Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lorenzo Biscardi | -- | 2423 | 2022-08-01 12:33:00 | | | |
| 2 | Sirius Huang | Meta information modification | 2423 | 2022-08-02 03:13:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zama, D.; Totaro, C.; Biscardi, L.; Rocca, A.; Turroni, S.; Brigidi, P.; Lanari, M. Role of the Gut–Lung Axis in RTIs. Encyclopedia. Available online: https://encyclopedia.pub/entry/25731 (accessed on 05 March 2026).
Zama D, Totaro C, Biscardi L, Rocca A, Turroni S, Brigidi P, et al. Role of the Gut–Lung Axis in RTIs. Encyclopedia. Available at: https://encyclopedia.pub/entry/25731. Accessed March 05, 2026.
Zama, Daniele, Camilla Totaro, Lorenzo Biscardi, Alessandro Rocca, Silvia Turroni, Patrizia Brigidi, Marcello Lanari. "Role of the Gut–Lung Axis in RTIs" Encyclopedia, https://encyclopedia.pub/entry/25731 (accessed March 05, 2026).
Zama, D., Totaro, C., Biscardi, L., Rocca, A., Turroni, S., Brigidi, P., & Lanari, M. (2022, August 01). Role of the Gut–Lung Axis in RTIs. In Encyclopedia. https://encyclopedia.pub/entry/25731
Zama, Daniele, et al. "Role of the Gut–Lung Axis in RTIs." Encyclopedia. Web. 01 August, 2022.
Copy Citation
Respiratory tract infections (RTIs) are a major cause of morbidity and mortality worldwide. These infections can range from mild upper respiratory tract infections to severe and life-threatening pneumonia. A plethora of studies have described the relationship between gut microbiota (GM) composition and function and the development of various human diseases, focusing especially on the role of GM in regulating the immune system. Although the underlying mechanisms are not yet fully understood, there is mounting evidence that GM can modulate the immune function in distant mucosal sites such as the respiratory system, and therefore play a role in the development of RTIs.
gut microbiota
gut–lung axis
respiratory tract infections
RTIs
1. Introduction
The concept that gut microbiota (GM) is vital not only for the gastrointestinal tract, but also for the overall health of the human organism, has been well studied and is widely accepted today [1][2][3][4][5][6]. On the other hand, the presence of microbes in body niches previously thought to be sterile, such as the lungs, has only been demonstrated in recent years and their relevance is still questionable [7][8][9]. The GM composition is dominated by mostly obligate anaerobic bacteria, with the main phyla being Firmicutes and Bacteroidetes, while others, such as Actinobacteria and Proteobacteria, are represented to a lesser extent.
In contrast, the airways harbor a distinct microbial ecosystem, featured by less diversity and richness and composed mainly of the genera Prevotella, Streptococcus, Veillonella, Fusobacterium, and Haemophilus [6][10]. Gut and lung microbial communities are known to influence each other during infections in an interesting, still not completely understood, crosstalk called the “gut-lung axis” (Table 1).
Table 1. Studies exploring the mechanisms through which the gut microbiota interacts with the lungs and affects the response to infections.
| Study, Year | Aim | Results |
|---|---|---|
| Trompette et al., 2018 [11] |
Effect of an HFD, through SCFA modulation in influenza-infected mice. |
|
| McAleer et al., 2017 [12] |
Effects of GM composition on lung immunity. |
|
| Maslowski et al., 2009 [13] |
The role of SCFAs in the regulation of the immune response by GPR43 activation. |
|
| Antunes et al., 2019 [10] |
The role of SCFAs in RSV infection. |
|
| Steed et al., 2017, [14] |
Evaluation of DAT in protecting from influenza through type I IFN. |
|
| Ichinohe et al., 2011 [15] |
The function of GM in influenza A-infected mice. |
|
| Thackray et al., 2018 [16] |
Effects of oral antibiotics in flaviviridae-infected mice. |
|
| Wypich et al., 2019 [6] |
Analysis of the gut–lung axis and its communication pathways. |
|
| Brown et al., 2017 [17] |
GM signaling that protects against respiratory infections. |
|
DAT: desaminotyrosine; GPCR: G protein-coupled receptor; GM: gut microbiota; GM-CSF: granulocyte–macrophage colony-stimulating factor; HFD: high-fiber diet; IL: interleukin; ILC: innate lymphoid cell; IFN: interferon; SCFA: short-chain fatty acid; TLR: Toll-like receptor, NLR: Nod-like receptor.
2. Innate Immunity
In the gut–lung axis, the key to the interaction could potentially be the many metabolites produced and/or influenced by GM, which have been shown to have a regulatory function of the immune system.
Among these, short-chain fatty acids (SCFAs), which include acetate, propionate, and butyrate, are end-products of the fermentation of fibers by commensal bacteria in the cecum and the colon. SCFAs not used for energy by intestinal epithelial cells are released into the circulation and reach distant sites, such as adipose tissue, liver, pancreas, lungs, brain, and bone marrow [4][6][18]. Butyrate administration has been shown to enhance the response to influenza infection, both by stimulating the production of macrophage precursors and by downregulating the damage due to the infiltration of neutrophils [6][11]. Trompette et al. demonstrated in mouse models that a high-fiber diet (HFD), resulting in the production of SCFAs in the gastrointestinal tract, is protective against influenza through two mechanisms described below. HFD mice showed enhanced bone marrow hematopoiesis of Ly6c-patrolling monocytes, which mature in macrophages with a limited ability to generate chemokine ligand 1 (CXCL1) chemokines in the lungs. The lower presence of CXCL1 resulted in a reduced recruitment of neutrophils to the airways and a subsequent lower release of mediators responsible for the damage in the lung tissue. Simultaneously, SCFAs boosted the function of CD8+ T cells that displayed an increased ability to kill virus-infected cells [11]. It should also be remembered that SCFAs are involved in the activation of G protein-coupled receptors (GPR40-43, also known respectively as free fatty acid receptors (FFAR1-3) [12][13][19]), which are necessary for the resolution of the inflammatory response, as seen in studies on GPR43-deficient mice [13][20]. In particular, Maslowski et al. demonstrated that in GPR43-deficient mice, SCFAs did not induce either the release of reactive oxygen species (ROS) from neutrophils or the enhancement of their phagocytic activity, as was instead noted in GPR43 mice.
Type I interferons (IFNs) are pleiotropic cytokines involved in another important signaling pathway in viral immunity. Increasing evidence shows that GM can regulate host immune homeostasis, as well as the response to injury and bacterial infections, through type I IFN signaling. In this regard, Antunes et al. studied the effects of acetate pre-treatment in RSV-infected mice. Acetate has been shown to modulate IFN-β production through type 1 IFN receptor engagement during RSV infection both in human pulmonary cells in vitro and in vivo in the lower airways of infected mice. This effect was not observed in the absence of the type 1 IFN receptor. Mice pre-treated with acetate showed an undetectable lung viral load, a reduced cell number in the bronchoalveolar lavage, and a decrease in inflammatory cells in the lungs.
Recently, Antunes et al. also demonstrated in animal models that pre-treatment with acetate led to faster recoveries from RSV infection by enhancing the expression of retinoic acid-inducible gene I (RIG-I) and interferon-stimulated genes. In RIG-I knockout mice these protective effects were not noted, proving that RIG-I plays a role in the recovery from RSV infections. Moreover, the high stool level of acetate was significantly associated with less severe bronchiolitis, higher oxygen saturation, and diminished lasting fever [10].
Similar results were found by administering propionate and butyrate, confirming the potential prophylactic role of SCFAs in RSV infection.
Desaminotyrosine (DAT) has also been shown to be a metabolite involved in modulating the immune response. DAT derives from flavonoids, a group of polyphenolic compounds enriched in certain foods, such as tea, citrus fruits and juices, and in wine [21]. The obligate anaerobe Clostridium orbiscindens is responsible for the metabolism of flavonoids with the consequent production of DAT. DAT protects against influenza through the amplification of type I IFN signaling [14] and is an essential mediator of phagocytes in the lung. Without DAT, the influenza virus causes inflammation and severe disease. GM-derived metabolites therefore have the potential to modulate the innate immune response by increasing the lung production of type I IFN. These findings imply that prior colonization by specific bacteria and a flavonoid-enriched diet are both key components that modulate immune response to influenza infection.
There are also other known immunomodulatory gut metabolites, such as indole derivatives, niacin, urolithin A, and pyruvic and lactic acids, which may affect respiratory health, but their role is not yet understood [6].
In addition, GM commensal bacteria appear to be able to directly prime innate immunity in response to an influenza infection in the lungs. Ichinohe et al., for example, showed that GM composition can regulate virus-specific CD4+ and CD8+ T cell generation and antibody responses following respiratory influenza virus infection [15]. Oral antibiotic treatment resulted in defective CD4+ T, CD8+ T, and B-cell immunity following intranasal infection with influenza virus, while distal (rectal) inoculation of Toll-like receptor (TLR) agonists (TLR9 and TLR3 agonists) could rehabilitate the pulmonary immune reaction to infection in antibiotic-treated mice. GM is also involved in the stimulation of the transcription and translation of pro-interleukin-1beta (ILs) and in the activation of the caspase that transforms pro-IL1beta into the mature one. In the study of Ichinohe et al., IL1beta levels in bronchoalveolar lavage were altered in influenza-infected mice that were treated with antibiotics. Therefore, GM integrity (i.e., maintenance of a eubiotic configuration) is required to maintain adequate pro-IL1beta expression, while antibiotic treatment for respiratory infections could have a deleterious effect on the immune response to the influenza virus.
Moreover, the integrity of GM results to be necessary for the correct function of dendritic cells (DCs). Negi et al. found that antibiotics’ treatment reduced the expression of macrophage-inducible C-type lectin (mincle) in lung DCs during Mycobacterium tuberculosis (Mtb) infections [22]. Specifically, DCs that expressed a lower presence of mincle were less capable of activating naïve CD 4 T cells with the result of the increased survival of Mtb. In vivo, the administration of mincle ligan restored the immune defect of lung DCs [22].
3. Adaptative Immunity
GM is also involved in adaptative immunity, enhancing the function of CD8+ T cells that play a key role against viral infections [11][15][16]. In particular, a greater availability of glucose, derived from the catabolic processes of GM, stimulates the effector function of these cells by increasing the differentiation and activation process [11][23].
Another piece of evidence of GM involvement comes from in vivo models with antibiotic exposure. Thackray et al. have shown that antibiotic-treated mice have an impaired response to flavivirus infection due to a decreased number of specific CD8+ T cells in the spleen and popliteal lymph nodes. Similarly, Ichinohe et al. showed reduced numbers of not only CD8+ T, but also of CD4+ T and B-cells in mice treated with oral antibiotics [15][16].
The role of GM in modulating the severity of RSV infection has been also demonstrated in animal models. Fonseca et al. observed that oral Lactobacillus johnsonii supplementation in mice led to an attenuated immune response to RSV. This effect was mediated by the recirculation of systemic metabolites including docosahexanoic acid, decreasing airway Th2 cytokines, dendritic cell function, and improving T-regulatory cells [24].
The gut–lung axis also appears to operate through the direct movement of cells from the gut to the respiratory system [6]. This migration of immune cells was demonstrated in the study by Huang et al., where, after connecting the circulation of two mice, labeled type 2 innate lymphoid cells (ILC2s) from a mouse were found in the lungs of both mice [25]. This did not occur in mice treated with antibiotics and contaminated with the gastrointestinal nematode Nippostrongylus brasiliensis [8], thus suggesting the relevance of an intact GM.
There is also evidence of a crucial role for GM against lung bacterial infections [17][26]. Brown et al. highlighted that GM can protect against Streptococcus pneumoniae and Klebsiella pneumoniae infections. To prove this, mice were treated with antibiotics and subsequently infected with the bacteria. In treated mice, increased lung bacterial counts and a diminished pulmonary production of granulocyte–macrophage colony-stimulating factor (GM-CSF) and chemokine ligands 1 and 2 (CXCL1 and CXCL2) were noted. To demonstrate the role of GM-CSF in the response to respiratory infections, mice were given GM-CSF-neutralizing antibodies, and this caused impaired alveolar macrophage development. Defects in bacterial clearance could be restored in antibiotic-treated mice by administering recombinant GM-CSF but not neutralizing CXCL1 and CXCL2. Moreover, antibiotic-induced GM disruption resulted in secondary IgA deficiency in both mice and humans, which led to an enhanced susceptibility to Pseudomonas aeruginosa-induced pneumonia [6]. Another confirmation of the important role of GM in respiratory bacterial infections comes from the study by Gauguet et al., who showed that the segmented filamentous bacteria present in GM play a role in defending against Staphylococcus aureus pneumonia by stimulating the production of the Th17 cytokine, IL22, and by enhancing the number of neutrophils in the lungs [27].
This fascinating immunological crosstalk along the gut–lung axis has not yet been fully explored, but the already known mechanisms underlying these interactions could be used in the near future for novel evidence-based strategies for the prevention and treatment of respiratory tract infections (RTIs) (Figure 1).
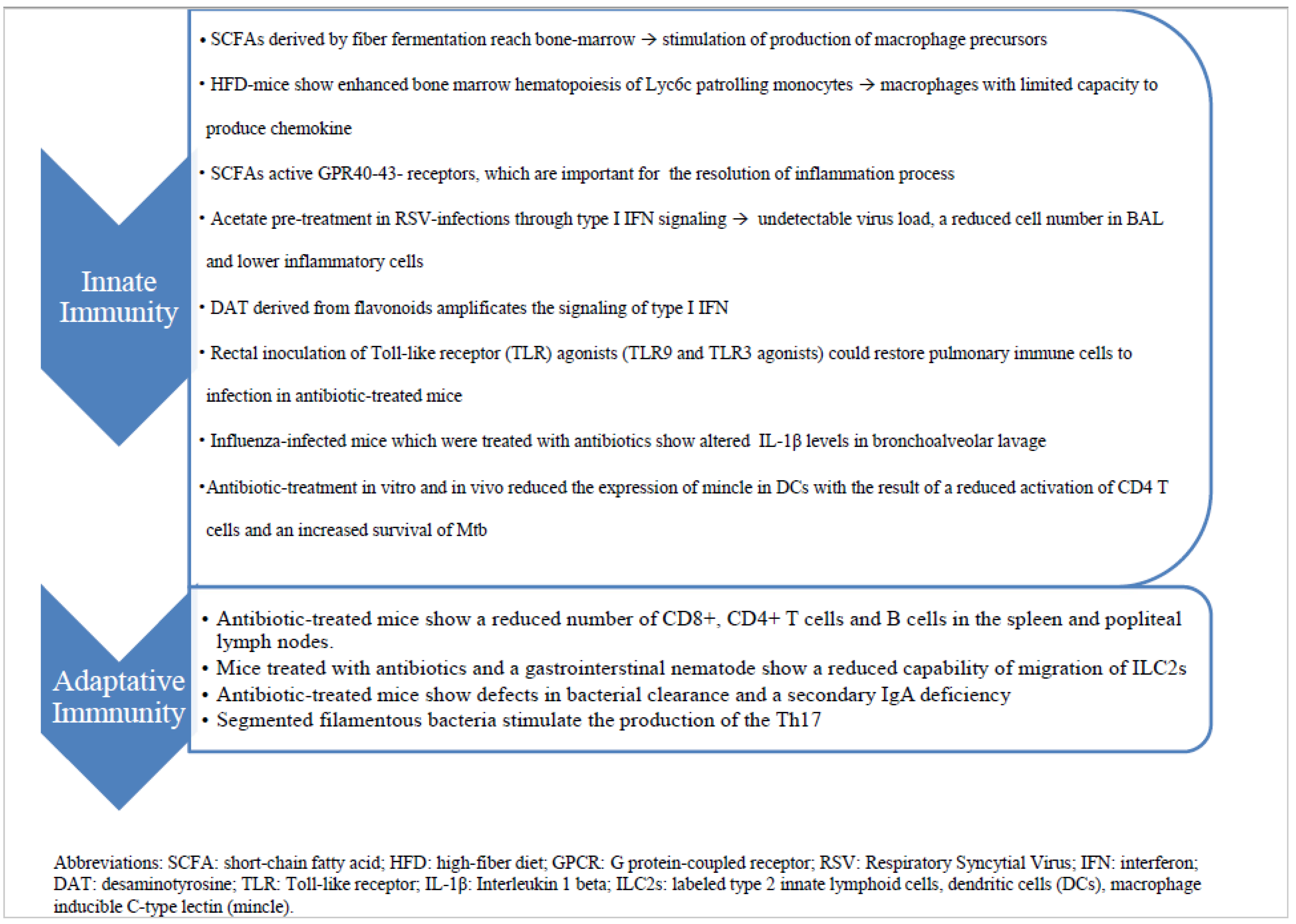
Figure 1. The diagram summarizes the immune mechanisms by which gut–lung axis works and potentially influences the answer to infections.
References
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63.
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400.
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota-Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2018, 61, 47–61.
- Sencio, V.; Machado, M.G.; Trottein, F. The lung–gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304.
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850.
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290.
- De Martino, M.; Ballotti, S. The child with recurrent respiratory infections: Normal or not? Pediatr. Allergy Immunol. 2007, 18, 13–18.
- Huang, Y.J.; Charlson, E.S.; Collman, R.G.; Colombini-Hatch, S.; Martinez, F.D.; Senior, R.M. The role of the lung microbiome in health and disease: A national heart, lung, and blood institute workshop report. Am. J. Respir. Crit. Care Med. 2013, 187, 1382–1387.
- Lee, Y.-T.; Kim, K.-H.; Hwang, H.S.; Lee, Y.; Kwon, Y.-M.; Ko, E.-J.; Jung, Y.-J.; Lee, Y.-N.; Kim, M.-C.; Kang, S.-M. Innate and adaptive cellular phenotypes contributing to pulmonary disease in mice after respiratory syncytial virus immunization and infection. Physiol. Behav. 2016, 176, 139–148.
- Antunes, K.H.; Stein, R.T.; Franceschina, C.; da Silva, E.F.; de Freitas, D.N.; Silveira, J.; Mocellin, M.; Leitão, L.; Fachi, J.L.; Pral, L.P.; et al. Short-chain fatty acid acetate triggers antiviral response mediated by RIG-I in cells from infants with respiratory syncytial virus bronchiolitis. EBioMedicine 2022, 77, 103891.
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di, Y.; Schilter, H.C.; Rolph, M.S.; MacKay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502.
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359.
- Thackray, L.B.; Handley, S.A.; Gorman, M.J.; Poddar, S.; Briseño, C.G.; Theisen, D.J.; Tan, Q.; Hykes, B.L., Jr.; Lucas, T.M.; Desai, C.; et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep. 2018, 22, 3440–3453.
- Brown, R.L.; Sequeira, R.P.; Clarke, T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017, 8, 1512.
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345.
- Hirasawa, A.; Hara, T.; Katsuma, S.; Adachi, T.; Tsujimoto, G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 2008, 31, 1847–1851.
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; Dos Santos, A.Á.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273.
- Ock, K.C.; Sang, J.C.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252.
- Negi, S.; Pahari, S.; Bashir, H.; Agrewala, J.N. Gut microbiota regulates mincle mediated activation of lung dendritic cells to protect against mycobacterium tuberculosis. Front. Immunol. 2019, 10, 1142.
- Jacobs, S.R.; Herman, C.E.; MacIver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J. Immunol. 2008, 180, 4476–4486.
- Yagi, K.; Asai, N.; Huffnagle, G.B.; Lukacs, N.W.; Fonseca, W. Early-Life Lung and Gut Microbiota Development and Respiratory Syncytial Virus Infection. Front. Immunol. 2022, 13, 1–10.
- Huang, Y.; Mao, K.; Chen, X.; Sun, M.-A.; Kawabe, T.; Li, W.; Usher, N.; Zhu, J.; Urban, J.F., Jr.; Paul, W.E.; et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 2020, 359, 114–119.
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509.
- Gauguet, S.; D’Ortona, S.; Ahnger-Pier, K.; Duan, B.; Surana, N.K.; Lu, R.; Cywes-Bentley, C.; Gadjeva, M.; Shan, Q.; Priebe, G.P.; et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect. Immun. 2015, 83, 4003–4014.
More
Information
Subjects:
Pediatrics; Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
769
Revisions:
2 times
(View History)
Update Date:
02 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





