be divided into: (1) defined cultures with multiple strains (e.g., Lc. lactis subsp. lactis and Lc. lactis subsp. cremoris in Camembert and Brie cheeses); (2) defined cultures with a single strain (e.g., S. thermophilus in Mozzarella cheese); (3) defined mixed cultures (e.g., S. thermophilus, Lb. helveticus, Lb. delbrueckii subsp. lactis, Lb. delbrueckii subsp. bulgaricus and Propionibacterium shermanii in Emmental and Gruyere cheeses); and (4) undefined mixed cultures (e.g., whey starter in Italian cheeses such as Parmigiano Reggiano) [7][11][21][23][23,27,110,112].
For instance, the cultures used for the production of Gouda cheese were isolated from an undefined starter culture traditionally used for the production of this cheese, consisting of L. lactis subsp. cremoris, L. lactis subsp. lactis biovar diacetylactis, and Ln. mesenteroides [18][23][95,112].
1.1.3. Natural Whey Starter (NWS)
Natural whey starter cultures (NWS) consist of an undefined culture of LAB and are mostly acid producers [24][113]. This type of starter is commonly used in the production of traditional artisanal cheeses using the back-slopping technique, which requires the inoculation of milk with whey or fermented milk from the previous day [10][26].
2. LAB as Adjunct Cultures
2.1. Selected Adjunct Cultures
Adjunct cultures can be defined as those added to cheese for purposes other than acid production, even though they often consist of microorganisms derived from ingredients (raw milk) or the cheese-making environment [3][97]. Adjunct cultures, selected from adventitious LAB, also called non-starter LAB (NSLAB), can therefore be added with starter to accelerate the ripening process and produce the desired flavor [21][110]. These cultures are selected to survive cheese curd cooking temperatures and participate in flavor development at a later stage of cheese ripening. Mesophilic cultures such as L. casei and L. paracasei are traditionally added with the starter to improve the flavor of dairy products [21][110]. They can also mitigate defects caused by contaminating adventitious LAB by inhibiting their development [2][84].
2.2. Natural Adjunct Cultures
Natural adjunct cultures are often adventitious cultures LAB, which are not part of the added starter culture [25][26][114,115]. Such adventitious LAB are usually difficult to grow in milk and do not contribute to acid production [3][97], but are critical for the final flavor and texture of the cheese [12][15][102,105]. These bacteria can grow with energy sources other than lactose, and are more resistant to environmental stress [7][27][27,116]. Adventitious LAB are present at very low concentrations in the curd but their populations start to increase during the first months of ripening and eventually become the dominant microbiota of longer ripened cheeses [7][10][27][28][3,26,27,116].
The composition of adventitious LAB varies depending on cheese type, the mode of processing, and ripening time [2][25][29][30][84,114,117,118]. The development of adventitious LAB during cheese ripening can be attributed in part to their ability to utilize available nutrient sources [10][26]. As lactose is metabolized during the first weeks of ripening, adventitious LAB can obtain energy from compounds such as lactic acid, citric acid, ribose, fatty acids, glycerol, and amino acids [10][31][26,119]. Because LAB possess a variety of hydrolytic enzymes convenient for cheese proteolysis and lipolysis, they are able to grow and act during cheese ripening [2][27][29][32][84,116,117,120].
2.3. Charaterization of Adventitious NSLAB
The adventitious NSLAB are a particularly heterogeneous group, and include mesophilic lactobacilli, enterococci, pediococci, and Leuconostoc [6][25][26][100,114,115]. Mesophilic lactobacilli are the predominant and most important group in the microbiota of NSLAB [33][121]. Among the mesophilic lactobacilli, facultative heterofermenters are the most abundant in NSLAB [25][114], mainly L. casei subsp. casei, L. casei subsp. pseudoplantarum, L. paracasei subsp. paracasei, L. plantarum, L. rhamnosus, L. curvatus [3][27][97,116], and L. pentosus [12][102]. The obligate heterofermentative species commonly found in cheese are: L. fermentum, L. buchneri, L. parabuchneri, and L. brevis [12][102], although other species of facultative or obligate heterofermentative lactobacilli also occur [27][116].
The most common pediococci found in cheese are Pediococcus acidilactici and P. pentosaceus [3][97]. Among enterococci, Enterococcus durans, E. faecalis, and E. faecium are most abundant in cheese [12][34][102,122]. Within the genus Leuconostoc, the species Ln. mesenteroides, Ln. peseudomesenteroides, and Ln. citreum have been detected in artisanal cheeses produced from raw milk [34][35][36][37][122,123,124,125].
The origin of NSBAL can vary, but the main source is raw milk [25][26][33][114,115,121] and, to a lesser extent, whey used as starter—NWS [33][121]. The microbial diversity of raw milk cheeses depends on the microbiota of the milk, the ingredients utilized, and the processes used in cheese production [25][26][27][33][114,115,116,121]. The cheese processing environment can also be a potential source of NSBAL, especially in the case of mesophilic bacteria, which can survive the processing environment and on the equipment itself, even after cleaning and disinfection, due to their ability to form biofilms [10][26].
NSBALs are generally associated with raw milk but are also present in cheese produced from pasteurized milk. The presence of NSBAL in cheese produced from pasteurized milk is due to airborne contamination, contact with equipment and/or ingredients used in cheese making, or thermoduric strains that survive pasteurization [32][120].
When artisanal cheese is produced without direct inoculation with starter cultures, the microorganisms involved in fermentation are derived from starting material and environmental sources [38][126]. Therefore, the inherent and unique flavors known in cheeses produced from raw milk are the result of a diverse indigenous microbiota [10][26]. These NSBALs dominate the microbiota of many aged cheeses and play a key role in the development of flavor and aroma throughout ripening. For instance, they contribute to the release of small peptides and amino acids, which in turn can be converted into alcohols, aldehydes, esters, and sulfur compounds that are associated with specific flavors and aromas of the ripened cheese [2][84].
3. Antimicrobial Activity of LAB
LAB can be used to inhibit or destroy undesirable microorganisms in foods, increase their safety, and extend their shelf life [39][127]. The use of LAB as bioprotective agents also ensures food quality and safety without the need to resort to chemical preservatives [40][128].
3.1. Antibacterial Activity
In the dairy industry, the main bacterial pathogens that need to be controlled are those that can survive and multiply in products produced from raw milk, or that arise from contamination after pasteurization, such as Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, and Salmonella spp. [40][41][42][128,129,130].
The use of LAB as starter cultures in food fermentation promotes food preservation through rapid acid production [43][131]. In addition to lowering pH, some LAB species/strains possess antibacterial properties resulting from a combination of factors, including competitive growth and the production of a variety of antibacterial compounds [44][132]. Antibacterial compounds produced by LAB include various organic acids, such as lactic acid, acetic acid, formic acid, and propionic acid, as well as such other compounds as diacetyl, acetoin, hydrogen peroxide, reuterin, and bacteriocins [45][46][47][48][49][50][51][133,134,135,136,137,138,139].
The efficacy of LAB to inhibit various bacterial pathogens has been demonstrated in several food matrices, including cheese, meat, and vegetables [52][140]. In fermented milk, the application of a bacteriocin-producing strain of Lc. lactis ssp. lactis reduced L. monocytogenes contamination to undetectable levels [53][141]. Several other studies have also shown a reduction in L. monocytogenes in various cheeses by using Lc. lactis strains that produce bacteriocins [54][55][17,142]. Such bacteriocin-producing LAB species as L. plantarum, Streptococcus spp. and Enterococcus spp. have been shown to reduce L. monocytogenes and S. aureus contamination in various dairy products [40][54][56][57][58][59][17,128,143,144,145,146].
3.2. Antifungal Activity
Molds and yeasts are ubiquitous contaminants of dairy products, especially under conditions that favor their growth [42][48][130,136]. In the case of cheese, fungal contamination occurs in all types of cheese, although more readily in soft and unripened cheeses [60][61][147,148].
Fungal spoilage causes visible or invisible sensory defects in cheese, such as the visible growth of the fungus on the surface, and the production of metabolites that lead to noticeable and unpleasant changes in aroma, flavor, and texture, thus resulting in a loss of product quality [42][47][61][62][63][130,135,148,149,150].
In addition to the major economic losses associated with spoilage, some fungi pose a threat to food safety due to their ability to produce mycotoxins [51][61][64][65][66][67][68][139,148,151,152,153,154,155]. Therefore, the risk of mycotoxins in cheese increases when toxigenic fungi are allowed to grow during production and storage [61][148]. Filamentous fungi belonging to the genera Aspergillus, Fusarium, and Penicillium can grow on the cheese surface and produce mycotoxins that are highly toxic [45][50][63][69][70][71][133,138,150,156,157,158]. Some mycotoxins are present only in the fungus, while most are excreted in food [72][159]. Aflatoxins are considered one of the most important and well-known classes of mycotoxins in food [72][73][159,160]. These compounds have numerous and diverse toxic properties, including carcinogenic, teratogenic, mutagenic, nephrotoxic, hepatotoxic, neurotoxic, immunosuppressive, and estrogenic effects, even when ingested at low concentrations [45][47][61][73][133,135,148,160].
Some LAB species/strains have shown activity against common cheese spoilage molds [46][134]. The antifungal activity of LAB is attributed to multiple compounds acting individually or in synergy to provide multiple barriers against spoilage molds [46][65][134,152]. Some LAB species are also able to reduce mycotoxins produced by molds [71][158].
The LAB best known for their ability to prevent or retard the growth of toxinogenic fungi belong to the genera Lactococcus and Lactobacillus and, to a lesser extent, Pediococcus and Leuconostoc [45][133]. The antifungal activity of the genus Lactobacillus has been extensively studied, with particular emphasis on the species L. plantarum [45][63][133,150]. Different strains of L. plantarum and its metabolites have been tested in a variety of foods, where they were able to inhibit various fungal species belonging to the genera Aspergillus, Penicillium, Rhizopus, and Rhodotorula [63][66][150,153]. In addition to L. plantarum, other species such as L. casei, L. paracasei, and L. brevis have also shown antifungal activity against a broad spectrum of spoilage molds [62][149].
3.3. Antimicrobial Metabolites Produced by LAB
3.3.1. Organic Acids
The antimicrobial activity of LAB is associated with the production of organic acids, mainly lactic and acetic acids, but also formic, propionic, butyric, phenyllactic, hydroxy-phenyllactic, and indole-3-lactic acids, among others [73][160]. The most extensively studied acids are lactic, acetic, propionic, and phenyllactic acids [65][152].
Organic acids lower pH and create unfavorable conditions for the growth of many potentially pathogenic microorganisms [73][160]. In addition to their effects on pH, the undissociated form can diffuse across the cell membrane of the target organism, dissociate within the cell, and lower the cytoplasmic pH. Therefore, the most important parameter that determines the antimicrobial activity of an organic acid is pKa, because when pH < pKa, the undissociated form enters the cell and consequently neutralizes the electrochemical proton gradient, leading to the death of the susceptible organisms [45][47][74][133,135,161].
Similar to lactic acid, acetic and propionic acids interact with cell membranes to neutralize the electrochemical proton gradient; however, the effect of these acids is often dependent on the pH reduction achieved [74][161].
Phenyllactic acid has been described as an antimicrobial compound that exhibits a broad spectrum of antibacterial and antifungal activities [75][162]. This acid contributes to microbial inhibition in synergy with other compounds produced by LAB [74][75][161,162]. Phenyllactic acid can retard the growth of many fungi, including species belonging to the genera Aspergillus, Fusarium, and Penicillium. However, many studies have reported that very high concentrations of this acid are required to inhibit fungal growth, thus making it less suitable as antifungal agent in foods [76][163].
3.3.2. Hydrogen Peroxide
Hydrogen peroxide (H2O2) is produced by most LAB in the presence of oxygen [45][47][133,135]. Since LAB are unable to produce catalase, they cannot degrade hydrogen peroxide, so it accumulates in the medium, where it exerts a strong oxidizing effect on the lipid membrane, while destroying the basic molecular structures of the cell proteins of the target organisms [45][47][74][133,135,161].
The bactericidal action of hydrogen peroxide has been shown to be effective in reducing spoilage bacteria and pathogens such as E. coli, L. ivanovii, and S. aureus [77][164].
3.3.3. Diacetyl
Diacetyl (also known as 2,3-butanedione) is an aromatic compound, characterized by its buttery taste when associated with dairy products [78][165]. Diacetyl is produced by some LAB strains during citrate fermentation and is present in many dairy products such as cheese [34][79][122,166]. Diacetyl has been shown to exert antifungal and antibacterial effects at low pH [80][81][167,168]. However, the amounts of diacetyl required to exert antimicrobial activity significantly alter the taste and flavor of the final product [74][161].
3.3.4. Reuterin
Reuterin was first described as produced by L. reuteri, and is an antimicrobial compound with a broad spectrum of activity [65][74][152,161]. It consists of acrolein and 3-hydroxypropionaldehyde (3-HPA), which can be further metabolized to 1,3-propanediol and 3-hydroxypropionic acid (3-HP) [82][169]. This low molecular weight compound is capable of inhibiting the growth of a wide range of microorganisms, and is one of the most intensively studied antifungal compounds [74][75][161,162].
Reuterin is produced by several LAB under anaerobic conditions via the fermentation of glycerol [50][65][74][138,152,161]. The main LAB producers of reuterin are lactobacilli, including the species L. reuteri, L. brevis, L. buchneri, L. collinoids, and L. coryniformis [45][133].
Gram-positive bacteria are generally more resistant to reuterin than Gram-negative strains, including common food pathogens such as E. coli, Salmonella, and L. monocytogenes [82][169]. In target organisms, reuterin can suppress ribonuclease activity [45][50][133,138] or induce oxidative stress by modifying thiol groups in proteins and glutathione [83][170]. In fungi, reuterin inhibits the growth of species belonging to the genera Fusarium, Aspergillus, and Penicillium [45][50][84][133,138,171].
3.3.5. Fatty Acids
Fatty acids may also possess antibacterial and antifungal activity. The length of the fatty acid chain appears to play an important role in antimicrobial activity, with lauric (C12) and capric (C10) acids showing the best antimicrobial results [85][172].
LAB can produce several types of fatty acids that improve the sensory quality of fermented products. Caproic acid is one of these fatty acids and it has strong antifungal activity. It can act synergistically with propionic, butyric, or valeric acid [50][138].
According to Crowley et al. [75][162], antifungal fatty acids cleave the lipid bilayers of fungal membranes, thus causing a loss of membrane integrity. The increase in fluidity increases membrane permeability, leading to the uncontrolled release of electrolytes and intracellular proteins, as well as the cytoplasmic disintegration of fungal cells.
Some strains of lactobacilli can produce hydroxylated fatty acids from linoleic acid [86][173]. Sjogren et al. [87][174] found that hydroxylated fatty acids possess strong antifungal activity against a broad spectrum of yeasts and molds.
3.3.6. Cyclic Dipeptides
Cyclic dipeptides include several types of diketopiperazines such as the 2,5-diketopiperazines, which are among the most abundant peptide derivatives in nature [75][162]. They can be formed in foods by chemical reactions during thermal processing, or by yeast and LAB during fermentation [88][175].
Several bioactive properties are attributed to these dipeptides, including antimicrobial and antitumor activities [75][162]. The broad spectrum of antimicrobial effects of cyclic dipeptides produced by LAB has been demonstrated in several studies [46][89][90][134,176,177].
3.3.7. Bacteriocins
In recent years, bacteriocins have attracted considerable interest as a safe alternative to chemical preservatives for being rapidly hydrolyzed in the human gastrointestinal tract [91][92][93][94][95][178,179,180,181,182].
Bacteriocins are peptides with antimicrobial activity, synthesized by bacteria in ribosomes. These peptides often exhibit a narrow inhibitory spectrum and inhibit taxonomically-close bacteria [56][96][97][98][143,183,184,185]. The most common mechanisms used by bacteriocins to kill other microorganisms include the formation of pores in the cell membrane or the inhibition of cell wall synthesis [99][186]. Most bacteriocins produced by LAB, especially those that inhibit Gram-positive bacteria, exert their antimicrobial effects by forming pores in the membrane of target cells, thereby depleting the transmembrane potential and/or pH gradient, which eventually leads to loss of cell contents [100][101][187,188].
Bacteriocins are produced by only a few strains of different bacterial species, including LAB [102][189]. Some of these bacteriocins are effective against important foodborne pathogens, such as L. monocytogenes, S. aureus, Pseudomonas aeruginosa, and Salmonella enterica, as well as other spoilage microorganisms [91][102][103][104][105][178,189,190,191,192]. Some studies have shown that LAB can also produce bacteriocins with antifungal activity. Although Lactococcus, Streptococcus, and Pediococcus have been reported to produce bacteriocin-like peptides against a variety of fungi, Lactobacillus strains have been most commonly associated with the production of antifungal peptides/proteins [75][162]. However, the mode of action of protein compounds in inhibiting fungal growth by LAB is not completely clear [45][65][75][133,152,162].
The only bacteriocins commercially available at present are nisin A, produced by Lc. lactis, and pediocin, produced by P. acidilactici [98][106][51,185]. Nisin has a broad spectrum of antimicrobial inhibition, and inhibits the growth of most Gram-positive bacteria that contaminate food, such as L. monocytogenes, S. aureus, and Clostridium perfringens [107][193]. However, the efficacy of nisin has some limitations, since it cannot be used in foods with neutral or alkaline pH, or in foods that require LAB for fermentation [108][194]. Other bacteriocins, such as enterocins, have been shown to be more effective than nisin in inhibiting L. monocytogenes [108][194]. Bacteriocins that are effective against this bacterium are important for use in foods, especially cheeses produced from raw milk, as they may be contaminated with this pathogen [109][195].
Bacteriocins produced by LAB are often active over a wide pH range, resist high temperatures, and inhibit the growth of a variety of food spoilage and pathogenic bacteria. In addition, bacteriocins are sensitive to digestive proteases such as pancreatin, trypsin, and chymotrypsin, and therefore have no negative effects on the gut microbiota [108][194]. Since they are not toxic to eukaryotic cells and become inactive toward proteolytic enzymes (e.g., digestive proteases), bacteriocins are generally considered safe substances [110][111][196,197].
4. Probiotic Potential of LAB
Consumers are becoming increasingly aware of the beneficial effects of probiotics, and this has led to greater demand for probiotic products worldwide [112][113][198,199]. Most microorganisms residing in the gastrointestinal tract are harmless or otherwise beneficial to the host, thus resulting in a generally harmonious and symbiotic relationship [114][200]. The potential benefits of consuming probiotics are primarily due to positive changes in the gut microbiota, known to play a key role upon the immune system [115][201].
In 2002, the Food and Agriculture Organization of the United Nations (OAA) and the World Health Organization (WHO) defined probiotics as “live microorganisms that, when ingested and administered in sufficient quantities, have health benefits for the host” [116][202]. Therefore, probiotics are preparations of viable and non-pathogenic microorganisms included in foods or dietary supplements that interact directly with the gastrointestinal microbiota and immune system, so as to produce health-promoting effects [117][118][203,204]. According to these definitions, a large number of LAB strains have been proposed as probiotics [116][118][202,204].
In addition to modulating the immune system, the positive health effects of taking probiotics include: the improvement of lactose tolerance and digestion [113][119][199,205], the prevention and treatment of gastrointestinal infections [120][206], the prevention of colorectal cancer [117][121][203,207], reduction in blood cholesterol levels [122][123][124][208,209,210], and the improvement of mental health via the gut-brain axis [125][211].
Probiotic LAB strains used in the production of fermented foods or pharmaceuticals must be recognized as safe for human use and possess GRAS or QPS status [115][126][201,212]. Probiotic microorganisms must not only fulfill safety aspects, but also have functional and technological properties that are of interest. These include ease of propagation and incorporation in food, long-term survival, and clinically valid and documented beneficial health effects [115][201]. The safety and efficacy of probiotics must be scientifically proven in advance for each strain and product [127][213].
4.1. LAB Used as Probiotics
Most microorganisms currently recognized as probiotics belong to the LAB group [115][116][128][201,202,214]. This is not at all surprising, because LAB are part of the natural microbiota of the healthy gastrointestinal tract of humans and animals [129][130][215,216].
A large amount of LAB, which can be classified as probiotics, are also present in milk and fermented dairy products, such as cheese, yogurt, and fermented milk [131][132][133][217,218,219]. As mentioned earlier, LAB can ferment various sugars and produce organic acids such as lactate and acetate, as well as other antimicrobial metabolites such as hydrogen peroxide and bacteriocins, all of which can effectively inhibit the growth of pathogenic organisms in the gut [119][134][205,220].
Species belonging to the genera Lactobacillus and Bifidobacterium are most commonly used as probiotics because they play a very important role in maintaining proper intestinal function and stimulating the host immune system [126][135][136][212,221,222]. Other genera with species that exhibit probiotic properties include Pediococcus, Lactococcus, and Enterococcus [137][138][139][48,79,223].
The most commonly used probiotic lactobacilli species in the food industry are L. acidophilus, L. plantarum, L. rhamnosus, L. paracasei, L. casei, L. gasseri, L. johnsonii, L. reuteri [130][135][216,221], L. fermentum, L. salivarius [126][212], and L. delbrueckii subsp. bulgaricus [115][201]. As for the genus Bifidobacteria, the most common species in food applications are: B. adolescentis, B. animalis subsp. lactis, B. bifidum, B. breve, B. longum subsp. longum, and B. longum subsp. children [126][212].
4.2. Mechanisms of Action of Probiotics
The mechanisms of action may vary from one probiotic strain to another, but in most cases a combination of activities is likely, making the researchtudy of the responsible mechanisms a difficult and complex task [140][1]. Furthermore, the response to probiotic treatment may be specific to each individual. Several studies have shown that the gut microbiota can influence the expected effect of treatments, as it may vary greatly from person to person [141][224].
Several mechanisms of action have been proposed for the therapeutic effect of probiotics, as shown in Figure 12. Probiotics may be active in preventing gastrointestinal infections by making it more difficult for pathogens to colonize the gastrointestinal tract, either by competing for nutrients or by competing for receptors. In this case, probiotics compete for a limited number of receptors on the surface of the intestinal epithelium [141][142][224,225]. The release of antimicrobial compounds such as organic acids, hydrogen peroxide, and bacteriocins may also exert antagonistic effects against pathogenic organisms [143][144][226,227].
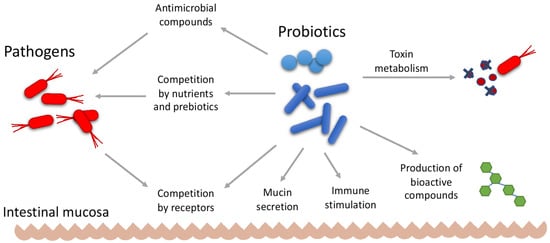
Figure 12.
Mechanisms of action of probiotics.
Probiotics may also act by strengthening and increasing the intestinal mucosal barrier. Increased mucin secretion enhances the binding of probiotics to the intestinal mucosa. This effect competitively prevents the binding of enteropathogens to the receptors of the epithelium. The stabilization of the intestinal barrier permeability limits pathogen colonization, eliminates foreign antigens that have invaded the mucosa, and regulates antigen-specific immune responses [141][142][224,225]. The use of appropriate strains of probiotics may be helpful in eliminating bacteria associated with colorectal cancer, thereby reducing the risk of developing this disease. Some studies have shown promising results regarding the use of probiotics as a prevention strategy for colorectal cancer; however, clinical trials are still needed to demonstrate this therapeutic effect [145][228].
The modulation of the host immune system represents another form of probiotic effect. Some LAB strains can modulate innate and acquired immune responses by binding to specific receptors on immune cells and other tissues such as intestinal epithelial tissue, and by stimulating the production of cytokines, T cells, the activation of dendritic cells and macrophages, and the production of specific antibodies [146][147][148][229,230,231].
With the increasing recognition of the importance of healthy gut microbiota in the development of autoimmune diseases, many studies have focused on the immunomodulatory effects of some probiotic strains [147][149][150][230,232,233]. In one of these studies, a Ln. citreum strain isolated from an artisanal cheese was shown to have an immunomodulatory effect due to its ability to decrease the production of proinflammatory cytokines (IL-8) by intestinal cells [148][231]. In animal studies, oral ingestion of this bacterium in an asthma model (nasal administration of an allergen) resulted in immune tolerance to the allergen [148][231].
Probiotic LAB may also be involved in the synthesis of neurotransmitters and neuromodulators. For example, certain species of Lactobacillus and Bifidobacterium produce γ-aminobutyric acid (GABA), Streptococcus spp. and Enterococcus spp. produce serotonin, and Lactobacillus spp. produce acetylcholine [151][152][234,235]. The gut microbiota is also involved in modulating the expression of neurochemical receptors and modulating the brain-gut axis, leading to psychotropic, antidepressant, and anxiolytic effects [153][236]. Several studies in animal models have unfolded the therapeutic effect associated to the administration of probiotic LAB strains upon cognitive processes and a reduction in psychophysiological markers of anxiety and depression [154][237]. As a result of the potential effect of probiotics on improving mental health, the term “psychobiotics” has been proposed [155][238]. Psychobiotics refer to a group of probiotics that are able to produce and release neuroactive substances such as GABA and serotonin. These act through the brain-gut axis, and exert antidepressant effects by altering emotional, cognitive, and neuronal indices [153][156][236,239].
4.3. Bioactive Compounds Produced by Probiotic LAB
Probiotics can increase the availability of nutrients and produce bioactive soluble factors (byproducts of metabolism) that are beneficial to the host and are referred to as postbiotics [157][240].
Fermented dairy products, especially cheese, may contain substances that have beneficial effects on human health [158][159][29,32]. In the last decade, fundamental studies have opened a new field of research dealing with bioactive compounds from food. Bioactive compounds are components of ready-to-eat foods that can exert a regulatory effect in the human body, regardless of their nutritional function [160][241].
The proteolysis of milk proteins by LAB during milk fermentation and cheese ripening can result in peptides with bioactive properties that confer immunostimulatory, opioid, or angiotensin I-converting enzyme (ACE) inhibitory activity [161][242]. Numerous studies have shown that milk fermented by Lactobacillus spp. can exert beneficial effects in controlling cardiovascular disease caused by hypertension via the production of ACE-inhibitory peptides [162][243].
Bioactive compounds produced by probiotic microorganisms also include vitamins (thiamine, riboflavin, cobalamin, folic acid, and vitamin K), enzymes (lactase or β-galactosidase), bioactive peptides (from the hydrolysis of proteins), conjugated linoleic acid (CLA), short-chain fatty acids (SCFA), gamma-aminobutyric acid (GABA), exopolysaccharides (EPS), and antimicrobial compounds such as bacteriocins (Figure 23) [159][163][32,244]. Some of these compounds stand out for their potential, yet poorly studied effects on human health.
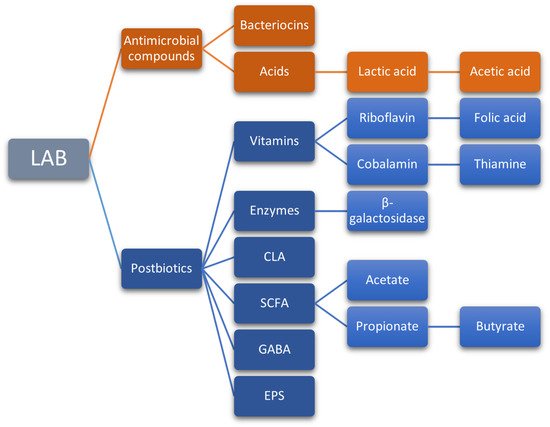
Figure 23.
Main bioactive compounds produced by probiotic LAB.
