
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yonghui Dong | -- | 939 | 2022-07-29 10:13:11 | | | |
| 2 | Beatrix Zheng | + 1908 word(s) | 2847 | 2022-08-01 03:02:44 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 2847 | 2022-08-01 03:15:17 | | | | |
| 4 | Beatrix Zheng | + 9 word(s) | 2856 | 2022-08-01 03:16:21 | | |
Video Upload Options
Anthocyanidin is a kind of water-soluble natural pigment that widely exists in natural plants. Like other natural flavonoids, anthocyanin has a C6-C3-C6 carbon skeleton. Due to the different carbon substituents (-OH, -OCH3) on the B ring, different types of anthocyanins were derived. The six common anthocyanins were Pelargonidin (Pg), Cyanidin (Cy), Delphinidin (Dp), Peonidin (Pn), Petunidin (Pt), and Malvidin (Mv). In addition to giving food a variety of bright colors, anthocyanin also has important biological activities, such as antioxidant, anti-inflammatory, and anti-aging effects, among others. A large number of studies have shown that dietary anthocyanins have a good preventive effect on cardiovascular diseases.
1. Structure of Anthocyanin
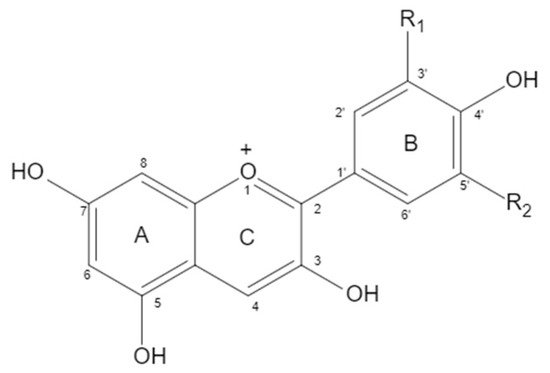
| Anthocyanin | Proportion | Substituents | |
|---|---|---|---|
| R1 | R2 | ||
| Cyanidin | 50% | OH | H |
| Delphindin | 12% | OH | OH |
| Pelargonidin | 12% | H | H |
| Peonidin | 12% | OCH3 | H |
| Petunidin | 7% | OCH3 | OH |
| Malvidin | 7% | OCH3 | OCH3 |
2. Physiological Activities of Anthocyanins
2.1. Anti-Cancer
2.2. Anti-Inflammatory
2.3. Anti-Oxidation
2.4. Protective Effect on the Liver
2.5. Lowering Blood Glucose
2.6. Anti-Aging
2.7. Other Effects
| Effects | Source | Mechanisms | Ref. | |
|---|---|---|---|---|
| Anti-cancer | colon cancer | Purple grape anthocyanins |
|
[8] |
| colon cancer | Cyanidin-3-O-rutinoside |
|
[9] | |
| colon cancer | Purple and red maize anthocyanins |
|
[10] | |
| breast cancer | Black sweet cherry anthocyanins |
|
[3] | |
| melanoma cancer | Hibiscus calyx anthocyanin |
|
[11] | |
| Purple sweet potato anthocyanin |
|
[12] | ||
| Anti-inflammatory |
|
[15] | ||
|
[16] [19] |
|||
| Cyanidin-3-glucoside |
|
[17] | ||
| Geranium pigment-3-O-glucoside in strawberry |
|
[21] | ||
| Hibiscus anthocyanin |
|
[22] | ||
| Anti-oxidation | Purple corn anthocyanin |
|
[25] | |
| Cyanidins in radish buds |
|
[26] | ||
| Mahonia aquifolium anthocyanin |
|
[27] | ||
| Black rice anthocyanin extract (cyanidin-3-O-glucoside) |
|
[28] | ||
| Blueberry anthocyanins |
|
[29] | ||
| Protective effect on liver | Cyanidin-3-O-glucoside |
|
[31] | |
| Riceberry bran anthocyanin |
|
[32] | ||
| Purple sweet potato anthocyanin |
|
[33] | ||
| Purple sweet potato anthocyanin |
|
[34] | ||
| Lowering blood glucose | Purple corn anthocyanins |
|
[47] | |
| Mulberry anthocyanin |
|
[36] | ||
| Anti-aging | Purple sweet potato anthocyanin |
|
[40] | |
| Cy-3-glu Pg-3-glu |
|
[41] | ||
| Ribes meyeri anthocyanins |
|
[42] | ||
|
[43] | |||
References
- Goncalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688.
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69.
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710.
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214.
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261.
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560.
- Long, H.L.; Zhang, F.F.; Wang, H.L.; Yang, W.S.; Hou, H.T.; Yu, J.K.; Liu, B. Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262.
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909.
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierma, E.; Clapper, M.L.; Carvalho, R.F.; Barbisa, L.F. Lyophilized acai pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 121, 237–245.
- Mazewski, C.; Liang, K.; de Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265.
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631.
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917.
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165.
- Akiyama, S.; Nesumi, A.; Maeda-Yamamoto, M.; Uehara, M.; Murakami, A. Effects of anthocyanin-rich tea “Sunrouge” on dextran sodium sulfate-induced colitis in mice. Biofactors 2012, 38, 226–233.
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer effects of bioactive berry compounds. Phytochem. Rev. 2013, 13, 295–322.
- Hou, D.X.; Kai, K.; Li, J.J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 2004, 25, 29–36.
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966.
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425.
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-kappaB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016, 23, 846–855.
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-gamma gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39.
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simoes, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65.
- Karunarathne, W.; Lee, K.T.; Choi, Y.H.; Jin, C.Y.; Kim, G.Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-kappaB signaling pathway. Phytomedicine 2020, 76, 153237.
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151.
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604.
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118.
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397–406.
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 2017, 35, 166–174.
- Lu, X.; Zhou, Y.; Wu, T.; Hao, L. Ameliorative effect of black rice anthocyanin on senescent mice induced by D-galactose. Food Funct. 2014, 5, 2892–2897.
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of Blueberry Anthocyanins Malvidin and Glycosides on the Antioxidant Properties in Endothelial Cells. Oxid. Med. Cell. Longev. 2016, 2016, 1591803.
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24.
- Jiang, X.; Guo, H.; Shen, T.; Tang, X.; Yang, Y.; Ling, W. Cyanidin-3-O-beta-glucoside Purified from Black Rice Protects Mice against Hepatic Fibrosis Induced by Carbon Tetrachloride via Inhibiting Hepatic Stellate Cell Activation. J. Agric. Food Chem. 2015, 63, 6221–6230.
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich Riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420.
- Zhang, Z.F.; Lu, J.; Zheng, Y.L.; Wu, D.M.; Hu, B.; Shan, Q.; Cheng, W.; Li, M.Q.; Sun, Y.Y. Purple sweet potato color attenuates hepatic insulin resistance via blocking oxidative stress and endoplasmic reticulum stress in high-fat-diet-treated mice. J. Nutr. Biochem. 2013, 24, 1008–1018.
- Cai, Z.; Song, L.; Qian, B.; Xu, W.; Ren, J.; Jing, P.; Oey, I. Understanding the effect of anthocyanins extracted from purple sweet potatoes on alcohol-induced liver injury in mice. Food Chem. 2018, 245, 463–470.
- Gowd, V.; Jia, Z.Q.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13.
- Hong, S.H.; Heo, J.I.; Kim, J.H.; Kwon, S.O.; Yeo, K.M.; Bakowska-Barczak, A.M.; Kolodziejczyk, P.; Ryu, O.H.; Choi, M.K.; Kang, Y.H.; et al. Antidiabetic and Beta Cell-Protection Activities of Purple Corn Anthocyanins. Biomol. Ther. 2013, 21, 284–289.
- Sarikaya, I.; Schierz, J.-H.; Sarikaya, A. Liver: Glucose metabolism and 18F-fluorodeoxyglucose PET findings in normal parenchyma and diseases. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 233–249.
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745.
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898.
- Zhao, J.G.; Yan, Q.Q.; Lu, L.Z.; Zhang, Y.Q. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr. Res. Pract. 2013, 7, 359–365.
- Wang, Y.; Zhang, D.; Liu, Y.; Wang, D.; Liu, J.; Ji, B. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J. Sci. Food Agric. 2015, 95, 936–944.
- Gao, J.M.; Wu, Y.T.; He, D.J.; Zhu, X.Q.; Li, H.B.; Liu, H.F.; Liu, H.L. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging 2020, 12, 17738–17753.
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J. Anthocyanins Delay Ageing-Related Degenerative Changes in the Liver. Plant Foods Hum. Nutr. 2017, 72, 425–431.
- Qin, S.; Sun, D.; Mu, J.; Ma, D.; Tang, R.; Zheng, Y. Purple sweet potato color improves hippocampal insulin resistance via down-regulating SOCS3 and galectin-3 in high-fat diet mice. Behav. Brain Res. 2019, 359, 370–377.
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003.
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287.
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219.




