Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ségolène De Waele | -- | 2813 | 2022-07-29 12:46:29 | | | |
| 2 | Jessie Wu | Meta information modification | 2813 | 2022-08-01 07:38:16 | | | | |
| 3 | Jessie Wu | Meta information modification | 2813 | 2022-08-01 07:45:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Waele, S.D.; Cras, P.; Crosiers, D. Apathy in Parkinson’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/25658 (accessed on 07 February 2026).
Waele SD, Cras P, Crosiers D. Apathy in Parkinson’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/25658. Accessed February 07, 2026.
Waele, Ségolène De, Patrick Cras, David Crosiers. "Apathy in Parkinson’s Disease" Encyclopedia, https://encyclopedia.pub/entry/25658 (accessed February 07, 2026).
Waele, S.D., Cras, P., & Crosiers, D. (2022, July 29). Apathy in Parkinson’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/25658
Waele, Ségolène De, et al. "Apathy in Parkinson’s Disease." Encyclopedia. Web. 29 July, 2022.
Copy Citation
Apathy is a neurobehavioural symptom affecting Parkinson’s disease patients of all disease stages. Apathy seems to be associated with a specific underlying non-motor disease subtype and reflects dysfunction of separate neural networks with distinct neurotransmitter systems.
Parkinson’s disease
apathy
neuropsychiatry
neuropsychiatric symptoms in Parkinson's disease
non-motor subtyping
non-motor symptoms
1. Pathophysiology
1.1. Psychological Model
To understand the pathophysiology of apathy it is necessary to evaluate how apathy arises as a behavioural concept. Apathy is essentially the reduction of voluntary, goal-directed behaviour (GDB) [1]. The neuro-cognitive formulation of how GDB arises is convoluted, so researchers provide an abbreviated model based on Brown and Pluck’s theory (Figure 1) [2]. The process of GDB emerges from an interaction of internal and external drives, intention, planning, motivation, and emotional state. Theoretically, interference in any of these processes can lead to apathy [1][2].
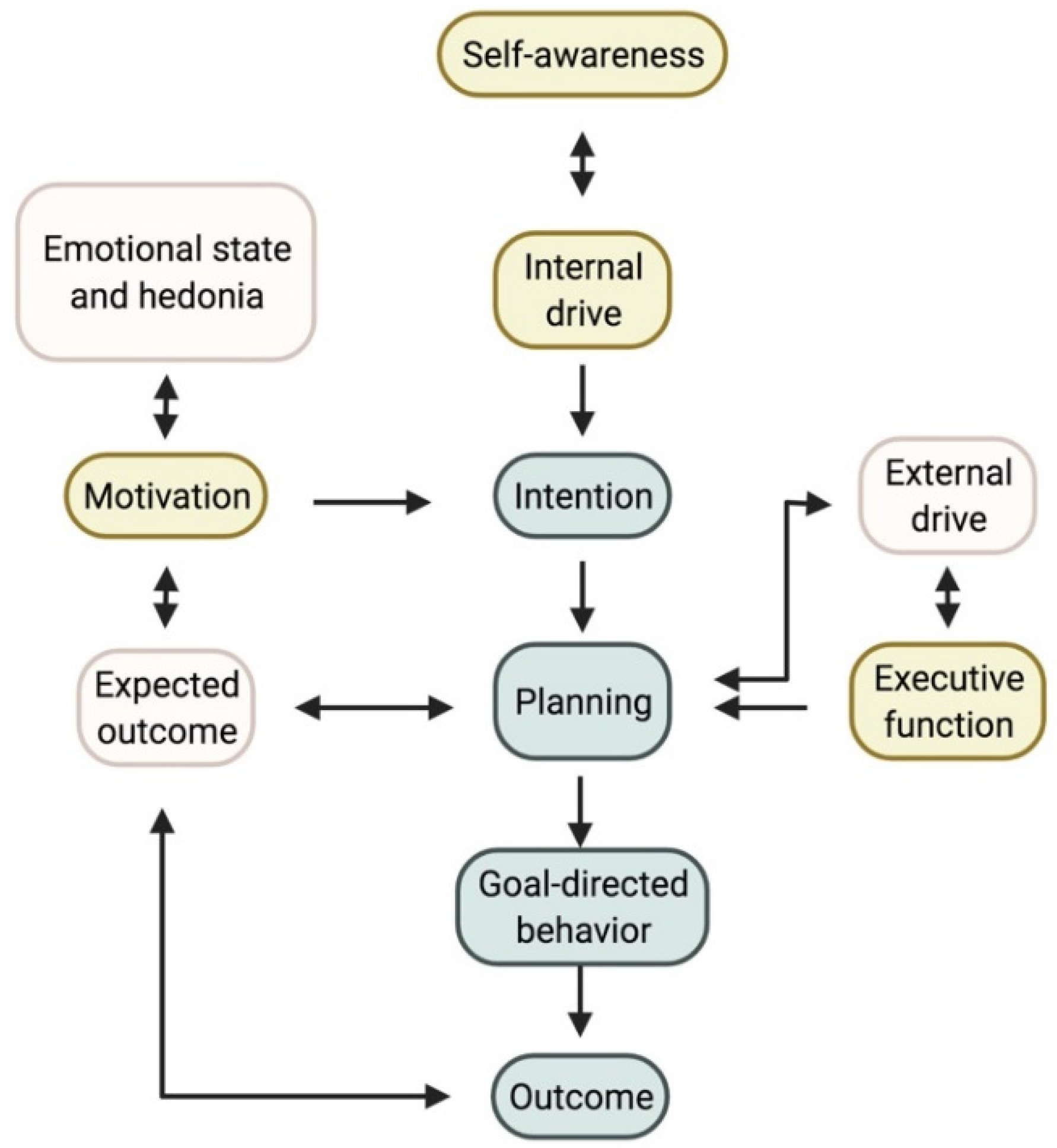
Figure 1. Proposed model for goal-directed behaviour. The three accepted subtypes and where they plug into the model are shown in yellow. Self-awareness, which is currently not yet considered a subtype, could interact with the internal drive and planning. Note on the left the importance of the hedonic state, which can affect those suffering from depression. Created with https://biorender.com/ (accessed on 27 June 2022).
Stuss and coworkers described three distinct subtypes of apathy. First, difficulties with self-activating thoughts or initiating the necessary motor functions for GDB are the predominant feature in the first type. Researchers summarized this as a reduction of ‘internal drive’. This reduced internal drive and response starkly contrast the preserved reaction to external drives and stimuli [1][3]. In an everyday setting, these patients do little unless instructed [4][5]. Daily productivity is low and there is no variation in activities in daily life [6], resulting in severe inertia that can be reversed successfully with external stimuli [1]. This subtype of apathy is sometimes referred to as ‘behavioural apathy’; however since all forms of apathy lead to a reduction in GDB, researchers suggest an alternative terminology [7]. Aphrenic apathy, derived from the Greek word for ‘inability to think’, is a more apt description of this subtype.
A disruption in the planning aspect of GDB leads to ‘cognitive’ apathy or cognitive inertia. Faulty executive processing lies at the basis here, rendering difficulties with planning, working memory, rule-finding, and set-shifting [1]. The underlying executive dysfunction makes it difficult to plan the actions needed to perform GDB [1][2][3]. Executive dysfunction does not always reflect underlying dementia but may herald it [8]. This clinically manifests as a decreased interest, or more accurately, a decreased (intellectual) curiosity [4]. These patients spend little or no time on leisure activities and have few interests. Often, they do not wish to pursue (new) hobbies or social engagements. They quickly give up on a task when facing difficulties, reflecting their executive dysfunction [4][6].
A final subtype of apathy is an underlying reward deficiency syndrome, in which a patient cannot relate the GDB to the (pleasurable) outcome or reward. This is the result of emotional blunting or reduced emotional resonance [1][3][4]. This third subtype is often referred to as ‘motivational’ apathy [1][3]. It results in a reduced emotional response, for instance when the patient is confronted with upsetting news or watching something humorous. Patients can also display a decreased concern for their families and often no longer inquire after their health and well-being spontaneously [1][6].
It is necessary to differentiate this apathy subtype from the symptoms of an underlying depression or mood disorder. Apathy can also be related to anhedonia, resulting in decreased GDB [2][9]. Apathy in patients suffering from a depressive episode can improve with adequate treatment of their mood disorder [10].
Aside from these three widely accepted apathy subtypes, a fourth dimension called ‘self-awareness’ was initially proposed by Stuss and coworkers [3]. These authors described self-awareness as a critical component of GDB. They defined it as ‘[…] a metacognitive ability, necessary to mediate information from a personal, social past and current history with projections to the future […]’ [3]. The Lille Apathy Rating Scale (LARS) was developed with this fourth dimension in mind, reflected by a fourth and independent cluster in their data analysis, separate from depression [6]. The question remains whether reduced self-awareness can be considered an underlying mechanism of apathy or a different construct altogether [4]. Self-awareness in essence organizes an individual’s understanding of a social environment and the function of this individual within it [11]. Clinically, impaired self-awareness can manifest as anosognosia, or reduced insight into one’s own physical limitations due to an illness. Reduced self-awareness has often been described in Parkinson's disease (PD) patients and is associated with cognitive decline [12][13][14]. Clinically, this may manifest itself in social interactions, where the patient might be quite headstrong in an argument, unwilling to concede to another’s point of view. This results from a decrease in self-reflection, making it difficult to assess one’s own faults accurately [6].
1.2. Neural Networks
It is often assumed that apathy results from a pure hypodopaminergic state, as it often can arise following dopamine withdrawal for Deep Brain Stimulation (DBS) surgery. Dopaminergic treatment has shown improvement in some patients, but a more complex model is required to explain the implicated neurotransmitter systems [15]. Despite adequate dopaminergic treatment, apathy occurs in PD patients, and the severity of apathy is independent of medication dosage [16][17]. Apathy can co-occur in PD patients suffering from impulse control disorders related to a hyperdopaminergic state [18][19]. Co-occurrence of apathy and impulse disorders was also reported in other neurological disorders [20]. Lastly, animal models and imaging studies in patients have shown the involvement of other neurotransmitter systems [21][22][23]. To further study the complex underlying physiology of apathy, the definition of the neuroanatomical correlates is an important starting point. Generally, the occurrence of apathy can be reduced to a dysfunctional circuitry between the frontal lobes and the basal ganglia. Within this circuitry, separate networks can be identified (see Figure 2) [3][24][25].
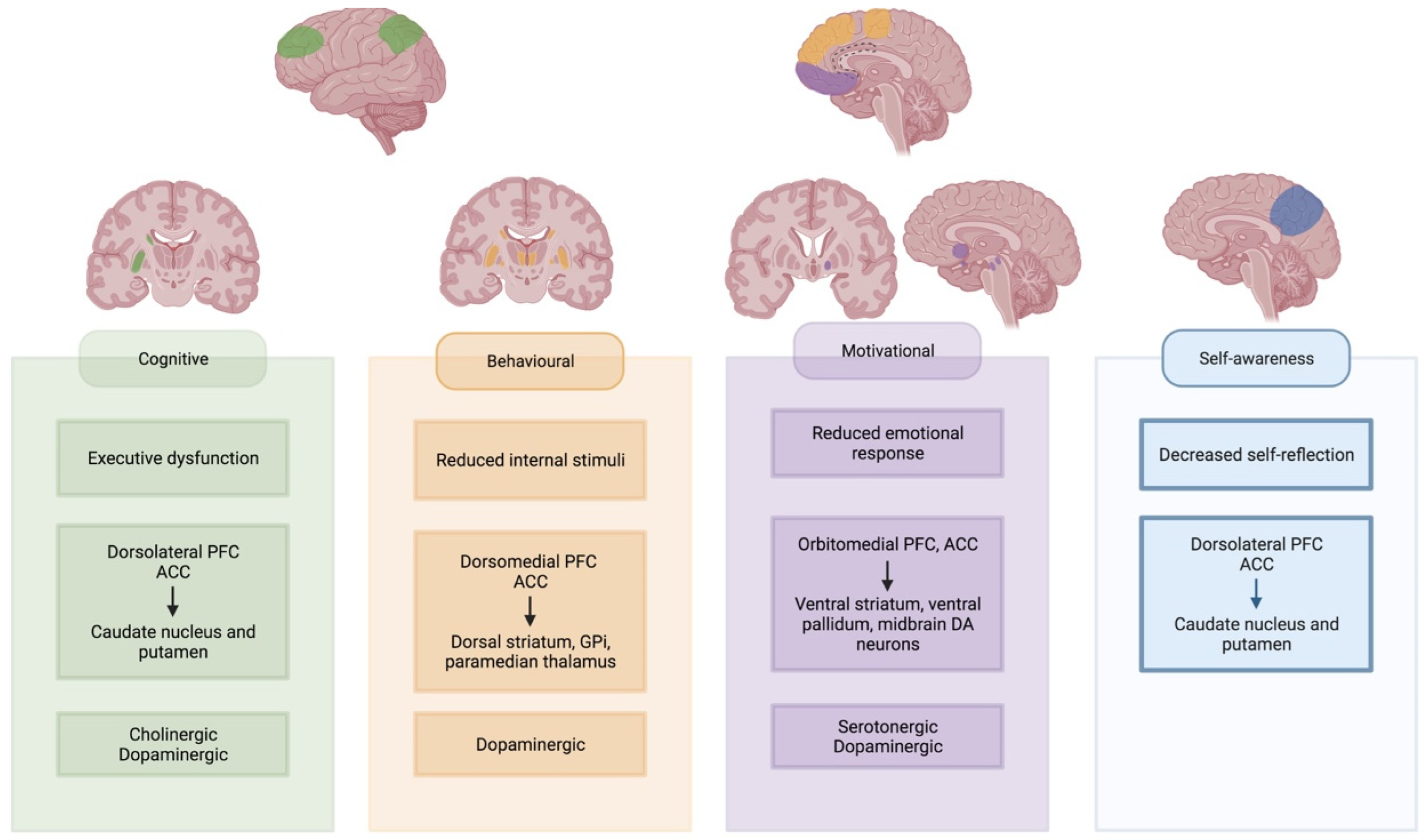
Figure 2. Neural networks underlying apathy subtypes. Involved cortical regions and basal ganglia regions are highlighted for each subtype. Created with https://biorender.com/ (accessed on 27 June 2022). Abbreviations: PFC: prefrontal cortex; ACC: anterior cingulate cortex; Gpi: internal globus pallidus; DA: dopaminergic.
‘Behavioural’ or ‘aphrenic’ apathy is often equated to a lack of initiation or internal drive to perform the GDB [1][3]. It is often referred to as an auto-activation deficit, with a reduced response to internal stimuli [1]. This type of apathy is often considered the most severe form. It has been described in bilateral dysfunction of the pathway between the dorsomedial prefrontal cortex (dmPFC) and anterior cingulate cortex (ACC) with the dorsal striatum, paramedian thalamus and the internal part of the globus pallidus (GPi) [26][27][28][29][30][31][32][33][34]. Similar syndromes have been described in uni- or bilateral lesions of the supplementary motor area (SMA) [5]. These are regions of interest in the ‘lateral orbitofrontal cortex’ circuit as described by Garrett and colleagues, which also receives input from temporal gryi and projects to the substantia nigra pars reticulata [25]. This circuit is partially dopamine-mediated, as evidenced by reduced dopaminergic binding and response to DRT [26][27][30][35].
Executive dysfunction leads to ‘cognitive’ apathy, where planning difficulties interfere with GDB. The dorsolateral PFC, cooperating with the ACC, is vital to executive processing, resulting in apathetic behaviour when lesioned [1][25][36][37]. This region has projections to the lateral parts of the dorsal striatum [25][30][38][39][40]. The lateral dorsal striatum also receives input from the posterior parietal cortex [25]. Cognitive apathy has been linked to decreased functional connectivity (FC) between the orbitofrontal cortex (OFC) and the right putamen [41]. Researchers assume that the ‘dorsolateral PFC’ circuit is largely acetylcholine-mediated due to its implication in executive dysfunction [42]. In Alzheimer’s disease patients with predominant cognitive apathy, there was reduced response to dextroamphetamine administration, suggesting some possible dopaminergic involvement as well [43].
‘Motivational’ apathy is mediated by the mesocorticolimbic pathway or the reward system [1][3][44]. Involved regions are the orbitomedial PFC, the ACC, the ventral striatum, the ventral pallidum and the dopaminergic midbrain neurons [1][45]. This system is mediated by the amygdala, hippocampus, thalamus, lateral habenular nucleus, the dorsal PFC, as well as the pedunculopontine nucleus and raphe nucleus in the brainstem [45][46]. Patients with ‘motivational’ apathy according to the LARS showed altered FC between the left inferior frontal gyrus and the left pallidum. There was an increased FC between the left inferior frontal gyrus and the right caudate [41]. Apathetic PD patients showed selective impairment of reward processing, reducing their ability to differentiate between favourable and unfavourable outcomes [47]. Dopamine plays an important role in this circuit, yet its relation to manifest apathy is complex [48]. Administration of dopamine agonists blunts reward sensitivity in healthy adults, while use in apathetic patients shows promise as a potential therapy [49][50]. Yet, some studies found no difference in dopaminergic uptake between apathetic and non-apathetic patients [22]. Serotonin could act as a modulator, with reduced uptake found in critical parts of the mesocorticolimbic pathway in apathetic PD patients [22][23]. The uptake reduction was proportional to apathy’s severity [22]. Reduced serotonergic uptake in the raphe nucleus was also associated with the presence and severity of apathy in possible prodromal PD patients [51].
In abbreviated and modified model in Figure 1, researhcers propose a new role for self-awareness in developing GDB. Recent imaging findings have identified common underlying brain regions and networks in patients with reduced self-awareness and apathy. The precuneus is part of the default-mode network and plays an important role in self-awareness [52][53][54]. Studies found that isolated apathy in PD was associated with atrophy and hypometabolism of the precuneus compared to healthy controls [55][56]. Other regions of interest in self-awareness are the ACC, the posterior cingulate cortex (PCC), the temporoparietal junction, the ventromedial and dorsolateral prefrontal cortex and the insula [57][58][59].
It is unlikely that a different type of apathy develops in each patient. Patients with typical auto-activation deficit lesions were also shown to have reduced reward sensitivity [35][60]. One study found that apathy profiles differed, depending on disease stage. In stable PD patients, defined by the authors as well-controlled motor symptoms without fluctuations and absence of dementia, there was a trend towards decreased intellectual curiosity or ‘cognitive’ apathy. In PD patients with motor fluctuations without dementia, mostly intellectual curiosity and action initiation were inhibited. In PDD, both domains as well as self-awareness were decreased. Interestingly, in all groups, motivational apathy, as measured by the emotion subscore of the LARS, did not differ significantly from healthy controls [61]. Another study found a predominant decrease in intellectual curiosity in early-stage PD patients [62]. These findings suggest that apathy subtypes might have a distinct temporal profile.
1.3. Imaging Biomarkers
Aside from neurotransmitter changes in different networks, additional imaging biomarkers of apathy have been investigated. Changes have been reported in grey-matter volume (GMV), white and grey matter integrity, FC and network analysis, regional homogeneity (ReHo), glucose metabolism, and resting activity pattern.
Decrease in GMV in the subgenual AAC, left superior temporal, left precuneus, inferior parietal, right superior frontal, and the dorsolateral part of the caput of the left caudate nucleus is related to the presence of apathy. Severity of apathy was related to morphological abnormalities in the superior cerebellar peduncle decussation, bilateral posterior cerebellum and vermis, left superior frontal gyrus, and left nucleus accumbens [22][55][63]. GMV increases were noted in the left superior frontal gyrus and cerebellar vermis [55]. Other imaging studies, however, could not confirm these changes [24][64][65]. The connectivity between the parietal cortex and frontal lobes might explain part of these findings, as frontal lesions lead to parietal hyperactivity [54]. Input of temporal gyri has also been described in the ‘lateral orbitofrontal cortex’ circuit of the basal ganglia, implicated in ‘behavioural’ apathy [25].
Fractional anisotropy (FA) was significantly decreased in the genu and body of the corpus callosum, bilaterally in the anterior corona radiata and the left superior part of the corona radiata and left cingulum in apathetic PD patients. The grade of integrity was related to apathy severity [66]. Another study found reduced FA in the anterior thalamic fibres, the cingulate bundle, and the corpus callosum’s interhemispheric connections and projection fibres. FA was also decreased bilaterally in the medial thalamus [22].
FC was reduced between the left ventral striatum and left frontal lobe in apathetic PD patients. Reduced FC between ventral and dorsal striatum and left frontal lobe, between the limbic region of the left frontal lobe and left striatum, between the caudal and rostral frontal lobe and right striatum and in between subdivisions of the left frontal lobe was related to increased severity of apathy [24] A regional network analysis could not find differences in connectivity between apathetic and non-apathetic PD patients [64].
Analysis of low-frequency function (ALFF), which evaluates the resting state of the entire brain, showed decreased ALFF signal in the left supplementary motor region, left inferior parietal love, left fusiform gyrus, and bilaterally in the cerebellum [67].
ReHo measures synchronization of local neural activity. In apathetic PD patients, ReHo was decreased in right caudate and dorsal ACC [68]. Some studies found reduced glucose metabolism in the precuneus bilaterally and right lingual gyrus and increased metabolism in the middle frontal gyrus in apathetic patients [55]. Additionally, the severity of white matter hyperintensities on FLAIR sequence also showed a link to apathy in PD, independent of depression [69]. These findings suggest top-down control from other cortical regions and support the involvement of the parietal cortex in certain subtypes [25][54].
2. Treatment
2.1. Pharmacological
As discussed above, many neurotransmitters are involved in the underlying process of apathy. Evidence has been found of dopaminergic, serotonergic, and cholinergic involvement in PD. [17][21][26][27][30][35].
Use of dopamine replacement therapy (DRT) has shown promising results, and administration of dopamine agonists is often most successful. A recent meta-analysis concluded that using rotigotine improved apathy scores, which was not confirmed in a more recent placebo-controlled study [70][71]. Other dopamine agonists such as pramipexole or apomorphine might also be beneficial, as patients score better on the items ‘intellectual curiosity’ and ‘self-awareness’ after administration [50][72]. Global apathy scores improved in those receiving apomorphine when combined with rotigotine [72][73]. Rotigotine and pramipexol were effective in reversing an auto-activation deficit in a case series [74].
DRT is assumed to improve apathy in the long term, as evidenced by the decreasing prevalence after the introduction of medication [75]. Apathy scores do not differ significantly in ON or OFF stages, showing no significant response to DRT in the acute phase [76]. No studies comparing different DRT strategies in these patients are available.
Results on serotonergic treatment are scarce. Selective serotonin receptor inhibitors are known to induce flat affect and apathy, both in healthy individuals and PD patients [77]. A cross-over study in 25 PD subjects with 5-hydroxytryptophan, a precursor of serotonin, had no significant impact on apathy scores [78]. Use of both selective serotonin and serotonin noradrenaline reuptake inhibitors (SSRI and SNRI respectively) did not significantly alter apathy scores compared to baselines [79].
In those already receiving optimized dopaminergic treatment without PDD, add-on of rivastigmine improved apathy scores [17]. Although rivastigmine was reported to improve apathy in PDD in a few case reports, a more extensive patient series showed no improvement in this group [42][80]. Use of rivastigmine decreased caregiver distress associated with apathy [81]. Galantamine might be effective in apathetic PDD patients [82].
Other strategies include the use of stimulants. Administration of dextroamphetamine in a PD sample with cognitive decline improved apathy scores in nearly a third of patients. Most of these patients were already receiving cholinesterase inhibitors [83]. Singular positive reports have been published on the use of methylphenidate, istradefylline, MAO inhibitors, yokukansan, and exenatide [84][85][86][87][88][89]. Bupropion and choline alphoscerate, a cholinergic precursor, was shown to be effective in treating apathy in other neurodegenerative diseases [90][91][92]. A case report of a patient suffering from an auto-activation deficit reported spectacular improvement of symptoms following administration of tricyclic antidepressants [93].
2.2. Non-Pharmacological
Non-pharmacological treatment options have garnered increasing interest. Exercise especially is beneficial in the treatment of both motor and NMS [94][95]. For the treatment of apathy, exercise and physical activity may also prove useful. A longitudinal study found that patients with baseline higher activity levels had improved apathy scores at follow-up. Apathy scores at baseline were not related to activity level [96]. Others however found very little difference between those following an intensive exercise schedule with sessions thrice a week and those without intervention. Only those following individual therapy showed slight improvement [97]. Apathy scores did improve in patients following biweekly Nordic walking sessions over 12 weeks, compared to control patients [98]. Though there is some evidence for a positive effect of dance, a recent meta-analysis concluded it was not superior to self-directed exercise or the best medical treatment [99][100]. There is need for structured research into the matter, wherein different physical activities and interventions are systematically researched and compared. As current evidence does not support one type of physical activity above another, it is advised to tailor the type of physical exercise to the patients’ needs and preferences [101].
A small body of evidence exists for using repetitive transcranial magnetic stimulation (rTMS). A cross-over study found that rTMS over the supplementary motor area improved apathy scores compared to placebo [102]. Stimulation of the M1 area in the precentral gyrus showed similar improvement [103]. Benefit was also found after targeting the dorsolateral PFC, after which both apathy and emotional processing improved [104]. Cognitive rehabilitation is beneficial in treating apathy in the healthy elderly, but no such benefits were observed in PD patients [105][106][107]. A pilot project showed slight benefits in the short term, but longitudinal data is not available [108].
References
- Levy, R.; Dubois, B. Apathy and the Functional Anatomy of the Prefrontal Cortex-Basal Ganglia Circuits. Cereb. Cortex 2006, 16, 916–928.
- Brown, R.G.; Pluck, G. Negative Symptoms: The “pathology” of Motivation and Goal-Directed Behaviour. Trends Neurosci. 2000, 23, 412–417.
- Stuss, D.T.; Van Reekum, R.; Murphy, K.J. Differentiation of States and Causes of Apathy. In The Neuropsychology of Emotion; Borod, J.C., Ed.; Series in Affective Science; Oxford University Press: Oxford, UK, 2000; pp. 340–363. ISBN 0-19-511464-7. (Hardcover).
- Robert, P.; Onyike, C.U.; Leentjens, A.F.G.; Dujardin, K.; Aalten, P.; Starkstein, S.; Verhey, F.R.J.; Yessavage, J.; Clement, J.P.; Drapier, D.; et al. Proposed Diagnostic Criteria for Apathy in Alzheimer’s Disease and Other Neuropsychiatric Disorders. Eur. Psychiatry 2009, 24, 98–104.
- Laplane, D.; Dubois, B. Auto-Activation Deficit: A Basal Ganglia Related Syndrome. Mov. Disord. 2001, 16, 810–814.
- Sockeel, P.; Dujardin, K.; Devos, D.; Denève, C.; Destée, A.; Defebvre, L. The Lille Apathy Rating Scale (LARS), a New Instrument for Detecting and Quantifying Apathy: Validation in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 579–584.
- Lazcano-Ocampo, C.; Wan, Y.M.; van Wamelen, D.J.; Batzu, L.; Boura, I.; Titova, N.; Leta, V.; Qamar, M.; Martinez-Martin, P.; Ray Chaudhuri, K. Identifying and Responding to Fatigue and Apathy in Parkinson’s Disease: A Review of Current Practice. Expert Rev. Neurother. 2020, 20, 477–495.
- Levy, G.; Jacobs, D.M.; Tang, M.X.; Côté, L.J.; Louis, E.D.; Alfaro, B.; Mejia, H.; Stern, Y.; Marder, K. Memory and Executive Function Impairment Predict Dementia in Parkinson’s Disease. Mov. Disord. 2002, 17, 1221–1226.
- Marin, R.S. Apathy: A Neuropsychiatric Syndrome. J. Neuropsychiatry Clin. Neurosci. 1991, 3, 243–254.
- Yuen, G.S.; Gunning, F.M.; Woods, E.; Klimstra, S.A.; Hoptman, M.J.; Alexopoulos, G.S. Neuroanatomical Correlates of Apathy in Late-Life Depression and Antidepressant Treatment Response. J. Affect. Disord. 2014, 166, 179–186.
- Hull, J.G.; Levy, A.S. The Organizational Functions of the Self: An Alternative to the Duval and Wicklund Model of Self-Awareness. J. Pers. Soc. Psychol. 1979, 37, 756–768.
- Leritz, E.; Loftis, C.; Crucian, G.; Friedman, W.; Bowers, D. Self-Awareness of Deficits in Parkinson Disease. Clin. Neuropsychol. 2004, 18, 352–361.
- Orfei, M.D.; Assogna, F.; Pellicano, C.; Pontieri, F.E.; Caltagirone, C.; Pierantozzi, M.; Stefani, A.; Spalletta, G. Anosognosia for Cognitive and Behavioral Symptoms in Parkinson’s Disease with Mild Dementia and Mild Cognitive Impairment: Frequency and Neuropsychological/Neuropsychiatric Correlates. Park. Relat. Disord. 2018, 54, 62–67.
- Maier, F.; Prigatano, G.P.; Kalbe, E.; Barbe, M.T.; Eggers, C.; Lewis, C.J.; Burns, R.S.; Morrone-Strupinsky, J.; Moguel-Cobos, G.; Fink, G.R.; et al. Impaired Self-Awareness of Motor Deficits in Parkinson’s Disease: Association with Motor Asymmetry and Motor Phenotypes. Mov. Disord. 2012, 27, 1443–1446.
- Ou, R.; Hou, Y.; Wei, Q.; Lin, J.; Liu, K.; Zhang, L.; Jiang, Z.; Cao, B.; Zhao, B.; Song, W.; et al. Longitudinal Evolution of Non-Motor Symptoms in Early Parkinson’s Disease: A 3-Year Prospective Cohort Study. NPJ Park. Dis. 2021, 7, 1–6.
- De La Riva, P.; Smith, K.; Xie, S.X.S.X.; Weintraub, D. Course of Psychiatric Symptoms and Global Cognition in Early Parkinson Disease. Neurology 2014, 83, 1096–1103.
- Devos, D.; Moreau, C.; Maltête, D.; Lefaucheur, R.; Kreisler, A.; Eusebio, A.; Defer, G.; Ouk, T.; Azulay, J.P.; Krystkowiak, P.; et al. Rivastigmine in Apathetic but Dementia and Depression-Free Patients with Parkinson’s Disease: A Double-Blind, Placebo-Controlled, Randomised Clinical Trial. J. Neurol. Neurosurg. Psychiatry 2014, 85, 668–674.
- Drew, D.S.; Muhammed, K.; Baig, F.; Kelly, M.; Saleh, Y.; Sarangmat, N.; Okai, D.; Hu, M.; Manohar, S.; Husain, M. Dopamine and Reward Hypersensitivity in Parkinson’s Disease with Impulse Control Disorder. Brain 2020, 143, 2502–2518.
- Scott, B.M.; Eisinger, R.S.; Burns, M.R.; Lopes, J.; Okun, M.S.; Gunduz, A.; Bowers, D. Co-Occurrence of Apathy and Impulse Control Disorders in Parkinson Disease. Neurology 2020, 95, e2769–e2780.
- Lansdall, C.J.; Coyle-Gilchrist, I.T.S.; Jones, P.S.; Rodríguez, P.V.; Wilcox, A.; Wehmann, E.; Dick, K.M.; Robbins, T.W.; Rowe, J.B. Apathy and Impulsivity in Frontotemporal Lobar Degeneration Syndromes. Brain 2017, 140, 1792–1807.
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Le Bars, D.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The Prominent Role of Serotonergic Degeneration in Apathy, Anxiety and Depression in de Novo Parkinson’s Disease. Brain 2016, 139, 2486–2502.
- Prange, S.; Metereau, E.; Maillet, A.; Lhommée, E.; Klinger, H.; Pelissier, P.; Ibarrola, D.; Heckemann, R.A.; Castrioto, A.; Tremblay, L.; et al. Early Limbic Microstructural Alterations in Apathy and Depression in de Novo Parkinson’s Disease. Mov. Disord. 2019, 34, 1644–1654.
- Maillet, A.; Météreau, E.; Tremblay, L.; Favre, E.; Klinger, H.; Lhommée, E.; Le Bars, D.; Castrioto, A.; Prange, S.; Sgambato, V.; et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Mov. Disord. 2021, 36, 2888–2900.
- Baggio, H.C.; Segura, B.; Garrido-Millan, J.L.; Marti, M.J.; Compta, Y.; Valldeoriola, F.; Tolosa, E.; Junque, C. Resting-State Frontostriatal Functional Connectivity in Parkinson’s Disease-Related Apathy. Mov. Disord. 2015, 30, 671–679.
- Alexander, G.E.; Crutcher, M.D.; Delong, M.R. Basal Ganglia-Thalamocortical Circuits: Parallel Substrates for Motor, Oculomotor, Prefrontal and Limbic Functions. Prog. Brain Res. 1990, 85, 119–149.
- Cottencin, O.; Guardia, D.; Warembourg, F.; Gaudry, C.; Goudemand, M. Methadone Overdose, Auto-Activation Deficit, and Catatonia:A Case Study. Prim. Care Companion J. Clin. Psychiatry 2009, 11, 275–276.
- Leroy, A.; Petyt, G.; Pignon, B.; Vaiva, G.; Jardri, R.; Amad, A. Research Letter: Auto-Activation Deficit in Schizophrenia: A Case Report. Psychol. Med. 2018, 48, 525–527.
- Ali-Cherif, A.; Royere, M.L.; Gosset, A.; Poncet, M.; Salamon, G.; Khalil, R. Behavior and mental activity disorders after carbon monoxide poisoning. Bilateral pallidal lesions. Rev. Neurol. 1984, 140, 401–405.
- Leu-Semenescu, S.; Uguccioni, G.; Golmard, J.L.; Czernecki, V.; Yelnik, J.; Dubois, B.; Forgeot D’Arc, B.; Grabli, D.; Levy, R.; Arnulf, I. Can We Still Dream When the Mind Is Blank? Sleep and Dream Mentations in Auto-Activation Deficit. Brain 2013, 136, 3076–3084.
- David, R.; Koulibaly, M.; Benoit, M.; Garcia, R.; Caci, H.; Darcourt, J.; Robert, P. Striatal Dopamine Transporter Levels Correlate with Apathy in Neurodegenerative Diseases. A SPECT Study with Partial Volume Effect Correction. Clin. Neurol. Neurosurg. 2008, 110, 19–24.
- Mega, M.S.; Cohenour, R.C. Akinetic Mutism: Disconnection of Frontal-Subcortical Circuits. Neuropsychiatry. Neuropsychol. Behav. Neurol. 1997, 10, 254–259.
- Bogousslavsky, J.; Regli, F.; Delaloye, B.; Delaloye-Bischof, A.; Assal, G.; Uske, A. Loss of Psychic Self-Activation with Bithalamic Infarction. Acta Neurol. Scand. 1991, 83, 309–316.
- Laplane, D. La perte d’auto-activation psychique. Rev. Neurol. 1990, 146, 397–404.
- Habib, M. Athymhormia and Disorders of Motivation in Basal Ganglia Disease. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 509–524.
- Adam, R.; Leff, A.; Sinha, N.; Turner, C.; Bays, P.; Draganski, B.; Husain, M. Dopamine Reverses Reward Insensitivity in Apathy Following Globus Pallidus Lesions. Cortex 2013, 49, 1292–1303.
- Laine, M.; Tuokkola, T.; Hiltunen, J.; Vorobyev, V.; Bliss, I.; Baddeley, A.; Rinne, J.O. Central Executive Function in Mild Cognitive Impairment: A PET Activation Study: Cognition and Neurosciences. Scand. J. Psychol. 2009, 50, 33–40.
- Kondo, H.; Osaka, N.; Osaka, M. Cooperation of the Anterior Cingulate Cortex and Dorsolateral Prefrontal Cortex for Attention Shifting. Neuroimage 2004, 23, 670–679.
- Baker, S.C.; Rogers, R.D.; Owen, A.M.; Frith, C.D.; Dolan, R.J.; Frackowiak, R.S.J.; Robbins, T.W. Neural Systems Engaged by Planning: A PET Study of the Tower of London Task. Neuropsychologia 1996, 34, 515–526.
- Levy, R.; Friedman, H.R.; Davachi, L.; Goldman-Rakic, P.S. Differential Activation of the Caudate Nucleus in Primates Performing Spatial and Nonspatial Working Memory Tasks. J. Neurosci. 1997, 17, 3870–3882.
- Owen, A.M. Planning and Spatial Working Memory: A Positron Emission Tomography Study in Humans. Eur. J. Neurosci. 1996, 8, 353–364.
- Lucas-Jiménez, O.; Ojeda, N.; Peña, J.; Cabrera-Zubizarreta, A.; Díez-Cirarda, M.; Gómez-Esteban, J.C.; Gómez-Beldarrain, M.Á.; Ibarretxe-Bilbao, N. Apathy and Brain Alterations in Parkinson’s Disease: A Multimodal Imaging Study. Ann. Clin. Transl. Neurol. 2018, 5, 803–814.
- Moretti, R.; Caruso, P.; Dal Ben, M. Rivastigmine as a Symptomatic Treatment for Apathy in Parkinson’s Dementia Complex: New Aspects for This Riddle. Parkinsons. Dis. 2017, 2017.
- Lanctôt, K.L.; Herrmann, N.; Black, S.E.; Ryan, M.; Rothenburg, L.S.; Liu, B.A.; Busto, U.E. Apathy Associated with Alzheimer Disease: Use of Dextroamphetamine Challenge. Am. J. Geriatr. Psychiatry 2008, 16, 551–557.
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What Can Rodent Models Tell Us about Apathy and Associated Neuropsychiatric Symptoms in Parkinson’s Disease? Transl. Psychiatry 2016, 6, e753.
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2010, 35, 4–26.
- Hong, S.; Hikosaka, O. Pedunculopontine Tegmental Nucleus Neurons Provide Reward, Sensorimotor, and Alerting Signals to Midbrain Dopamine Neurons. Physiol. Behav. 2016, 176, 139–148.
- Martínez-Horta, S.; Riba, J.; Fernández De Bobadilla, R.; Pagonabarraga, J.; Pascual-Sedano, B.; Antonijoan, R.M.; Romero, S.; Ngel Mañanas, M.A.; García-Sanchez, C.; Kulisevsky, J. Apathy in Parkinson’s Disease: Neurophysiological Evidence of Impaired Incentive Processing. J. Neurosci. 2014, 17, 5918–5926.
- Schultz, W. Behavioral Dopamine Signals. Trends Neurosci. 2007, 30, 203–210.
- Riba, J.; Krämer, U.M.; Heldmann, M.; Richter, S.; Münte, T.F. Dopamine Agonist Increases Risk Taking but Blunts Reward-Related Brain Activity. PLoS ONE 2008, 3, e2479.
- Leentjens, A.F.G.; Koester, J.; Fruh, B.; Shephard, D.T.S.; Barone, P.; Houben, J.J.G. The Effect of Pramipexole on Mood and Motivational Symptoms in Parkinson’s Disease: A Meta-Analysis of Placebo-Controlled Studies. Clin. Ther. 2009, 31, 89–98.
- Barber, T.R.; Griffanti, L.; Muhammed, K.; Drew, D.S.; Bradley, K.M.; McGowan, D.R.; Crabbe, M.; Lo, C.; MacKay, C.E.; Husain, M.; et al. Apathy in Rapid Eye Movement Sleep Behaviour Disorder Is Associated with Serotonin Depletion in the Dorsal Raphe Nucleus. Brain 2018, 141, 2848–2854.
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583.
- Shulman, G.L.; Fiez, J.A.; Corbetta, M.; Buckner, R.L.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J. Cogn. Neurosci. 1997, 9, 648–663.
- Mesulam, M.-M. Frontal Cortex and Behavior. Ann. Neurol. 1986, 19, 320–325.
- Shin, J.H.; Shin, S.A.; Lee, J.Y.; Nam, H.; Lim, J.S.; Kim, Y.K. Precuneus Degeneration and Isolated Apathy in Patients with Parkinson’s Disease. Neurosci. Lett. 2017, 653, 250–257.
- Reijnders, J.S.A.M.; Scholtissen, B.; Weber, W.E.J.; Aalten, P.; Verhey, F.R.J.; Leentjens, A.F.G. Neuroanatomical Correlates of Apathy in Parkinson’s Disease: A Magnetic Resonance Imaging Study Using Voxel-Based Morphometry. Mov. Disord. 2010, 25, 2318–2325.
- Newen, A.; Vogeley, K. Self-Representation: Searching for a Neural Signature of Self-Consciousness. Conscious. Cogn. 2003, 12, 529–543.
- Morin, A. Self-Awareness Part 2: Neuroanatomy and Importance of Inner Speech. Soc. Personal. Psychol. Compass 2011, 5, 1004–1017.
- Robert, G.; Le Jeune, F.; Dondaine, T.; Drapier, S.; Péron, J.; Lozachmeur, C.; Sauleau, P.; Houvenaghel, J.F.; Travers, D.; Millet, B.; et al. Apathy and Impaired Emotional Facial Recognition Networks Overlap in Parkinson’s Disease: A PET Study with Conjunction Analyses. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1153–1158.
- Schmidt, L.; D’Arc, B.F.; Lafargue, G.; Galanaud, D.; Czernecki, V.; Grabli, D.; Schüpbach, M.; Hartmann, A.; Lévy, R.; Dubois, B.; et al. Disconnecting Force from Money: Effects of Basal Ganglia Damage on Incentive Motivation. Brain 2008, 131, 1303–1310.
- Dujardin, K.; Sockeel, P.; Devos, D.; Delliaux, M.; Krystkowiak, P.; Destée, A.; Defebvre, L. Characteristics of Apathy in Parkinson’s Disease. Mov. Disord. 2007, 22, 778–784.
- Dujardin, K.; Langlois, C.; Plomhause, L.; Carette, A.S.; Delliaux, M.; Duhamel, A.; Defebvre, L. Apathy in Untreated Early-Stage Parkinson Disease: Relationship with Other Non-Motor Symptoms. Mov. Disord. 2014, 29, 1796–1801.
- Carriere, N.; Besson, P.; Dujardin, K.; Duhamel, A.; Defebvre, L.; Delmaire, C.; Devos, D. Apathy in Parkinson’s Disease Is Associated with Nucleus Accumbens Atrophy: A Magnetic Resonance Imaging Shape Analysis. Mov. Disord. 2014, 29, 897–903.
- Wen, M.C.; Wen, M.C.; Thiery, A.; Tseng, W.Y.I.; Kok, T.; Xu, Z.; Chua, S.T.; Tan, L.C.S.; Tan, L.C.S.; Tan, L.C.S. Apathy Is Associated with White Matter Network Disruption and Specific Cognitive Deficits in Parkinson’s Disease. Psychol. Med. 2020, 52, 264–273.
- Alzahrani, H.; Antonini, A.; Venneri, A. Apathy in Mild Parkinson’s Disease: Neuropsychological and Neuroimaging Evidence. J. Parkinsons. Dis. 2016, 6, 821–832.
- Zhang, Y.; Wu, J.; Wu, W.; Liu, R.; Pang, L.; Guan, D.; Xu, Y. Reduction of White Matter Integrity Correlates with Apathy in Parkinson’s Disease. Int. J. Neurosci. 2018, 128, 25–31.
- Skidmore, F.M.; Yang, M.; Baxter, L.; von Deneen, K.; Collingwood, J.; He, G.; Tandon, R.; Korenkevych, D.; Savenkov, A.; Heilman, K.M.; et al. Apathy, Depression, and Motor Symptoms Have Distinct and Separable Resting Activity Patterns in Idiopathic Parkinson Disease. Neuroimage 2013, 81, 484–495.
- Sun, H.H.; Pan, P.L.; Hu, J.B.; Chen, J.; Wang, X.Y.; Liu, C.F. Alterations of Regional Homogeneity in Parkinson’s Disease with “Pure” Apathy: A Resting-State FMRI Study. J. Affect. Disord. 2020, 274, 792–798.
- Zhang, Y.; Zhang, G.Y.; Zhang, Z.E.; He, A.Q.; Gan, J.; Liu, Z. White Matter Hyperintensities: A Marker for Apathy in Parkinson’s Disease without Dementia? Ann. Clin. Transl. Neurol. 2020, 7, 1692–1701.
- Wang, H.T.; Wang, L.; He, Y.; Yu, G. Rotigotine Transdermal Patch for the Treatment of Neuropsychiatric Symptoms in Parkinson’s Disease: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Neurol. Sci. 2018, 393, 31–38.
- Castrioto, A.; Thobois, S.; Anheim, M.; Quesada, J.L.; Lhommée, E.; Klinger, H.; Bichon, A.; Schmitt, E.; Durif, F.; Azulay, J.P.; et al. A Randomized Controlled Double-Blind Study of Rotigotine on Neuropsychiatric Symptoms in de Novo PD. NPJ Park. Dis. 2020, 6, 1–6.
- Auffret, M.; Le Jeune, F.; Maurus, A.; Drapier, S.; Houvenaghel, J.F.; Robert, G.H.; Sauleau, P.; Vérin, M. Apomorphine Pump in Advanced Parkinson’s Disease: Effects on Motor and Nonmotor Symptoms with Brain Metabolism Correlations. J. Neurol. Sci. 2017, 372, 279–287.
- Todorova, A.; Martinez-Martin, P.; Martin, A.; Rizos, A.; Reddy, P.; Chaudhuri, K.R. Daytime Apomorphine Infusion Combined with Transdermal Rotigotine Patch Therapy Is Tolerated at 2 Years: A 24-h Treatment Option in Parkinson’s Disease. Basal Ganglia 2013, 3, 127–130.
- Blundo, C.; Gerace, C. Dopamine Agonists Can Improve Pure Apathy Associated with Lesions of the Prefrontal-Basal Ganglia Functional System. Neurol. Sci. 2015, 36, 1197–1201.
- Ou, R.; Lin, J.; Liu, K.; Jiang, Z.; Wei, Q.; Hou, Y.; Zhang, L.; Cao, B.; Zhao, B.; Song, W.; et al. Evolution of Apathy in Early Parkinson’s Disease: A 4-Years Prospective Cohort Study. Front. Aging Neurosci. 2021, 12, 1–9.
- Ganjavi, H.; Macdonald, P.A. On-off Effects of Dopaminergic Therapy on Psychiatric Symptoms in Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e134–e139.
- Zahodne, L.B.; Bernal-Pacheco, O.; Bowers, D.; Ward, H.; Oyama, G.; Limotai, N.; Velez-Lago, F.; Rodriguez, R.L.; Malaty, I.; McFarland, N.R.; et al. Are Selective Serotonin Reuptake Inhibitors Associated with Greater Apathy in Parkinson’s Disease? J. Neuropsychiatry Clin. Neurosci. 2012, 24, 326–330.
- Meloni, M.; Puligheddu, M.; Carta, M.; Cannas, A.; Figorilli, M.; Defazio, G. Efficacy and Safety of 5-Hydroxytryptophan on Depression and Apathy in Parkinson’s Disease: A Preliminary Finding. Eur. J. Neurol. 2020, 27, 779–786.
- Takahashi, M.; Tabu, H.; Ozaki, A.; Hamano, T.; Takeshima, T. Antidepressants for Depression, Apathy, and Gait Instability in Parkinson’s Disease: A Multicenter Randomized Study. Intern. Med. 2019, 58, 361–368.
- Bullock, R.; Cameron, A. Rivastigmine for the Treatment of Dementia and Visual Hallucinations Associated with Parkinson’s Disease: A Case Series. Curr. Med. Res. Opin. 2002, 18, 258–264.
- Oh, Y.-S.; Kim, J.-S.; Lee, P.H. Effect of Rivastigmine on Behavioral and Psychiatric Symptoms of Parkinson’s Disease Dementia. J. Mov. Disord. 2015, 8, 98–102.
- Litvinenko, I.V.; Odinak, M.M.; Mogil’naya, V.I.; Emelin, A.Y.U. Efficacy and Safety of Galantamine (Reminyl) for Dementia in Patients with Parkinson’s Disease (an Open Controlled Trial). Neurosci. Behav. Physiol. 2008, 38, 937–945.
- Lanctot, K.L.; Chau, S.A.; Herrmann, N.; Drye, L.T.; Rosenberg, P.B.; Scherer, R.W.; Black, S.E.; Vaidya, V.; Bachman, D.L.; Mintzer, J.E. Effect of Methylphenidate on Attention in Apathetic AD Patients in a Randomized, Placebo-Controlled Trial. Int. Psychogeriatr. 2014, 26, 239–246.
- Nagayama, H.; Kano, O.; Murakami, H.; Ono, K.; Hamada, M.; Toda, T.; Sengoku, R.; Shimo, Y.; Hattori, N. Effect of Istradefylline on Mood Disorders in Parkinson’s Disease. J. Neurol. Sci. 2019, 396, 78–83.
- Krishna, R.; Ali, M.; Moustafa, A.A. Effects of Combined MAO-B Inhibitors and Levodopa vs Monotherapy in Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 180.
- Hatano, T.; Hattori, N.; Kawanabe, T.; Terayama, Y.; Suzuki, N.; Iwasaki, Y.; Fujioka, T. An Exploratory Study of the Efficacy and Safety of Yokukansan for Neuropsychiatric Symptoms in Patients with Parkinson’s Disease. J. Neural Transm. 2014, 121, 275–281.
- Athauda, D.; MacLagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Skene, S.S.; Chowdhury, K.; Aviles-Olmos, I.; Limousin, P.; Foltynie, T. What Effects Might Exenatide Have on Non-Motor Symptoms in Parkinson’s Disease: A Post Hoc Analysis. J. Parkinsons. Dis. 2018, 8, 247–258.
- Smith, K.M.; Eyal, E.; Weintraub, D. Combined Rasagiline and Antidepressant Use in Parkinson Disease in the ADAGIO Study: Effects on Nonmotor Symptoms and Tolerability. JAMA Neurol. 2015, 72, 88–95.
- Chatterjee, A.; Fahn, S. Methylphenidate Treats Apathy in Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 461–462.
- Gelderblom, H.; Wüstenberg, T.; McLean, T.; Mütze, L.; Fischer, W.; Saft, C.; Hoffmann, R.; Süssmuth, S.; Schlattmann, P.; Van Duijn, E.; et al. Bupropion for the Treatment of Apathy in Huntington’s Disease: A Multicenter, Randomised, Double-Blind, Placebocontrolled, Prospective Crossover Trial. PLoS ONE 2017, 12, 0173872.
- Maier, F.; Spottke, A.; Bach, J.P.; Bartels, C.; Buerger, K.; Dodel, R.; Fellgiebel, A.; Fliessbach, K.; Frölich, L.; Hausner, L.; et al. Bupropion for the Treatment of Apathy in Alzheimer Disease: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e206027.
- Rea, R.; Carotenuto, A.; Traini, E.; Fasanaro, A.M.; Manzo, V.; Amenta, F. Apathy Treatment in Alzheimer’s Disease: Interim Results of the ASCOMALVA Trial. J. Alzheimers. Dis. 2015, 48, 377–383.
- Laplane, D.; Baulac, M.; Widlocher, D.; Dubois, B. Pure Psychic Akinesia with Bilateral Lesions of Basal Ganglia. J. Neurol. Neurosurg. Psychiatry 1984, 47, 377–385.
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The Effectiveness of Exercise Interventions for People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2008, 23, 631–640.
- Bloem, B.R.; de Vries, N.M.; Ebersbach, G. Nonpharmacological Treatments for Patients with Parkinson’s Disease. Mov. Disord. 2015, 30, 1504–1520.
- Ng, S.Y.-E.; Chia, N.S.-Y.; Abbas, M.M.; Saffari, E.S.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L.; Tan, E.-K.; et al. Physical Activity Improves Anxiety and Apathy in Early Parkinson’s Disease: A Longitudinal Follow-Up Study. Front. Neurol. 2021, 11, 625897.
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serdar, A.; Horak, F.B. Effects of Group, Individual, and Home Exercise in Persons With Parkinson Disease: A Randomized Clinical Trial. J. Neurol. Phys. Ther. 2015, 39, 204–212.
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A.; et al. Effects of a Nordic Walking Program on Motor and Non-Motor Symptoms, Functional Performance and Body Composition in Patients with Parkinson’s Disease. NeuroRehabilitation 2015, 37, 245–254.
- Cai, W.; Wang, Y.; Juan, Z.; Cong, Y.; Niu, Y.; Yang, J.; Huang, S. Effects of Dance Therapy on Non-Motor Symptoms in Patients with Parkinson ’ s Disease: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2021, 34, 1201–1208.
- Subramanian, I. Complementary and Alternative Medicine and Exercise in Nonmotor Symptoms of Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 1163–1188.
- Manera, V.; Abrahams, S.; Agüera-Ortiz, L.; Bremond, F.; David, R.; Fairchild, K.; Gros, A.; Hanon, C.; Husain, M.; König, A.; et al. Recommendations for the Nonpharmacological Treatment of Apathy in Brain Disorders. Am. J. Geriatr. Psychiatry 2020, 28, 410–420.
- Oguro, H.; Nakagawa, T. Randomized Trial of Repetitive Transcranial Magnetic Stimulation for Apathy and Depression in Parkinson’s Disease. J. Neurol. Neurophysiol. 2014, 5, 1–6.
- Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Oshino, S.; Morris, S.; Kageyama, Y.; Yokoe, M.; Yoshimine, T.; Saitoh, Y. High-Frequency Repetitive Transcranial Magnetic Stimulation over the Primary Foot Motor Area in Parkinson’s Disease. Brain Stimul. 2013, 6, 884–891.
- Wei, W.; Yi, X.; Ruan, J.; Duan, X.; Luo, H. The Efficacy of Repetitive Transcranial Magnetic Stimulation on Emotional Processing in Apathetic Patients with Parkinson’s Disease: A Placebo-Controlled ERP Study. J. Affect. Disord. 2021, 282, 776–785.
- Montoya-Murillo, G.; Ibarretxe-Bilbao, N.; Peña, J.; Ojeda, N. Effects of Cognitive Rehabilitation on Cognition, Apathy, Quality of Life, and Subjective Complaints in the Elderly: A Randomized Controlled Trial. Am. J. Geriatr. Psychiatry 2020, 28, 518–529.
- Díez-Cirarda, M.; Ojeda, N.; Peña, J.; Cabrera-Zubizarreta, A.; Lucas-Jiménez, O.; Gómez-Esteban, J.C.; Gómez-Beldarrain, M.; Ibarretxe-Bilbao, N. Long-Term Effects of Cognitive Rehabilitation on Brain, Functional Outcome and Cognition in Parkinson’s Disease. Eur. J. Neurol. 2018, 25, 5–12.
- Peña, J.; Ibarretxe-Bilbao, N.; García-Gorostiaga, I.; Gomez-Beldarrain, M.A.; Díez-Cirarda, M.; Ojeda, N. Improving Functional Disability and Cognition in Parkinson Disease Randomized Controlled Trial. Neurology 2014, 83, 2167–2174.
- Butterfield, L.C.; Cimino, C.R.; Salazar, R.; Sanchez-Ramos, J.; Bowers, D.; Okun, M.S. The Parkinson’s Active Living (PAL) Program: A Behavioral Intervention Targeting Apathy in Parkinsons Disease. J. Geriatr. Psychiatry Neurol. 2017, 30, 11–25.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Neurodegeneration
Revisions:
3 times
(View History)
Update Date:
01 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




