Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hossam S. El-Beltagi | -- | 3604 | 2022-07-29 19:09:44 | | | |
| 2 | Catherine Yang | Meta information modification | 3604 | 2022-08-01 03:34:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds. Encyclopedia. Available online: https://encyclopedia.pub/entry/25679 (accessed on 07 March 2026).
El-Beltagi HS, Mohamed AA, Mohamed HI, Ramadan KMA, Barqawi AA, Mansour AT. Phytochemical and Potential Properties of Seaweeds. Encyclopedia. Available at: https://encyclopedia.pub/entry/25679. Accessed March 07, 2026.
El-Beltagi, Hossam S., Amal A. Mohamed, Heba I. Mohamed, Khaled M. A. Ramadan, Aminah A. Barqawi, Abdallah Tageldein Mansour. "Phytochemical and Potential Properties of Seaweeds" Encyclopedia, https://encyclopedia.pub/entry/25679 (accessed March 07, 2026).
El-Beltagi, H.S., Mohamed, A.A., Mohamed, H.I., Ramadan, K.M.A., Barqawi, A.A., & Mansour, A.T. (2022, July 29). Phytochemical and Potential Properties of Seaweeds. In Encyclopedia. https://encyclopedia.pub/entry/25679
El-Beltagi, Hossam S., et al. "Phytochemical and Potential Properties of Seaweeds." Encyclopedia. Web. 29 July, 2022.
Copy Citation
Seaweeds have been employed as source of highly bioactive secondary metabolites that could act as key medicinal components. Seaweeds have many uses: they are consumed as fodder, and have been used in medicines, cosmetics, energy, fertilizers, and industrial agar and alginate biosynthesis. The beneficial effects of seaweed are mostly due to the presence of minerals, vitamins, phenols, polysaccharides, and sterols, as well as several other bioactive compounds. These compounds seem to have antioxidant, anti-inflammatory, anti-cancer, antimicrobial, and anti-diabetic activities.
antioxidant activity
functional foods
health benefits
seaweeds
1. Introduction
Seaweeds have received lot of attention in recent years because of their incredible potential. Seaweeds are essential nutritional sources and traditional medicine components [1]. Marine macroalgae, sometimes known as seaweeds, are microscopic, multicellular, photosynthetic eukaryotic creatures. Based on their coloration and depending on their taxonomic classification, they can be classified into three groups: Rhodophyta (red), Phaeophyceae (brown), and Chlorophyta (green). The global variety of all algae (micro and macro) is estimated to consist of over 164,000 species with roughly 9800 of them being seaweeds, just 0.17% of which have been domesticated for commercial exploitation [2]. In recent years, seaweed has gained in popularity, making it a more versatile food item that may be used directly or indirectly in preparation of dishes or beverages [3]. Many types of seaweed are edible, they provide the body with a different variety of vitamins and critical minerals (including iodine) when consumed as food, and some are also high in protein and polysaccharides [4].
Seaweeds are now used in several industrial products as raw materials such as agar, algin, and carrageenan, but they are still widely consumed as food in several nations [5]. Seaweeds are frequently subjected to harsh environmental conditions with no visible damage; as a result, the seaweed generates a wide variety of metabolites (xanthophylls, tocopherols, and polysaccharides) to defend itself from biotic and abiotic factors such as herbivory or mechanical aggression at sea [6]. Please note that the content and diversity of seaweed metabolites are influenced by abiotic and biotic factors such as species, life stage, nutrient enrichment, reproductive status, light intensity exposure, salinity, phylogenetic diversity, herbivory intensity, and time of collection; thus, fully exploiting algal diversity and complexity necessitates knowledge of environmental impacts as well as a thorough understanding of biological and biochemical variability [7][8].
Seaweeds and their products are particularly low in calories but high in vitamins A, B, B2, and C, minerals, and chelated micro-minerals (selenium, chromium, nickel, and arsenic), as well as polyunsaturated fatty acids, bioactive metabolites, and amino acids [9]. Although current research revealed that the amount of specific secondary metabolites dictates the effective bioactive potential of seaweeds, phenolic molecules are prevalent among these secondary metabolites [10]. Furthermore, integrating seaweed into one’s daily diet has been linked to a lower risk of a range of disorders, including digestive health and chronic diseases such as diabetes, cancer, or cardiovascular disease, according to research mentioned by [11]. As a result, incorporating seaweed components into the production of novel natural drugs is one of the goals of marine pharmaceuticals, a new discipline of pharmacology that has evolved in recent decades.
2. Seaweed Resources
The word “seaweed” has no taxonomic importance; rather, it is a popular term for the common large marine algae.
2.1. Brown Seaweeds
Phaeophyceae have not been well investigated, despite the fact that they have been shown to offer several health benefits. Fucoxanthin (Fuco), the principal marine carotenoid (Car), is a commercially important component of brown seaweeds, in addition to sodium alginate. Fuco contains anti-inflammatory properties. The presence of the xanthophyll pigment fucoxanthin, which is higher than chlorophyll-a, chlorophyll-c, -carotene, and other xanthophylls, gives these seaweeds their brown color [12]. Because of its bigger size and ease of collecting, brown seaweed is used in animal feed more often than other algae species. Brown algae are the largest seaweeds, with some species reaching up to 35–45 m in length and a wide range of shapes. Ascophyllum, Laminaria, Saccharina, Macrocystis, Nereocystis, and Sargassum are the most prevalent genera. Sargassum as a member of brown seaweeds is low in protein, but high in carbs and easily accessible minerals. They are high in beta-carotene and vitamins, and they are free of anti-nutrients [13].
2.2. Red Seaweeds
These algae are red because of the pigments phycoerythrin and phycocyanin. The walls are made of carrageenan and cellulose agar. Both of these polysaccharides with a lengthy chain are commonly employed in the industry. Coralline algae, which secrete calcium carbonate on the surface of their cells, are an important category of red algae. Chondrus, Porphyra, Pyropia, and Palmaria are some of the most common red algae genera. The antioxidant activity of Phaeophyta (brown seaweeds) is higher than that of green and red algae [14].
2.3. Green Seaweeds
The majority of the species are aquatic, living in both freshwater and marine habitats. The green color of these algae is due to chlorophyll-a or chlorophyll-b. Some of them are terrestrial, meaning they grow in soil, trees, or rocks. Ulva is one of the most common green seaweeds. Ulva, Cladophora, Enteromorpha, and Chaetomorpha are the most common genera. Green algae thrive in regions with lots of light, including shallow waterways and tide pools. Ulva sp. has a high protein content (typically > 15%) and a low energy content and is abundant in both soluble and insoluble dietary fiber (glucans) [15]. The main types of seaweeds are shown in Figure 1.

Figure 1. Three example species of brown (a) red (b) and green (c) seaweeds. Adapted from ref. [16] obtained from mdpi journals.
3. Bioactive Compounds
The chemical composition of algae varies depending on the species, cultivation location, meteorological conditions, and harvesting period. Because of the broad diversity of compounds produced by seaweeds, they are currently considered to be prospective organisms for contributing new physiologically active chemicals for the production of novel food (nutraceutical), cosmetic (cosmeceutical), and medical compounds. Polyphenolic compounds, carotenoids, minerals, vitamins, phlorotannins, peptides, tocotrienols, proteins, tocopherols, and carbohydrates (polysaccharides) are considered to be a great variety of bioactive compounds (Figure 2).
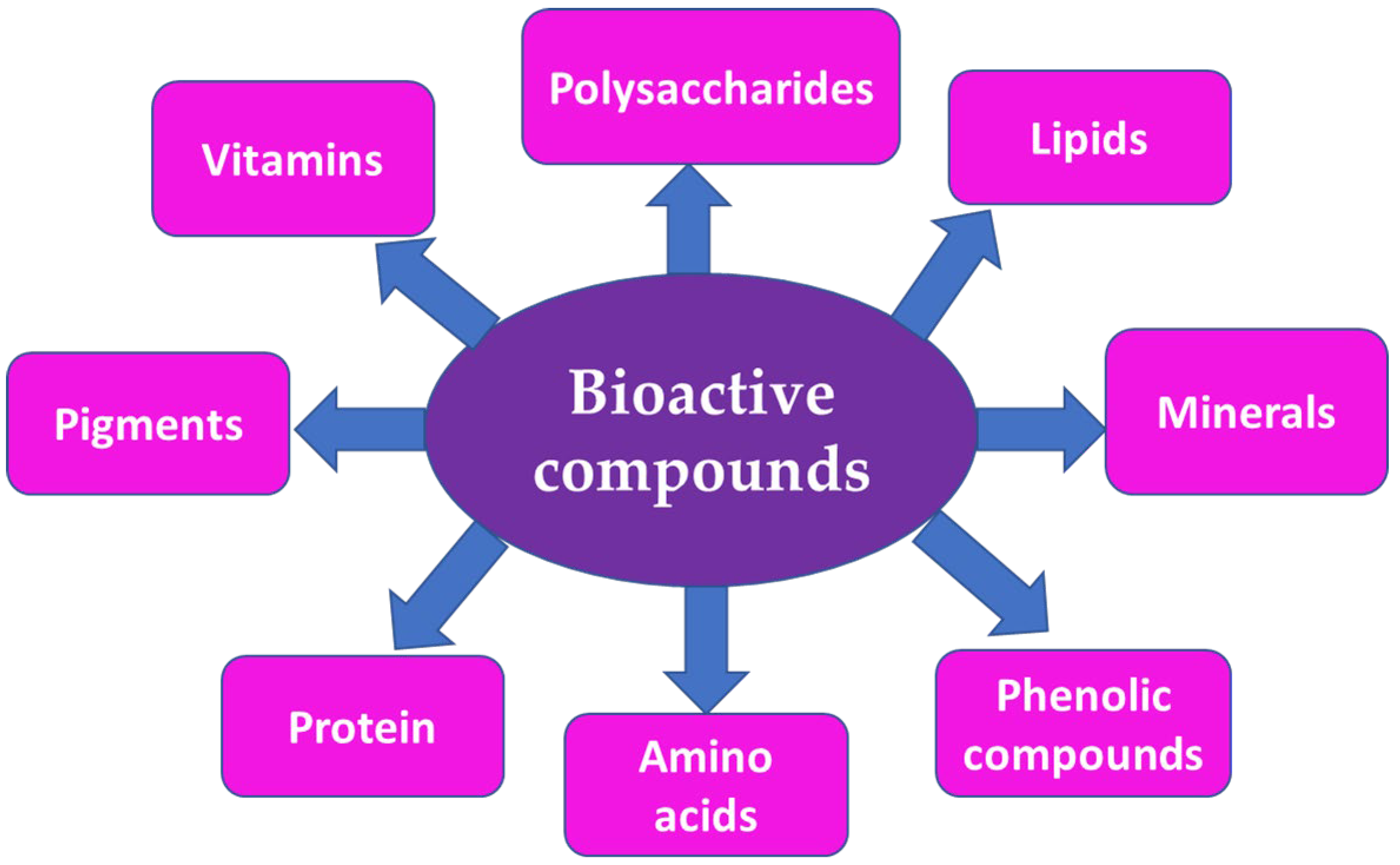
Figure 2. Main bioactive compounds from marine seaweeds.
3. Biological Activities
3.1. Antioxidant Activity
An imbalance in the creation and neutralization of free radicals causes oxidative stress, which leads to a variety of degenerative illnesses [17]. Several free radicals, particularly reactive oxygen species (ROS), were created in living organisms as a result of metabolic activity, and hence have an impact on health (Figure 3). ROS were formed in form of hydrogen peroxide (H2O2), superoxide radical (O2−), hydroxyl radical (·OH), or nitric oxide (NO). Oxidative stress causes unconscious or prominent enzyme activation, as well as oxidative damage for cellular systems [18]. ROS attack or damage important macromolecules including lipids membrane, proteins, or DNA, resulting in a variety of conditions include inflammatory or neurodegenerative diseases, diabetes mellitus, cancer, or severe tissue injuries [19][20] (Figure 3).
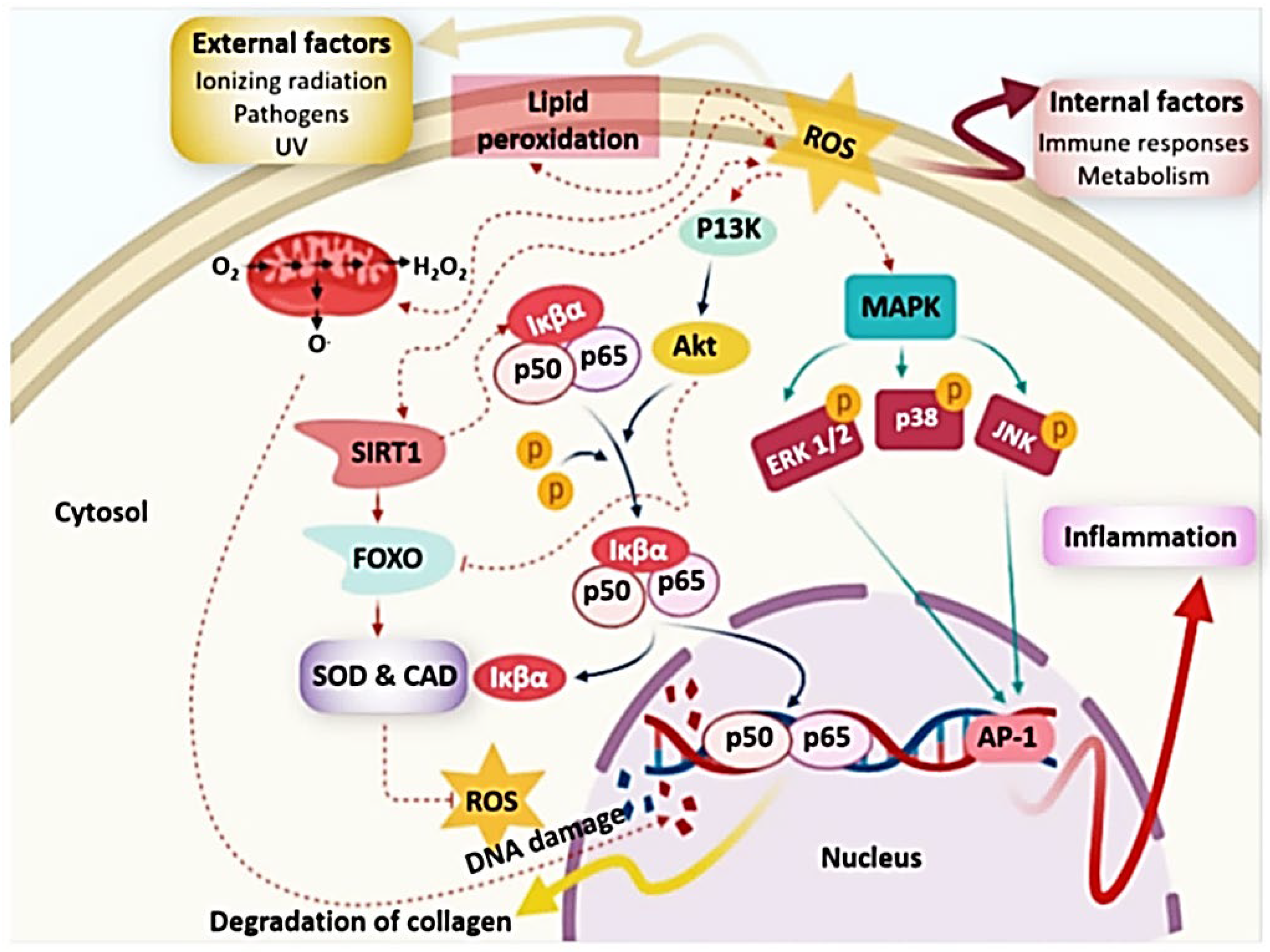
Figure 3. Damage caused via reactive oxygen species (ROS). Adapted from ref. [21] obtained from mdpi journals.
Antioxidants may have a favorable impact on human health because they may protect the body from damage caused via reactive oxygen species (ROS) [22]. To determine the antioxidant activity of marine derived bioactive peptides, researchers used electron spin resonance spectroscopy as well as intracellular free-radical scavenging assays.
ROS can produce several detrimental biological events, such as DNA oxidative lesions, membrane peroxidation, structural changes in proteins and functional carbohydrate, and so on. All of these structural and functional changes have direct clinical effects, speed up the aging process while also causing pathological phenomena, such as increased capillary permeability and impaired blood cell function [23]. All of these antioxidant systems behave differently depending on their structure and characteristics, whether hydrophilic or lipophilic, and where they are located (intracellular or extracellular, in cell or organelles membrane, in the cytoplasm, etc.). All of the above processes work in concert to establish a network that protects live cells from the damaging impacts of reactive oxygen species (ROS).
Figure 4 represents reactive oxygen species and neutralization with several biomolecules [24]. Hydrophobic amino acids in peptide chain contribute to their possible antioxidant effect [25]. Seaweeds also include nutraceutical and medicinal chemicals such phenols that have antioxidant activity. Polyphenols generated by seaweeds received special attention because their pharmacological action and broad range of health-promoting advantages, as polyphenols play a vital role in a variety of seaweed biological activities. Seaweed phenolic compounds are metabolites with hydroxylated aromatic rings that are chemically defined as molecules. In this context, Al-Amoudi et al. [14] stated that sulfated polysaccharides from three marine algae (Phaeophyta Sargassum crassifolia (S), Chlorophyta Ulva lactuca (U) and Rhodophyta Digenea simplex (D) exert antioxidant activity.
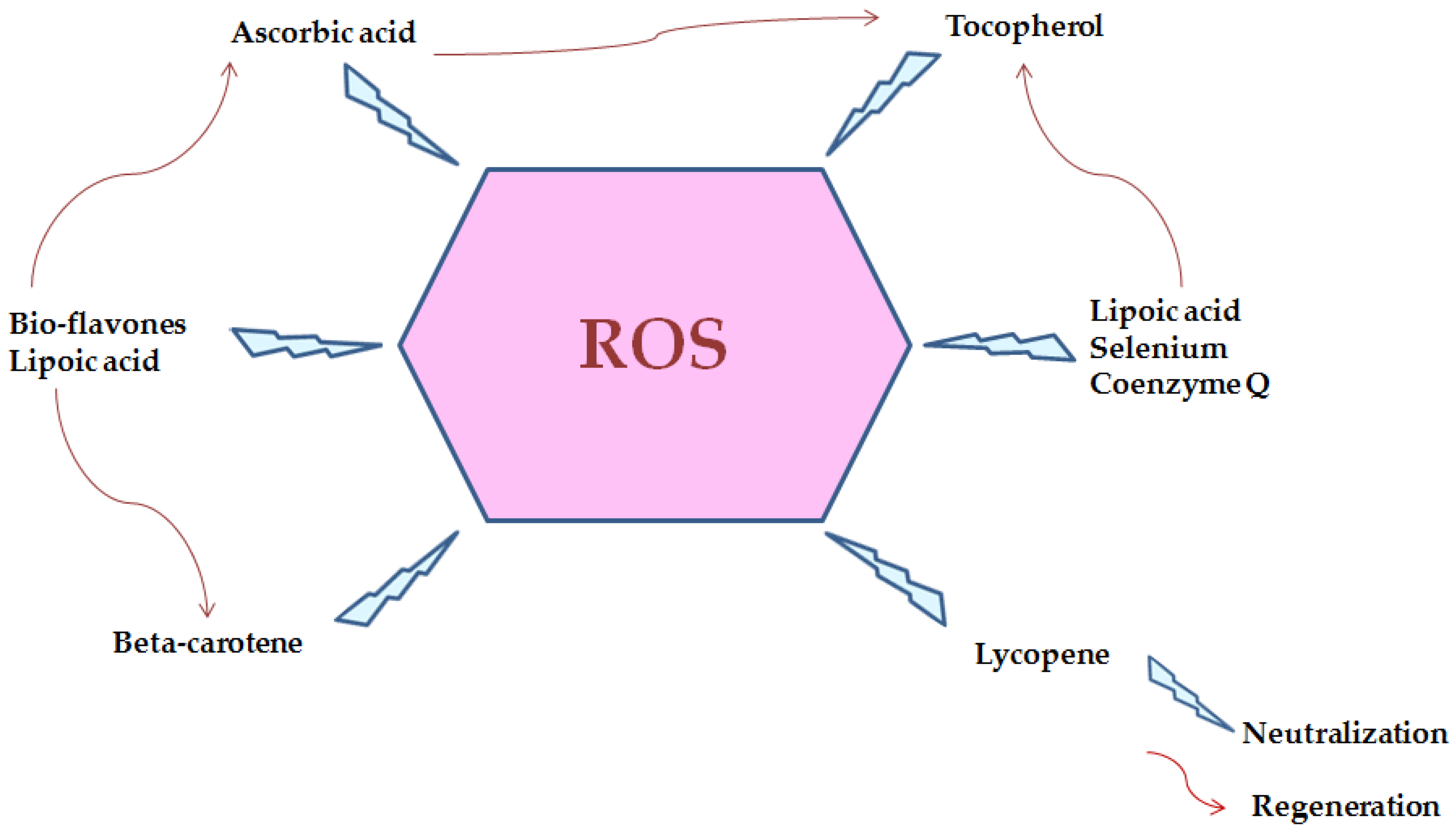
Figure 4. Reactive oxygen species and neutralization by several biomolecules.
3.2. Antimicrobial Activity
Susceptibility testing of harmful microorganisms (e.g., bacteria and fungi) in the presence of possible compounds of interest is the focus of antimicrobial activity assays. Microbial infections can cause life-threatening illnesses, resulting in millions of deaths each year. Despite the fact that the discovery of penicillin pushed many aggressive pathogenic bacteria back, many strains evolved and developed remarkable resistance mechanisms to most antibiotics [26]. Variable solvents have different antibacterial action depending on their solubility and polarity. As a result, chemical compounds isolated from various seaweeds should be optimized for antibacterial activity by selecting the optimal solvent system [27]. Micro-algal cell-free extracts are already being studied as food and feed additives in an attempt to replace synthetic antibacterial chemicals currently in use. According to Tuney et al. [28], the antibacterial action of the extract is attributable to various chemical agents found in the extract, such as flavonoids, triterpenoids, and other phenolic compounds or free hydroxyl groups. Extraction procedures, solvents used, and the time window in which samples were collected all have the potential to alter antibacterial activity [29]. A variety of organic solvents had previously been recommended for screening algae for antibacterial activity.
3.3. Anticancer Activity
Cancers are life-threatening diseases that are considered to be a major public health issue around the world [30][31]. Uncontrolled cell development spreads into the surrounding tissues, resulting in the formation of a tumor mass [32]. Much research has looked into the anticancer potential of natural compounds derived from seaweeds, as well as the signaling pathways involved in anticancer activity [33]. Because those secondary metabolites have no hazardous effects, they have seen a lot of progress in the treatment of numerous diseases, including cancer. Thymoquinone (TQ) is one of the most important bioactive elements of black seeds, and it has been found to have numerous health advantages, including cancer prevention and treatment. Following on this, Algotiml et al. [34] studied the effect of biosynthesized Red Sea marine algal silver nanoparticles AgNPs on anticancer and antibacterial properties and the authors stated that due to their relatively moderate side effects, marine resources are currently being increasingly examined for antibacterial and anticancer medication prospects.
In addition, kappa-carrageenan extracted from Hypnea musciformis (Hm-SP) decreased proliferation of MCF-7 or SH-SY5Y cancer cell lines [35]. Additionally, polysaccharides derived from Sargassum fusiforme (SFPS) reduced SPC-A-1 cell proliferation in vitro and tumor formation in vivo [36]. Additionally, Ji and Ji [37] found that commercial laminaran (400–1600 g/mL) inhibited the growth of human colon cancer LoVo cells through stimulating mitochondrial or DR pathways. Additionally, Fucoidans isolated from Undaria pinnatifida have anticancer potential comparable to commercial fucoidans in cell lines Hela (human cervical), PC-3 (human prostate), HepG2 (human hepatocellular liver carcinoma), or A549 (carcinomic human alveolar basal epithelial) [38]. Moreover, previous study reported that fucoidan isolated from Sargassum hemiphyllum may increase miR-29b expression in human hepatocellular carcinoma cells, which aids in the lowering of DNA methyltransferase 3B expression [39]. Moreover, Fucoidans from Fucus vesiculosus were revealed to have anticancer potential, inducing apoptosis in MC3 human mucoepidermoid carcinoma cells via caspase-dependent apoptosis signaling cascade [40] (Figure 5).
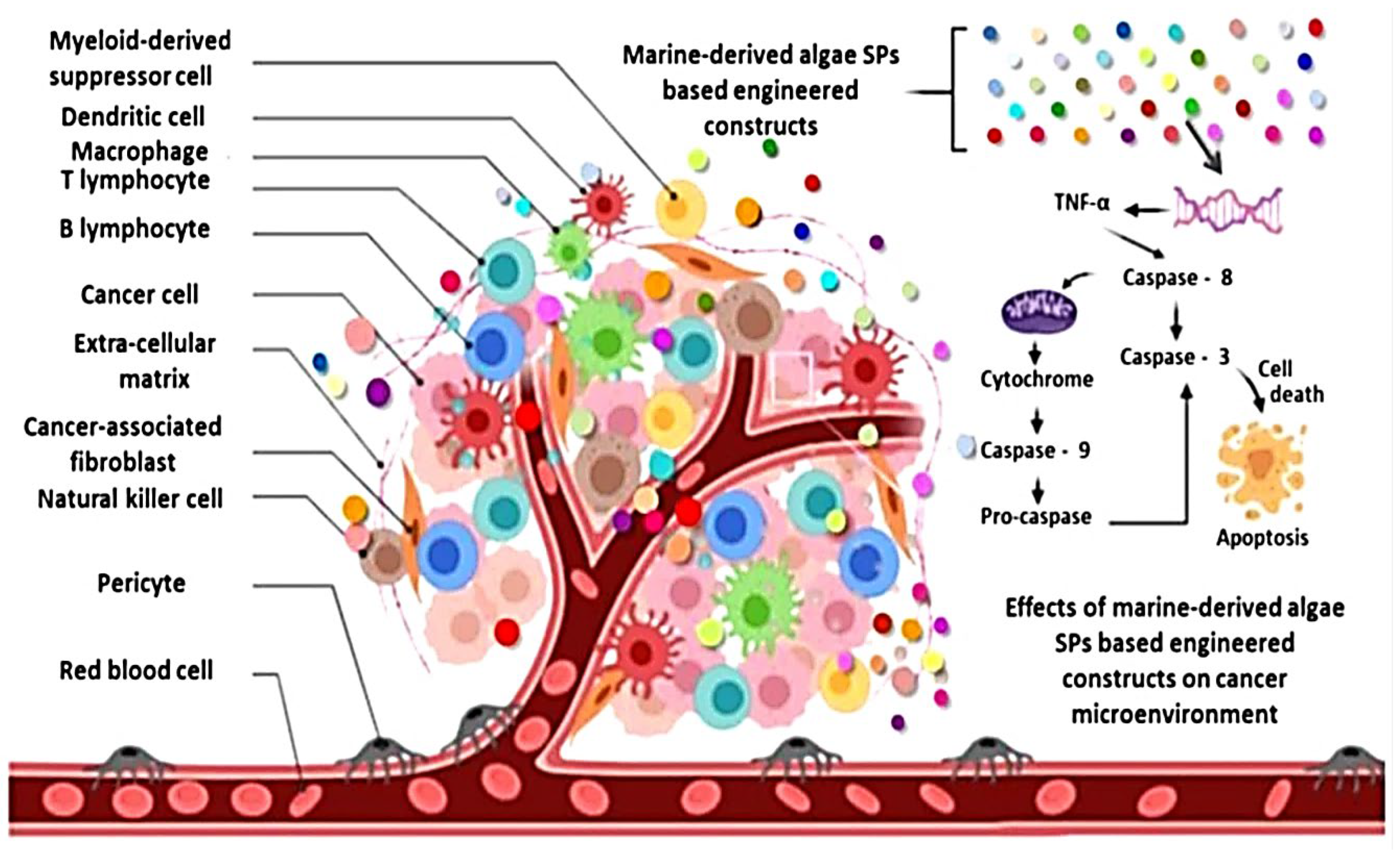
Figure 5. Demonstrate the ability of algal polysaccharide (SP)-based customized signals produced from sea algae to cause tumor cell death (apoptosis). Adapted from ref. [21] obtained from mdpi journals.
3.4. Antidiabetics Activity
As a result of an unhealthy lifestyle, obesity, and stress, diabetes is becoming a global illness. Additionally, obesity has been on the rise in Saudi Arabia as a result of changing lifestyles and socioeconomic status [40][41]. There is a close association between obesity and type 2 diabetes. Drugs that suppress the enzymes α-glucosidase and α-amylase, which break down starch into glucose before it is absorbed into the bloodstream, could be used to treat diabetes [42]. It is necessary to look for effective therapeutic natural medications with less side effects. Garcimartn et al. [43] showed that a α-glucosidase inhibitory effect on restructured pork treated with seaweeds such as Undaria pinnatifida, Himanthalia elongata, and Porphyra umbilicalis caused a reduction in the blood glucose absorption. Padina tetrastromatica phenolic extracts inhibited both α-glucosidase and α-amylase, with higher inhibition linked with a higher phenolic concentration in the extracts. The extracts inhibited α-glucosidase (IC50 value of 28.8 g mL−1) and -amylase (IC50 value of 47.2 g mL−1) by 38.9 and 26.8%, respectively [44]. Similarly, α-glucosidase inhibitory action was observed in methanol, ethanol, and acetone extracts of Durvillea antarctica, methanol extracts of Ulva sp., and acetone extracts of Lessonia spicata [45]. Methanol extracts of Padina tenuis (400 µg mL−1) and ethanol extract of Eucheuma denticulatum (10 mg mL−1) and Sargassum polycystum (10 mg mL−1) significantly inhibited α-amylase by 60%, 67%, and 46%, respectively [46]. Recently, the acetone extract (80%) of brown seaweed Turbinaria decurrens was studied for its antihyperglycemic effects in alloxan induced diabetic wistar male rats [47]. The results showed a significant reduction in postprandial blood glucose levels of seaweed extracts treated rats to 180.33 mg dL−1 and 225.33 mg dL−1 at the dose of 300 mg/kg body weight and 150 mg/kg body weight, respectively, compared to diabetic control (565.0 mg dL−1) and positive control (115.33 mg dL−1).
4. Seaweeds in Bio-Manufacturing Applications
Modern consumers are well aware of the nutritional value of food and the negative impact that synthetic preservatives may have worse effect on their health, so it is unsurprising that they prefer fresh and lightly preserved foods that are free of chemical preservatives, but contain natural compounds that may benefit their health [48].
4.1. Fertilizer and Soil Conditioners
Seaweed extracts have been frequently employed in agriculture in recent years to increase crop yield. This improvement is achieved by stimulating various physiological processes involved in plant growth and development, as well as improving final product quality (Figure 6). The use of traditional chemical fertilizers has expanded dramatically as result of world’s fast-growing population or ever-increasing food demand [49]. The usage of these chemical fertilizers, as well as their impacts, notably on environment, has become major source of worry [50]. As a result, farmers began to switch to organic farming rather than using synthetic agricultural fertilizers. Seaweeds are abundant or long-lasting resources discovered along the world’s coastlines, and they are important sources of food, feed, biofuels, cosmetics, fertilizers, nutraceuticals, and pharmaceuticals [51][52]. Due to their commercial importance or potential applications, seaweeds are used as fodder, cosmetics, human food, or biofertilizers [53]. Because of availability of various trace elements, vitamins, growth regulators, or amino acids, macroalgae extracts are currently being used as foliar sprays or presoaking for boosting growth or production of variety of plants, particularly crops [54]. Each year, more than 15 million tons of seaweed is produced, with much of it used as biofertilizers in agriculture or horticulture industries [55][56].
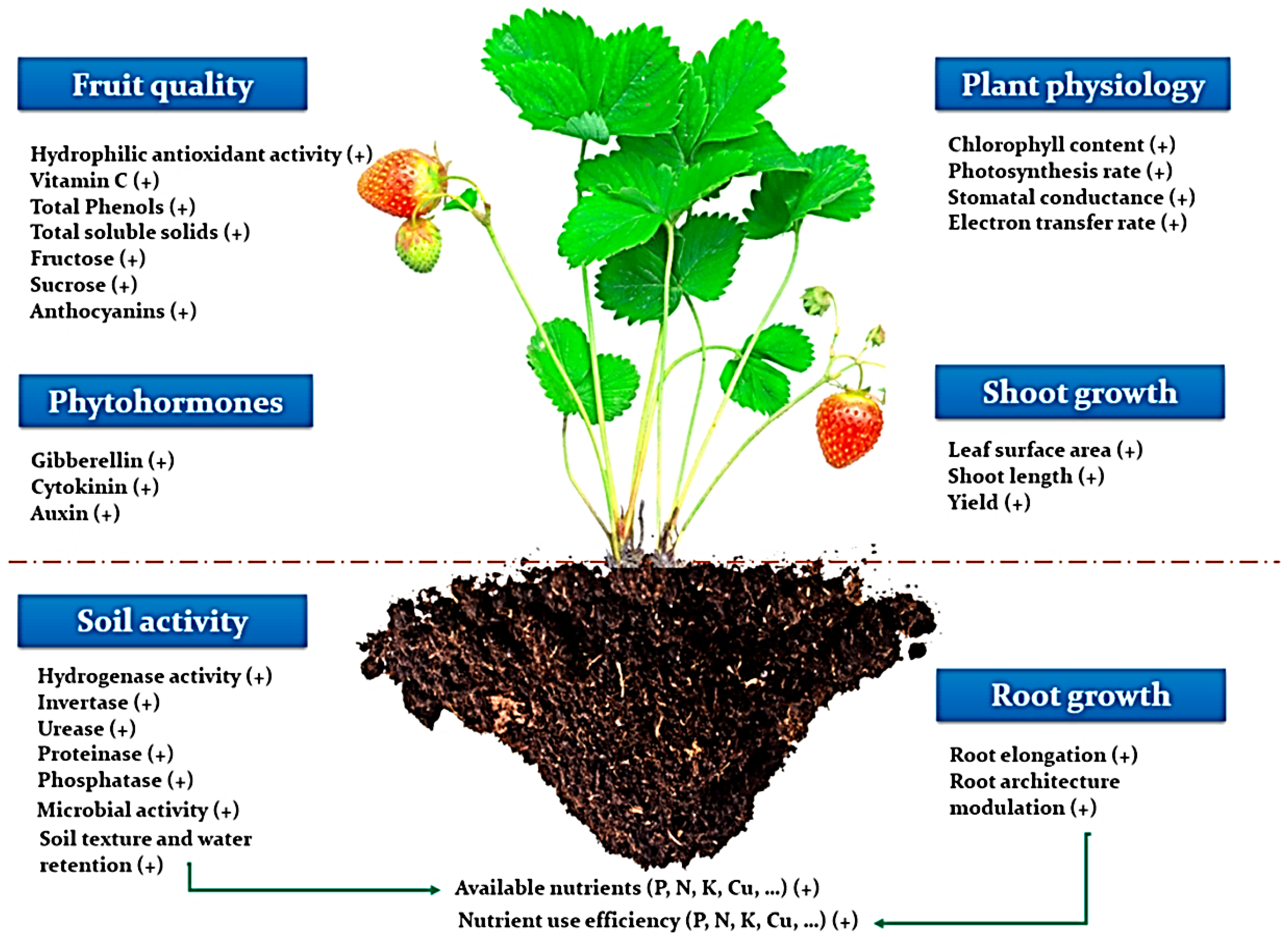
Figure 6. Illustration demonstrating beneficial effects of seaweed extracts on the entire soil-plant system. Such impacts include increased fruit quality and phytohormone content in plants, increased soil enzymatic activity, improved roots system, and overall physiological properties of plants. Adapted from ref. [57] obtained from mdpi journals.
4.2. Medical and Pharmaceutical Use
5.2.1. Biomedical Applications of Seaweeds
Bioactive chemicals found in seaweeds have features that make them appealing for biomedical applications. Many species of seaweeds have been employed in traditional medicine for a long time, notably in Asian nations, against goiter, nephritic disorders, anthelmintic, catarrh, and a few other ailments as medicaments or pharmaceutical auxiliaries, long before scientific study information [58]. Fucus vesiculosus has been used as a medicinal drug, primarily due to its iodine content, for obesity defects and goiters [58], for the treatment of sore knees [59], healing wounds [60], and also as herbal teas for their laxative effects [61].
Chondrus crispus (Rhodophyta) carrageens have been used as mucilage against diarrhea, dysentery, gastric ulcers, and as a component of several health teas, such as for colds, for a long time. Gelidium cartilagineum (Rhodophyta) has been used in pediatric medicine in Japan for colds and scrofula [62]. Ulva lactuca (Chlorophyta) has been used for gout and as an astringent in folk medicine [62]. Rhodophyta extracts are very promising natural chemicals that could be used in biomedicine. Many species of Asian seaweeds are employed in traditional medicine, including Gracilaria spp. (Rhodophyta), which is used as a laxative, Sargassum spp. (Phaeophyceae), which is used to treat Chinese influence, and Caloglossa spp., Codium spp., Dermonema spp., and Hypnea spp. (Rhodophyta) [63].
Carrageenans’ biological actions make them attractive candidates for future antitumoral therapeutics since they activate antitumor immunity [64]. Kappaphycus species (Rhodophyta), for example, are used to treat ulcers, headaches, and tumors [63]. Antitumoral efficacy of carrageenans derived from Kappaphycus striatum against human nasopharyngeal carcinoma, human gastric carcinoma, and cervical cancer cell lines [65]. The bioactivity of chemicals from various Laurencia species (Rhodophyta) was investigated. In vitro, certain halogenated metabolites of Laurencia papillosa showed action against Jurkat (acute lymphoblastic leukemia) human tumor cells [66]. Laurencia obtuse extracts, specifically three sesquiterpenes, have been extracted and tested against Ehrlich ascites cancer cells. The sesquiterpenes were found to have antitumoral action against Ehrlich ascites cells [67]. Gracilaria edulis ethanol extracts showed antitumor efficacy in mice with ascites tumors [68].
Undaria pinnatifida (Phaeophyceae) has anti-inflammatory qualities and can be used to treat postpartum depression in women. This alga can also be used to treat edema and as a diuretic. Celikler et al. [69] investigated the antigenotoxic effect of Ulva rigida extracts in human cells in vitro (Chlorophyta).
Seaweeds have been suggested as a way to avoid neurogen-erative illnesses in investigations over the last decade [70]. Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and Amyotrophic Lateral Sclerosis (ALS) are the most frequent [70]. According to Bauer et al., several studies highlighted the use of algal polysaccharides for the treatment of neurodegenerative illnesses [71]. Park et al. [72] found that mice treated with fucoidan extracts from Ecklonia cava had better memory and learning; consequently, the study implies favorable results in future human trials. In comparison to the control group, mice treated with polysaccharide isolated from Sargassum fusiforme demonstrated enhanced memory and cognition [73]. Dieckol and phlorofucofuroeckol, two phlorotannins from Ecklonia cava, are linked to an increase in the main central neurotransmitters in the brain, particularly Acetylcholine (ACh) [74]. Ahn et al. [75] investigated Eisenia bicyclis phlorotannins and found that 7-phloroeckol and phlorofucofuroeckol A were powerful neuroprotective agents against induced cytotoxicity, while eckol had a weaker impact.
4.2.2. Pharmaceutical Applications of Seaweeds
Bioactive chemicals from seaweeds are used in the pharmaceutical industry to help develop new formulations for revolutionary treatments and to replace synthetic components with natural ones. Bioactive chemicals found in seaweeds have important pharmacological properties, including anticoagulant, antioxidant, antiproliferative, antititumoral, anti-inflammatory, and antiviral effects [76].
Table 1. The potential pharmacological activity of brown, red and green seaweeds.
| Component | Properties/Activities | Seaweed | Doses | Models | References |
|---|---|---|---|---|---|
| Fucoxanthins | Antitumoral activity on lung cancer cells |
Laminaria japonica | 12.5–100 μM | Female and male (1:1 ratio) BALB/c nude mice (18–20 g; 6–8 weeks of age) | [77] |
| Antitumoral activity on MCF-7, HepG-2, HCT-116 cells | Colpomenia sinuosa, Sargassum prismaticum | 100 and 200 mg/kg | Paracetamol-administered rats (one dose of 1 g/kg) | [78] | |
| Antitumoral activity on SiHa, Malme-3M cells | Undaria pinnatifida | 1.5625, 6.25, 12.5, 25, 50, 80, 100 µM | Human cell lines | [79] | |
| Antimicrobial activity | Cladosiphon okamuranus | 2–2000 µg/mL. | Helicobacter pylori | [80] | |
| Antimicrobial activity | Laminaria japonica | 2, 3, 4, 5, 6, 7, and 7.5 mg/mL | Staphylococcus aureus, Escherichia coli | [81] | |
| Antimicrobial activity | Fucus vesiculosus | 2, 4, 6, 8 and 10 mg/mL | Staphylococcus aureus, Bacillus licheniformis, Escherichia coli, Staphylococcus epidermidis |
[82] | |
| Antiviral activity against ECHO-1, HIV-1, HSV-1, HSV-2 | Fucus evanescens | 200 μg/mL | Female outbred mice (16–20 g) | [83] | |
| Sulfate polysaccharide | Antiviral activity against HSV-1, HVS-2 |
Sargassum patens | 0.78–12.5 μg/mL | Vero cells (African green monkey kidney cell line) | [84] |
| Anti-obesity, antidiabetic activities | Gracilaria lemaneiformis | 5–10% Seaweed powder | Dawley laboratory rats (4 to 5 months old, 250–300 g) | [85] | |
| Phloroglucinol | Anti-inflammatory activity | Ecklonia cava | 1, 5, 10, 50 100 µM |
HT1080 and RAW264.7 cells |
[86] |
Fucoidans extracted from Laminaria cichorioides (Phaeophyceae) [87] and Fucus evanescens [88] behave like heparin in both in vitro and in vivo experiments, demonstrating anticoagulant activity by accelerating the development of antithrombin III to inhibit the effect against thrombin.
Seaweeds’ antiviral qualities make them an excellent alternative for improving the health of infected persons; also, their use in pharmaceuticals will provide new and natural antiviral drugs that can replace synthetic chemicals. Furthermore, when compared to the creation of synthetic antivirals, the use of bioactive components from seaweeds is less expensive [89]. Antiviral activity of macroalgae has been discovered to protect against a variety of viruses, including HIV, Herpes Simplex Virus (HSV), genital warts [90], and hepatitis C (HCV) [91]. HSV [92], Encephalomyocarditis virus, Influenza “A” virus [93], and human metapneumovirus [94] are only a few of the viruses that Chlorophyta species have been shown to be effective against. The antiviral action of macroalgae is linked to a variety of substances such as as fatty acids and diterpenes, but most notably to the presence of Seaweed bioactive compounds [95], which can inhibit virus multiplication or help the immune system combat viral infection.
4.3. Cosmetic Industry
Cosmetics and cosmeceuticals are commonplace therapies for improving the skin’s appearance and treating several dermatological problems. Seaweeds are a valuable component in product development because of their wide range of functional, sensory, and biological properties. Consumer demand for green or eco-friendly products has risen in recent years. This pattern can be seen in the globally competitive cosmetics industry, in need of natural, secure, or effective ingredients to make innovative skin care products [96]. The usage of seaweed-isolated compounds in cosmetic products rose steadily as a result of various scientific studies revealing prospective skincare properties of seaweed bio-actives. Biologically active substances include carotenoids, polysaccharides, phlorotannins, fatty acids, sterols, tocopherol, vitamins, phycocyanins, or phycobilins [97][98][99][100][101]. In this context, a Sargassum plagyophyllum extract was shown to have antioxidant and anti-collagenase that can considered to be potent pharmaceutical ingredient for anti-wrinkle cosmetics action [102][103][104][105]. As a form of polyphenol, phlorotannins contain a group of heterogeneous polymeric molecules with substantial chemical modifications and various chemical structures [106]. These molecules can play a key role in the interaction between the skin and UVR, such as preventing radiation from penetrating the skin and lowering inflammation, oxidative stress, DNA damage, and maintaining signaling pathways intact. They also attracted a lot of interest because of their participation in several phototoxic pathways and mechanisms [107]. Brown algae Sargassum fusiforme [108], Halidrys siliquosa [109], Padina australis [110], Sargassum coreanum [111], and Polycladia myrica [112] have been explored for using in cosmetic products.
References
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641.
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Science 2007, 316, 382–383. Available online: https://www.science.org/doi/10.1126/science.1138042 (accessed on 20 April 2007).
- Irkin, L.C.; Yayintas, Ö. Pharmacological Properties and Therapeutic Benefits of Seaweeds (A Review). Int. J. Trend Sci. Res. Dev. 2018, 2, 1126–1131.
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Chapman and Hall in Associate with Methuen: London, UK, 1980; p. 334.
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275.
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19.
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204.
- Alshehri, M.A.; Al Thabiani, A.; Alzahrani, O.; Ibrahim, A.A.S.; Osman, G.; Bahattab, O. DNA-barcoding and Species Identification for some Saudi Arabia Seaweeds using rbcL Gene. J. Pure Appl. Microbiol. 2019, 13, 2035–2044.
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Mar. Drugs 2013, 11, 3754–3776.
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250.
- Misurcová Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra species: A mini-review of its pharmacological and nutritional properties. J. Med. Food 2016, 19, 111–119.
- Ghosh, R.; Banerjee, K.; Mitra, A. Seaweeds in the Lower Gangetic Delta. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012.
- Misurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.-K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 567.
- Al-Amoudi, O.A.; Mutawie, H.H.; Patel, A.V.; Blunden, G. Chemical composition and antioxidant activities of Jeddah corniche algae. Saudi J. Biol. Sci. 2009, 16, 23–29.
- Edwards, M.; Hanniffy, D.; Heesch, S.; Hernández-Kantún, J.; Moniz, M.; Queguineur, B.; Ratcliff, J.; Soler-Vila, A.; Wan, A. Macroalgae Fact-Sheets; Soler-Vila, A., Moniz, M., Eds.; Irish Seaweed Research Group, Ryan Institute, NUI Galway: Galway, Ireland, 2012; p. 40.
- Mantri, V.A.; Kavale, M.G.; Kazi, M.A. Seaweed biodiversity of India: Reviewing current knowledge to identify gaps, challenges, and opportunities. Diversity 2020, 12, 13.
- Ul-Haq, I.; Butt, M.S.; Amjad, N.; Yasmin, I.; Suleria, H.A.R. Marine-Algal Bioactive Compounds: A Comprehensive Appraisal. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 71–80.
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 9, 12233.
- Sellimi, S.; Kadri, N.; Barragan-Montero, V.; Laouer, H.; Hajji, M.; Nasri, M. Fucans from a Tunisian brown seaweed Cystoseira barbata: Structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014, 66, 281–288.
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367.
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152.
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and control of its deleterious effects with melatonin and salicylic acid. Plants 2021, 28, 459.
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidney functions against lipidemic disease. Elec. J. Environ. Agricult. Food Chem. 2010, 9, 1117–1127.
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322.
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171.
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093.
- Salama, H.M.H.; Marraiki, N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi J. Biol. Sci. 2010, 17, 57–63.
- Tuney, I.; Cadirci, B.H.; Unal, D.; Sukatar, A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turkish J. Biol. 2006, 30, 171–175.
- Kandhasamy, M.; Arunachalam, K.D. Evaluation of in vitro antibacterial property of seaweeds of southeast coast of India. Afr. J. Biotechnol. 2008, 7, 1958–1961.
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 493–505.
- Hamed, M.M.; Abd El-Mobdy, M.A.; Kamel, M.T.; Mohamed, H.I.; Bayoumi, A.E. Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. Pharmacol. Online 2019, 2, 101–121.
- Hussain, E.; Wang, L.J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592.
- Gutierrez-Rodriguez, A.G.; Juarez-Portilla, C.; Olivares-Banuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447.
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022, 12, 2421.
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; De Almeida, A.A.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256.
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27.
- Ji, C.F.; Ji, Y.B. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol. Lett. 2014, 7, 1728–1732.
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydras. Polym. 2010, 81, 41–48.
- Yan, M.D.; Yao, C.J.; Chow, J.M.; Chang, C.L.; Hwang, P.A.; Chuang, S.E.; Whang-Peng, J.; Lai, G.M. Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar. Drugs 2015, 13, 6099–6116.
- Lee, H.E.; Choi, E.S.; Shin, J.; Lee, S.O.; Park, K.S.; Cho, N.P.; Cho, S.D. Fucoidan induces caspase-dependent apoptosis in MC3 human mucoepidermoid carcinoma cells. Exp. Ther. Med. 2014, 7, 228–232.
- Elrggal, M.E.; Alamer, S.I.; Alkahtani, S.A.; Alshrahili, M.A.; Alharbi, A.; Alghamdi, B.A.; Zaitoun, M.F. Dispensing Practices for Weight Management Products in Eastern Saudi Arabia: A Survey of Community Pharmacists. Int. J. Environ. Res. Public Health 2021, 18, 13146.
- Krentz, A.J.; Bailey, C.J. Oral Antidiabetic Agents-Current Role in Type 2 Diabetes Mellitus. Drugs 2005, 65, 385–411.
- Garcimartín, A.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Aqueous extracts and suspensions of restructured pork formulated with Undaria pinnatifida, Himanthaliaelongata and Porphyraumbilicalis distinctly affect the in vitro α-glucosidase activity and glucose diffusion. LWT Food Sci. Technol. 2015, 64, 720–726.
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of bioactives and in vitro evaluation of antioxidant and antidiabetic property of polyphenols of marine algae Padina tetrastromatica. Algal Res. 2021, 55, 102250.
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive polyphenols from southern chile seaweed as inhibitors of enzymes for starch digestion. Mar. Drugs 2020, 18, 353.
- Al-Araby, S.Q.; Rahman, M.A.; Chowdhury, M.A.; Das, R.R.; Chowdhury, T.A.; Hasan, C.M.M.; Afroze, M.; Hashem, M.A.; Hajjar, D.; Alelwani, W.; et al. Padina tenuis (marine alga) attenuates oxidative stress and streptozotocin-induced type 2 diabetic indices in Wistar albino rats. S. Afr. J. Bot. 2020, 128, 87–100.
- Abdel-Karim, O.H.; Abo-Shady, A.M.; Ismail, G.A.; Gheda, S.F. Potential effect of Turbinaria decurrens acetone extract on the biochemical and histological parameters of alloxan-induced diabetic rats. Int. J. Environ. Health Res. 2021, 202, 1–22.
- Jae-Llane, D.; Carlos Braisv, C. Versatility of the Humble Seaweed in Biomanufacturing. Procedia Manuf. 2019, 32, 87–94.
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubble and microbubble. J. Soil Sci. Plant Nutr. 2021, 21, 389–403.
- Eissa, M.A.; Nasralla, N.N.; Gomah, N.H.; Osman, D.M.; El-Derwy, Y.M. Evaluation of natural fertilizer extracted from expired dairy products as a soil amendment. J. Soil Sci. Plant Nutr. 2018, 18, 694–704.
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335.
- Nkemka, V.N.; Murto, M. Exploring strategies for seaweed hydrolysis: Effect on methane potential and heavy metal mobilisation. Process Biochem. 2012, 47, 2523–2526.
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45.
- Abdel Khalik, K.; Osman, G. Genetic analysis of Plectranthus L. (Lamiaceae) in Saudi Arabia based on RAPD and ISSR markers. Pak. J. Bot. 2017, 49, 1073–1084.
- Anisimov, M.; Chaikina, E.; Klykov, A.; Rasskazov, V. Effect of seaweeds extracts on the growth of seedling roots of buckwheat (Fagopyrum esculentum Moench) is depended on the season of algae collection. Agric. Sci. Dev. 2013, 2, 67–75.
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558.
- EL Boukhari, M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359.
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341.
- Myers, S.P.; O’Connor, J.; Fitton, J.H.; Brooks, L.; Rolfe, M.; Connellan, P.; Wohlmuth, H.; Cheras, P.A.; Morris, C. A combined Phase I and II open-label study on the Immunomodulatory effects of seaweed extract nutrient complex. Biol. Targets Ther. 2011, 5, 45–60.
- Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A.; Deters, A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100–107.
- EMA. Community Herbal Monograph on Fucus vesiculosus L., Thallus; EMA: Amsterdam, The Netherlands, 2012.
- Pezeshk, F.; Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Naseri, M. The effect of supplementing diets with extracts derived from three different species of macroalgae on growth, thermal stress resistance, antioxidant enzyme activities and skin colour of electric yellow cichlid (Labidochromis caeruleus). Aquac. Nutr. 2019, 25, 436–443.
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826.
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020, 246, 116568.
- Yuan, H.; Song, J. Preparation, structural characterization and in vitro antitumor activity of kappa-carrageenan oligosaccharide fraction from Kappaphycus striatum. J. Appl. Phycol. 2005, 17, 7–13.
- Tannoury, M.Y.; Saab, A.M.; Elia, J.M.; Harb, N.N.; Makhlouf, H.Y.; Diab-Assaf, M. In vitro cytotoxic activity of Laurencia papillosa, marine red algae from the Lebanese coast. J. Appl. Pharm. Sci. 2017, 7, 175–179.
- Patra, S.; Muthuraman, M.S. Gracilaria edulis extract induces apoptosis and inhibits tumor in Ehrlich Ascites tumor cells in vivo. BMC Complement. Altern. Med. 2013, 13, 331.
- Alarif, W.M.; Al-Lihaibi, S.S.; Ayyad, S.E.N.; Abdel-Rhman, M.H.; Badria, F.A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtusa as potential antitumor-antimicrobial agents. Eur. J. Med. Chem. 2012, 55, 462–466.
- Celikler, S.; Yildiz, G.; Vatan, O.; Bilaloglu, R. In vitro antigenotoxicity of Ulva rigida C. Agardh (Chlorophyceae) extract against induction of chromosome aberration, sister chromatid exchange and micronuclei by mutagenic agent MMC. Biomed. Environ. Sci. 2008, 21, 492–498.
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458.
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The application of seaweed polysaccharides and their derived products with potential for the treatment of Alzheimer’s disease. Mar. Drugs 2021, 19, 89.
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid_production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591.
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908.
- Myung, C.S.; Shin, H.C.; Hai, Y.B.; Soo, J.Y.; Bong, H.L.; Jong, S.K. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698.
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998.
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552.
- Mei, C.H.; Zhou, S.C.; Zhu, L.; Ming, J.X.; Zeng, F.D.; Xu, R. Antitumor effects of laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39.
- Atya, M.E.; El-Hawiet, A.; Alyeldeen, M.A.; Ghareeb, D.A.; Abdel-Daim, M.M.; El-Sadek, M.M. In vitro biological activities and in vivo hepatoprotective role of brown algae-isolated fucoidans. Environ. Sci. Pollut. Res. 2021, 28, 19664–19676.
- Wang, S.; Li, Y.; White, W.; Lu, J. Extracts from New Zealand Undaria pinnatifida containing fucoxanthin as potential functional biomaterials against cancer in vitro. J. Funct. Biomater. 2014, 5, 29–42.
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2003, 8, 59–65.
- Liu, M.; Liu, Y.; Cao, M.J.; Liu, G.M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305.
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the barents sea. Biology 2021, 10, 67.
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224.
- Zhu, W.; Chiu, L.C.M.; Ooi, V.E.C.; Chan, P.K.S.; Ang, P.O. Antiviral property and mode of action of a sulphated polysaccharide from Sargassum patens against Herpes simplex virus type 2. Int. J. Antimicrob. Agents 2004, 24, 279–283.
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Histopathological studies on liver, kidney and heart of normal and dietary induced hyperlipidaemic rats fed with tropical red seaweed Gracilaria changii. J. Funct. Foods 2015, 17, 202–213.
- Kim, M.M.; Kim, S.K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933.
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulfated Rhamnan. Mar. Drugs 2018, 16, 243.
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant Activity of Sulfated Ulvan Isolated from the Green Macroalga Ulva rigida. Mar. Drugs 2019, 17, 291.
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9.
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30.
- Gheda, S.F.; El-Adawi, H.I.; El-Deeb, N.M. Antiviral Profile of Brown and Red Seaweed Polysaccharides against Hepatitis C Virus. Iran. J. Pharm. Res. IJPR 2016, 15, 483–491.
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Braz. J. Pharmacogn. 2012, 22, 714–723.
- Lakshmi, V.; Goel, A.K.; Srivastava, M.N.; Raghubir, R. Bioactivity of marine organisms: Part X-Screening of some marine fauna from the Indian coasts. Indian J. Exp. Biol. 2006, 44, 754–756.
- Mendes, G.D.S.; Soares, A.R.; Martins, F.O.; De Albuquerque, M.C.M.; Costa, S.S.; Yoneshigue-Valentin, Y.; Gestinari, L.M.D.S.; Santos, N.; Romanos, M.T.V. Antiviral activity of the green marine alga Ulva fasciata on the replication of human metapneumovirus. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 3–10.
- Mohamed, M.E.; Kandeel, M.; Abd El-Lateef, H.M.; El-Beltagi, H.S.; Younis, N.S. The Protective effect of Anethole against Renal Ischemia/Reperfusion: The role of the TLR2,4/MYD88/NF_B pathway. Antioxidants 2022, 11, 535.
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an attractive source for cosmetics to counter environmental stress. Sci. Total Environ. 2021, 772, 144905.
- Gellenbeck, K.W. Utilization of algal materials for nutraceutical and cosmeceutical applications—What do manufacturers need to know? J. Appl. Phycol. 2012, 24, 309–313.
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory effect of papaya extract against chlorpyrifos-induced oxidative stress, immune suppression, endocrine disruption, and dna damage in female Clarias gariepinus. Int. J. Environ. Res. Public Health 2022, 19, 4640.
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried brown seaweed’s phytoremediation potential for methylene blue dye removal from aquatic environments. Polymers 2022, 14, 1375.
- El-Beltagi, H.S.; Mohamed, H.I.; Abou El-Enain, M.M. Role of secondary metabolites from seaweeds in the context of plant development and crop production. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; Pereira, L., Bahcevandziev, K., Joshi, N.H., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 64–79.
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresen. Environ. Bull. 2011, 20, 1084–1088.
- El-desoky, A.H.; Abdel-Rahman, A.H.; Rehab, F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134.
- Riani Mansauda, K.L.; Anwar, E.; Nurhayati, T. Antioxidant and anti-collagenase activity of Sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacogn. Mag. 2018, 10, 932–936.
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983.
- Unnikrishnan, P.S.; Jayasri, M.A. Marine algae as a prospective source for antidiabetic compounds—A Brief Review. Curr. Diabetes Rev. 2018, 14, 237–245.
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. In Vitro Cell. Dev. Biol. Plant. 2022.
- Wang, L.; Je, J.-G.; Yang, H.-W.; Jeon, Y.-J.; Lee, S. Dieckol, an algae-derived phenolic compound, suppresses UVB-induced skin damage in human dermal fibroblasts and its underlying mechanisms. Antioxidants 2021, 10, 352.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49.
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559.
- Thiyagarasaiyar, K.; Mahendra, C.K.; Goh, B.-H.; Gew, L.T.; Yow, Y.-Y. UVB radiation protective effect of brown Alga Padina australis: A potential cosmeceutical application of Malaysian Seaweed. Cosmetics 2021, 8, 58.
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161.
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Evaluation of fractions extracted from Polycladia myrica: Biological activities, UVR protective effect, and stability of cream formulation based on it. J. Appl. Phycol. 2022.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
01 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





