Spermidine is a polyamine with the chemical formula C7H19N3. It exists in ribosomes and living tissues and has various metabolic functions in living organisms. Spermidine was originally isolated from semen. Polyamines like spermidine, which are polycationic fatty amines and contain many functions, have important effects on cell survival. Spermidine synchronizes biological processes (such as Ca2+, Na+, K+ -ATPase), maintains membrane potential and controls intercellular pH and volume. Spermidine-mediated processes such as Ca2+ recruitment via the glutamatergic N-methyl-d-aspartate receptor (NMDA receptor). This receptor is associated with the activation pathways of nitric oxide synthase, cGMP/PKG and reduced Na+,K+-ATPase activity at cerebral cortex synapses.
Natural polyamines consist of spermine, spermidine, and putrescine
[1]. Spermidine is well-known as an anti-aging agent and exerts several protective effects
[2]. Numerous studies on human and animal models have shown it to delay the aging of the whole organism and extend the median lifespan
[3][4][3,4]. In the central nervous system, spermidine improved cognitive function in diabetic murine and drosophila aging models
[5][6][5,6] and ameliorated neuron injury in an ischemic preconditioned murine model
[7]. In the cardiovascular system, it controls high blood pressure and prevents heart failure in murine and human beings
[3][8][9][3,8,9]. In the immune system, it helps to maintain mucosal homeostasis of the intestine, which is under excessive inflammation
[10][11][12][10,11,12]. It also facilitates the regeneration and migration of epithelial cells, thus protecting barrier function in some cases, such as retinal pigment epithelial cells (RPEs) and colon epithelial cells
[10][13][14][15][10,13,14,15].
2. Metabolism of Spermidine
Spermidine [N-(3-aminopropyl)1,4-diaminobutane], a small molecule with a relative molecular weight of 145.25 Daltons, is abundant in a broad palette of plant and animal food
[16][24]. The amount of spermidine in the human body is maintained through absorption of nutrients, intracellular biosynthesis, and microbial production in the gut
[17][18][25,26]. Clinically, as a pharmacological caloric restriction mimetic (CRM), its low toxicity yet strong efficacy has been elucidated in clinical trials. Because of different dietary habits, there is regional variation in the recommended daily minimum of spermidine ranging from 5 to 15 mg
[4].
Spermidine is distributed in almost all human tissue and can be synthesized endogenously. In the internal environment, it exhibits a net positive charge, so it cannot transport directly across the cell membrane; therefore, exogenous spermidine is taken up from the extracellular space through active transportation and endocytosis
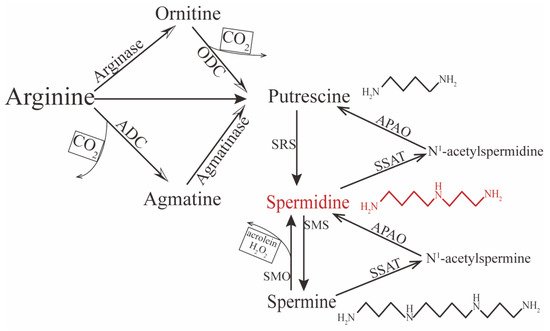
[19][20][27,28]. In contrast, endogenous spermidine can be synthesized via two principal pathways. One concerns the formation of putrescine (the precursor of spermidine) from L-arginine by two chemical reactions (
Figure 1). In mammalian cells, putrescine can be synthesized directly via ornithine decarboxylation under catalysis from the rate-limiting enzyme ornithine decarboxylase (ODC). The other pathway is decarboxylation of arginine to agmatine by arginine decarboxylase (ADC), which is then converted into putrescine via the enzyme agmatinase
[21][22][23][24][29,30,31,32]. Conversion between polyamines can also occur. For instance, spermine can gradually decompose into spermidine and putrescine under the catalysis of spermidine/spermine-N1-acetyltransferase (SSAT)—N1-acetylpolyamine oxidase (APAO)
[25][33]; it can also be directly converted to spermidine under the action of spermine oxidase (SMO), but this process may generate harmful compounds such as hydrogen peroxide (H
2O
2) and acrolein, which could be toxic and induce oxidative stress in excessive accumulation
[26][27][28][34,35,36]. Therefore, the spermidine level in vivo is determined by polyamines, including spermine and putrescine, predominantly initiated by the amino acids ornithine, methionine, and arginine.
Figure 1.
Polyamine’s synthesis and catabolism in mammals.
3. Biofunctions of Spermidine
Spermidine has a wide range of biofunctions: anti-aging, autophagy induction, cell proliferation activation, anti-inflammation, anti-oxidation, and ion channel regulation
[14][29][30][31][32][33][34][35][36][37][14,37,38,39,40,41,42,43,44,45].
Mechanically, aging enhances the formation of some proteins and organelles, which are regarded as damaged or unnecessary cytoplasmic components
[38][39][46,47]. Aging cells cannot degrade them, while autophagy delivers these components into lysosomes, which function as a complex intracellular degradation system
[40][48]. Therefore, autophagy induction has been recognized as an effective strategy to extend life, while autophagy deficiency may shorten it
[29][37]. Spermidine, a well-known autophagy inducer, upregulates autophagy-related genes
[41][42][23,49] and the expression of transcription factors (e.g., elF5A and TFEB
[43][50]). Spermidine can also inhibit protein acetylation by reducing the expression of E1A-associated protein p300 (EP300), which promotes the directly acetylation of multiple autophagy-essential proteins and stimulates the deacetylation of tubulin by indirectly inhibiting a-tubulin acetyltransferase 1 (aTAT1)
[44][45][46][51,52,53]. In addition, spermidine has been proven to be a precursor for the essential enzymatic modification of eIF5A
[47][54]. It may also play a positive role in stem cell function, such as the proliferation and migration of epithelial cells or slow-proliferating primary cultures
[13][31][48][49][13,17,39,55], by stimulating. them to differentiate and prevent their senescence
[41][23], partly through promoting entry into the S/G2-M phases of the cell cycle
[14][30][14,38]. Regarding anti-inflammation, spermidine downregulates proinflammatory cytokines such as IL-6, TNF-α, and IL-1β. It does this by inducing macrophage polarization and CD4
+ T cell differentiation in an autophagy-dependent manner to modulate the amount and function of immune cells
[32][33][50][40,41,56]. When cells or tissues are confronted with oxidative or endoplasmic reticulum stress, spermidine acts as an antioxidant as well as a specific [Ca
2+]
I chelator that rescues cells from Ca
2+ overload induced by oxidative stress thereby preventing apoptosis
[14][31][34][51][14,39,42,57]. It significantly reduced oxidative markers and endoplasmic reticulum stress-related proteins in cell models that simulated bone joint and lung fibrosis in an autophagy-dependent manner
[31][34][39,42]. The supplementation of spermidine also maintains mitochondrial homeostasis by upregulating the expression of sirtuin 1 (SIRT1), which deacetylates peroxisome proliferator-activated receptor γ coactivator-1 α (PGC-1α), a key regulator of mitochondrial biogenesis and function. As a result, the accumulation of ROS induced by H
2O
2 during the protection of cardiac aging is inhibited
[35][43]. Additionally, spermidine has the potential for neuroprotection by modulating the ion channel to inhibit the N-methyl-D-aspartate receptor (NMDAr). It is a glutamate receptor that mediates glutamate’s excitatory role in the pathogenesis of some diseases of the central nervous system (e.g., epilepsy) because it acts on NR2B subunits of NMDAr to inhibit Ca
2+ influx and excitotoxicity. NMDAr can also decrease inducible nitric oxide synthase (iNOS) and NO, which are triggered by NMDAr activation
[36][37][44,45].