
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wentao Han | -- | 1002 | 2022-08-02 14:05:52 | | | |
| 2 | Conner Chen | Meta information modification | 1002 | 2022-08-03 06:20:01 | | |
Video Upload Options
Spermidine, a natural polyamine, exists in almost all human tissues, exhibiting broad properties like anti-aging, autophagy induction, anti-inflammation, anti-oxidation, cell proliferation activation, and ion channel regulation.
1. Introduction
Spermidine is a polyamine with the chemical formula C7H19N3. It exists in ribosomes and living tissues and has various metabolic functions in living organisms. Spermidine was originally isolated from semen. Polyamines like spermidine, which are polycationic fatty amines and contain many functions, have important effects on cell survival. Spermidine synchronizes biological processes (such as Ca2+, Na+, K+ -ATPase), maintains membrane potential and controls intercellular pH and volume. Spermidine-mediated processes such as Ca2+ recruitment via the glutamatergic N-methyl-d-aspartate receptor (NMDA receptor). This receptor is associated with the activation pathways of nitric oxide synthase, cGMP/PKG and reduced Na+,K+-ATPase activity at cerebral cortex synapses.
2. Metabolism of Spermidine
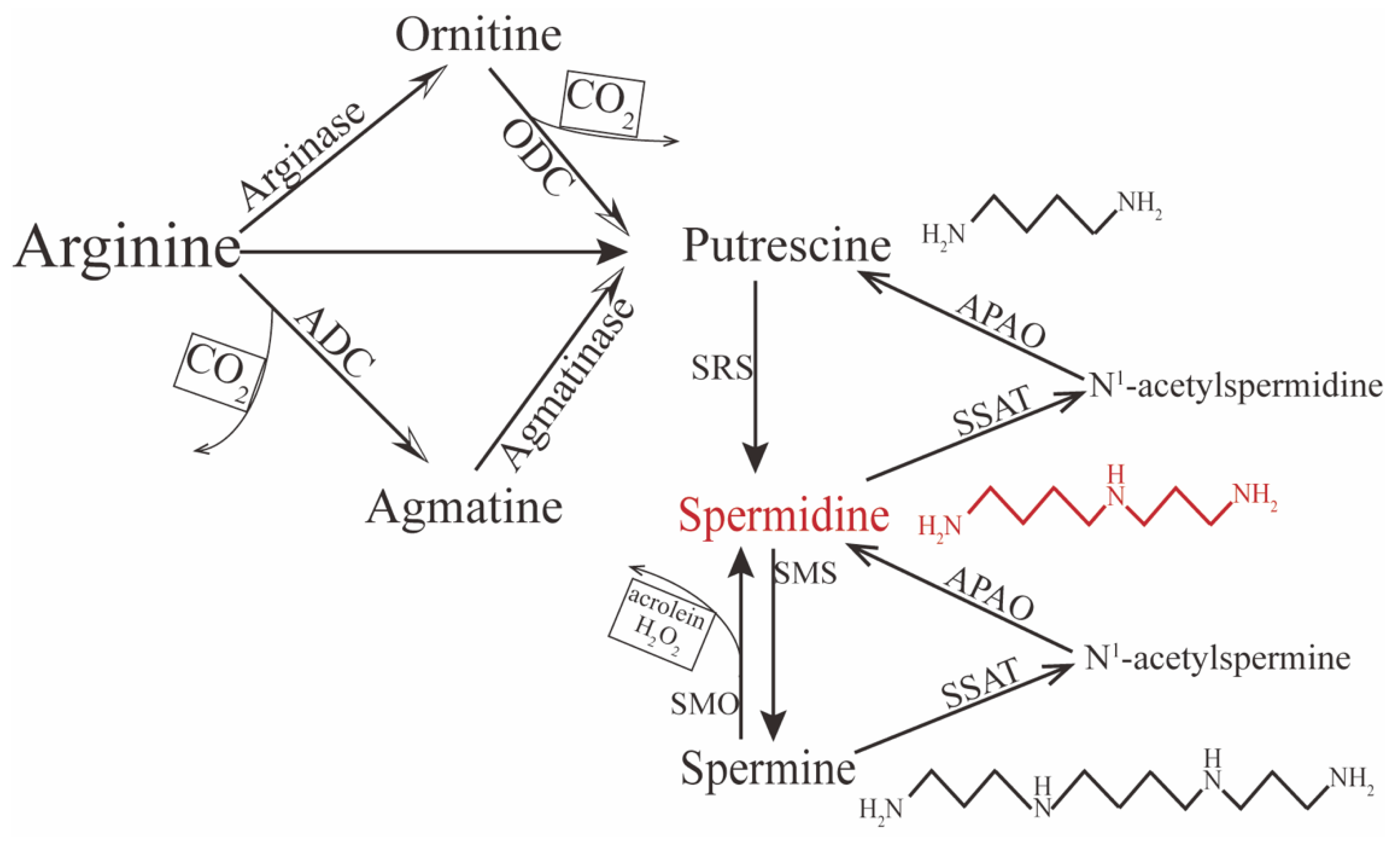
3. Biofunctions of Spermidine
References
- Patocka, J.; Kuehn, G.D. Natural Polyamines and Their Biological Consequence in Mammals. Acta Med. (Hradec Kral.) 2000, 43, 119–124.
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The Quest to Slow Ageing through Drug Discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532.
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438.
- Hofer, S.J.; Davinelli, S.; Bergmann, M.; Scapagnini, G.; Madeo, F. Caloric Restriction Mimetics in Nutrition and Clinical Trials. Front. Nutr. 2021, 8, 717343.
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Müller, M.; et al. Dietary Spermidine Improves Cognitive Function. Cell Rep. 2021, 35, 108985.
- Kang, X.; Li, C.; Xie, Y.; He, L.-L.; Xiao, F.; Zhan, K.-B.; Tang, Y.-Y.; Li, X.; Tang, X.-Q. Hippocampal Ornithine Decarboxylase/Spermidine Pathway Mediates H2S-Alleviated Cognitive Impairment in Diabetic Rats: Involving Enhancment of Hippocampal Autophagic Flux. J. Adv. Res. 2021, 27, 31–40.
- Zhang, Y.; Yin, J.; Zhang, L.; Qi, C.-C.; Ma, Z.-L.; Gao, L.-P.; Wang, D.-G.; Jing, Y.-H. Spermidine Preconditioning Ameliorates Laurate-Induced Brain Injury by Maintaining Mitochondrial Stability. Neurol. Res. 2017, 39, 248–258.
- Eisenberg, T.; Abdellatif, M.; Zimmermann, A.; Schroeder, S.; Pendl, T.; Harger, A.; Stekovic, S.; Schipke, J.; Magnes, C.; Schmidt, A.; et al. Dietary Spermidine for Lowering High Blood Pressure. Autophagy 2017, 13, 767–769.
- Messerer, J.; Wrede, C.; Schipke, J.; Brandenberger, C.; Abdellatif, M.; Eisenberg, T.; Madeo, F.; Sedej, S.; Mühlfeld, C. Spermidine Supplementation Influences Mitochondrial Number and Morphology in the Heart of Aged Mice. J. Anat. 2021, in press.
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic Polyamine Metabolism Regulates Epithelial Proliferation and Macrophage Differentiation in the Colon. Nat. Commun. 2021, 12, 2105.
- Ma, L.; Ni, L.; Yang, T.; Mao, P.; Huang, X.; Luo, Y.; Jiang, Z.; Hu, L.; Zhao, Y.; Fu, Z.; et al. Preventive and Therapeutic Spermidine Treatment Attenuates Acute Colitis in Mice. J. Agric. Food Chem. 2021, 69, 1864–1876.
- Morón, B.; Spalinger, M.; Kasper, S.; Atrott, K.; Frey-Wagner, I.; Fried, M.; McCole, D.F.; Rogler, G.; Scharl, M. Activation of Protein Tyrosine Phosphatase Non-Receptor Type 2 by Spermidine Exerts Anti-Inflammatory Effects in Human THP-1 Monocytes and in a Mouse Model of Acute Colitis. PLoS ONE 2013, 8, e73703.
- Johnson, D.A.; Fields, C.; Fallon, A.; Fitzgerald, M.E.C.; Viar, M.J.; Johnson, L.R. Polyamine-Dependent Migration of Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1228–1233.
- Kim, D.H.; Kim, J.-H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Cha, H.-J.; Park, C.; Hong, S.H.; Kim, G.-Y.; et al. Spermidine Attenuates Oxidative Stress-Induced Apoptosis via Blocking Ca2+ Overload in Retinal Pigment Epithelial Cells Independently of ROS. Int. J. Mol. Sci. 2021, 22, 1361.
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.-T.; Turner, D.J.; Bass, B.L.; Wang, J.-Y. Regulation of Adherens Junctions and Epithelial Paracellular Permeability: A Novel Function for Polyamines. Am. J. Physiol. Cell Physiol. 2003, 285, C1174–C1187.
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108.
- Hofer, S.J.; Liang, Y.; Zimmermann, A.; Schroeder, S.; Dengjel, J.; Kroemer, G.; Eisenberg, T.; Sigrist, S.J.; Madeo, F. Spermidine-Induced Hypusination Preserves Mitochondrial and Cognitive Function during Aging. Autophagy 2021, 17, 2037–2039.
- Kalač, P. Health Effects and Occurrence of Dietary Polyamines: A Review for the Period 2005-Mid 2013. Food Chem. 2014, 161, 27–39.
- Soulet, D.; Gagnon, B.; Rivest, S.; Audette, M.; Poulin, R. A Fluorescent Probe of Polyamine Transport Accumulates into Intracellular Acidic Vesicles via a Two-Step Mechanism. J. Biol. Chem. 2004, 279, 49355–49366.
- Moriyama, Y.; Hatano, R.; Moriyama, S.; Uehara, S. Vesicular Polyamine Transporter as a Novel Player in Amine-Mediated Chemical Transmission. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183208.
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912.
- Malpica-Nieves, C.J.; Rivera-Aponte, D.E.; Tejeda-Bayron, F.A.; Mayor, A.M.; Phanstiel, O.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The Involvement of Polyamine Uptake and Synthesis Pathways in the Proliferation of Neonatal Astrocytes. Amino Acids 2020, 52, 1169–1180.
- Fan, J.; Feng, Z.; Chen, N. Spermidine as a Target for Cancer Therapy. Pharmacol. Res. 2020, 159, 104943.
- Çelik, V.K.; Kapancık, S.; Kaçan, T.; Kaçan, S.B.; Kapancık, S.; Kılıçgün, H. Serum Levels of Polyamine Synthesis Enzymes Increase in Diabetic Patients with Breast Cancer. Endocr. Connect. 2017, 6, 574–579.
- Pegg, A.E.; Casero, R.A. Current Status of the Polyamine Research Field. Methods Mol. Biol. Clifton N. J. 2011, 720, 3–35.
- Alfarhan, M.; Liu, F.; Shan, S.; Pichavaram, P.; Somanath, P.R.; Narayanan, S.P. Pharmacological Inhibition of Spermine Oxidase Suppresses Excitotoxicity Induced Neuroinflammation in Mouse Retina. Int. J. Mol. Sci. 2022, 23, 2133.
- Muth, A.; Madan, M.; Archer, J.J.; Ocampo, N.; Rodriguez, L.; Phanstiel, O. Polyamine Transport Inhibitors: Design, Synthesis, and Combination Therapies with Difluoromethylornithine. J. Med. Chem. 2014, 57, 348–363.
- Wang, Y.; Devereux, W.; Woster, P.M.; Stewart, T.M.; Hacker, A.; Casero, R.A. Cloning and Characterization of a Human Polyamine Oxidase That Is Inducible by Polyamine Analogue Exposure. Cancer Res. 2001, 61, 5370–5373.
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the Hallmarks of Aging. Ageing Res. Rev. 2021, 72, 101468.
- Ramot, Y.; Tiede, S.; Bíró, T.; Abu Bakar, M.H.; Sugawara, K.; Philpott, M.P.; Harrison, W.; Pietilä, M.; Paus, R. Spermidine Promotes Human Hair Growth and Is a Novel Modulator of Human Epithelial Stem Cell Functions. PLoS ONE 2011, 6, e22564.
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzì, R.M. Spermidine Rescues the Deregulated Autophagic Response to Oxidative Stress of Osteoarthritic Chondrocytes. Free Radic. Biol. Med. 2020, 153, 159–172.
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine Endows Macrophages Anti-Inflammatory Properties by Inducing Mitochondrial Superoxide-Dependent AMPK Activation, Hif-1α Upregulation and Autophagy. Free Radic. Biol. Med. 2020, 161, 339–350.
- Carriche, G.M.; Almeida, L.; Stüve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. Regulating T-Cell Differentiation through the Polyamine Spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348.
- Baek, A.R.; Hong, J.; Song, K.S.; Jang, A.S.; Kim, D.J.; Chin, S.S.; Park, S.W. Spermidine Attenuates Bleomycin-Induced Lung Fibrosis by Inducing Autophagy and Inhibiting Endoplasmic Reticulum Stress (ERS)-Induced Cell Death in Mice. Exp. Mol. Med. 2020, 52, 2034–2045.
- Wang, J.; Li, S.; Wang, J.; Wu, F.; Chen, Y.; Zhang, H.; Guo, Y.; Lin, Y.; Li, L.; Yu, X.; et al. Spermidine Alleviates Cardiac Aging by Improving Mitochondrial Biogenesis and Function. Aging 2020, 12, 650–671.
- Jamwal, S.; Singh, S.; Kaur, N.; Kumar, P. Protective Effect of Spermidine against Excitotoxic Neuronal Death Induced by Quinolinic Acid in Rats: Possible Neurotransmitters and Neuroinflammatory Mechanism. Neurotox. Res. 2015, 28, 171–184.
- Ghosh, I.; Sankhe, R.; Mudgal, J.; Arora, D.; Nampoothiri, M. Spermidine, an Autophagy Inducer, as a Therapeutic Strategy in Neurological Disorders. Neuropeptides 2020, 83, 102083.
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The Machinery of Macroautophagy. Cell Res. 2014, 24, 24–41.
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741.
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the Cell Biology of Age-Related Disease. Nat. Cell Biol. 2018, 20, 1338–1348.
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, eaan2788.
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in Aging and Disease. Aging 2011, 3, 716–732.
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control EIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.
- Ni, Y.-Q.; Liu, Y.-S. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging Dis. 2021, 12, 1948–1963.
- Madeo, F.; Pietrocola, F.; Eisenberg, T.; Kroemer, G. Caloric Restriction Mimetics: Towards a Molecular Definition. Nat. Rev. Drug Discov. 2014, 13, 727–740.
- Mackeh, R.; Lorin, S.; Ratier, A.; Mejdoubi-Charef, N.; Baillet, A.; Bruneel, A.; Hamaï, A.; Codogno, P.; Poüs, C.; Perdiz, D. Reactive Oxygen Species, AMP-Activated Protein Kinase, and the Transcription Cofactor P300 Regulate α-Tubulin Acetyltransferase-1 (ATAT-1/MEC-17)-Dependent Microtubule Hyperacetylation during Cell Stress. J. Biol. Chem. 2014, 289, 11816–11828.
- Pällmann, N.; Braig, M.; Sievert, H.; Preukschas, M.; Hermans-Borgmeyer, I.; Schweizer, M.; Nagel, C.H.; Neumann, M.; Wild, P.; Haralambieva, E.; et al. Biological Relevance and Therapeutic Potential of the Hypusine Modification System. J. Biol. Chem. 2015, 290, 18343–18360.
- Lee, H.; Kim, D.H.; Hwangbo, H.; Kim, S.Y.; Ji, S.Y.; Kim, M.Y.; Shim, J.-H.; Leem, S.-H.; Hyun, J.W.; Kim, G.-Y.; et al. The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5. Pharmaceutics 2021, 13, 1439.
- Ghisalberti, C.A.; Borzì, R.M.; Cetrullo, S.; Flamigni, F.; Cairo, G. Soft TCPTP Agonism-Novel Target to Rescue Airway Epithelial Integrity by Exogenous Spermidine. Front. Pharmacol. 2016, 7, 147.
- Yuan, H.; Wu, S.-X.; Zhou, Y.-F.; Peng, F. Spermidine Inhibits Joints Inflammation and Macrophage Activation in Mice with Collagen-Induced Arthritis. J. Inflamm. Res. 2021, 14, 2713–2721.
- Yuan, X.; Tian, G.G.; Pei, X.; Hu, X.; Wu, J. Spermidine Induces Cytoprotective Autophagy of Female Germline Stem Cells in Vitro and Ameliorates Aging Caused by Oxidative Stress through Upregulated Sequestosome-1/P62 Expression. Cell Biosci. 2021, 11, 107.




