Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sheersha Pramanik | -- | 3200 | 2022-07-29 08:02:25 | | | |
| 2 | Camila Xu | + 5 word(s) | 3205 | 2022-07-29 08:57:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-Based Hydrogels. Encyclopedia. Available online: https://encyclopedia.pub/entry/25645 (accessed on 06 March 2026).
Ansari MJ, Rajendran RR, Mohanto S, Agarwal U, Panda K, Dhotre K, et al. Poly(N-isopropylacrylamide)-Based Hydrogels. Encyclopedia. Available at: https://encyclopedia.pub/entry/25645. Accessed March 06, 2026.
Ansari, Mohammad Javed, Rahul R. Rajendran, Sourav Mohanto, Unnati Agarwal, Kingshuk Panda, Kishore Dhotre, Ravi Manne, A. Deepak, Ameeduzzafar Zafar, Mohd Yasir, et al. "Poly(N-isopropylacrylamide)-Based Hydrogels" Encyclopedia, https://encyclopedia.pub/entry/25645 (accessed March 06, 2026).
Ansari, M.J., Rajendran, R.R., Mohanto, S., Agarwal, U., Panda, K., Dhotre, K., Manne, R., Deepak, A., Zafar, A., Yasir, M., & Pramanik, S. (2022, July 29). Poly(N-isopropylacrylamide)-Based Hydrogels. In Encyclopedia. https://encyclopedia.pub/entry/25645
Ansari, Mohammad Javed, et al. "Poly(N-isopropylacrylamide)-Based Hydrogels." Encyclopedia. Web. 29 July, 2022.
Copy Citation
Poly(N-isopropylacrylamide) (PNIPAM) is a widely utilized negative thermosensitive polymer (as it has increased solubility with lowering of temperature, causing volume phase transition by forming hydrogen bonds) that has currently sparkled a lot of scientific inquisitiveness. Smart hydrogels based on PNIPAM demonstrate distinct thermoresponsive features close to a lower critical solution temperature (LCST) that enhance their capability in various biomedical applications such as drug delivery, tissue engineering, and wound dressings.
PNIPAM
hydrogels
drug delivery
tissue engineering
1. Brief on Poly(N-isopropylacrylamide) (PNIPAM)-Based Hydrogel
Poly(N-isopropylacrylamide) (PNIPAM) is a widely utilized negative thermosensitive polymer (as it has increased solubility with lowering of temperature, causing volume phase transition by forming hydrogen bonds) that has currently sparkled a lot of scientific inquisitiveness [1][2]. In the aqueous medium, PNIPAM assembles a stretched spiral elastic shape; polymer molecules form hydrogen bonds with each other due to hydrogen bonding with molecules of water. It has both hydrophobic isopropyl (–CH(CH3)2) side groups and hydrophilic amide (–CONH–) groups in the structure. PNIPAM has an LCST of 32 °C, which is somewhat lower than human body temperature of 37 °C, and it can be modified by copolymerizing with some distinct hydrophobic or hydrophilic polymers to develop thermo-sensitive, in situ hydrogels. [3][4][5]. The temperature-responsive behaviors are primarily due to connections between these groups and solvent molecules. As a result, PNIPAM converts from solution to gel state as the LCST reaches human body temperature [6][7][8][9][10]. Additional hydrophilic monomers increase the polymer’s hydrophilicity and lead to more significant interactions with water, resulting in a higher LCST, whereas copolymerization with more hydrophobic monomers reduces LCST [11]. This property makes PNIPAM suitable for biomedical applications, e.g., controlled wound dressings, tissue engineering scaffolds, and drug delivery systems [12][13]. Notwithstanding these appealing benefits, PNIPAM-based hydrogels nevertheless have certain drawbacks, including limited biodegradability [14], weak mechanical strength [15], insufficient drug loading capacity [16], and immediate release [15][17][18], etc.; limiting the feasibility in drug delivery.
Several drugs can be efficiently encapsulated into the hydrophilic polymers by using a lower temperature to dissolve the drug in the solvent to ensure efficient delivery, raising the temperature slightly above the LCST for hydrogel appearance. Furthermore, maintaining precise temperature control permits site-specific drug delivery and delayed release. The PNIPAM solution can be rapidly applied to the skin and activated by utilizing body temperature to create a 3D hydrogel that can help restore tissues by encouraging cell proliferation and differentiation while preserving the homeostatic activity of cells. The temperature-dependent modulation of gel coating and reversible sol-to-gel can be utilized to develop adherence to the skin and its dissociation for wound treatment. Other features of PNIPAM, in conjunction with thermosensitivity, include adjustable geometries and nontoxicity, both of which are advantageous for biological applications [19]. Wound dressings play a crucial part in lesion care, and their effectiveness impacts how quickly a wound heals [20]. That a warm, moist environment promotes faster healing has been widely demonstrated in several empirical investigations in both in vivo and in vitro wound healing [21][22]. Various medical studies also recommend that dressings should be translucent to allow easy observation, and that they have the ability to retain fluids [23][24][25]. Aside from the properties described above, zwitterionic hydrogels have lately gained popularity as nonadherent wound dressings due to their high resistance to cell attachment, protein adsorption, and microbial adherence [26][27]. For wound dressing, PNIPAM can be utilized to retain the skin adhesion and differentiation of gel coatings. Its thermosensitivity is complemented by LCST adjustability, low toxicity, and [24] variable structure. [28], making it suitable for biomedical applications [19].
Many contemporary tissue engineering techniques rely on the use of a material scaffold. These scaffolds act as an artificial extracellular matrix (ECM), allowing cells to be organized into a three-dimensional framework and stimulating the growth and creation of desirable tissue [29]. The scaffold material and qualities required vary greatly depending on the tissue of concern and the individual application [30]. Thermoresponsive polymers are extensively employed in tissue engineering in two ways: platforms for cell proliferation and injectable solutions for in situ scaffolding [31].
The primary disadvantage of PNIPAM-based hydrogels in drug delivery is the incorporation of hydrophobic drugs in the water-loving polymeric core [32], since mostly hydrophobic drugs are typically and effectively used in disease treatment. Along with this, the tensile strength [15] of these hydrogels is weak, which may sometimes lead to early drug release before arrival at the specific site [33]. Several researchers are working painstakingly to improve the mechanical characteristics of PNIPAM hydrogels to fix the reported shortcomings. Hybridization of PNIPAM-based composite hydrogels with appropriate materials such as inorganic/metal nanoparticles [34], organic self-assemblies [16], and several other polymeric material incorporations/composite formations are some reported viable techniques for achieving this goal [35][36]. A novel polymeric block could regulate the thermal sensitivity of the resulting hydrogel by adjusting the hydrophilic/hydrophobic equilibrium of PNIPAM. Alternatively, a novel polymeric component can affect the hydrogel network’s chemical rigidity and morphology, modulating the copolymeric hydrogel’s mechanical properties [37][38][39]. The successful composite formation of PNIPAM with various materials, e.g., hyaluronic acid (HA) [40][41], zinc oxide (ZnO) nanoparticles [42], silver nanoparticles [43], hydroxyapatite (HAp) nanoparticles [44], carbon nanoparticles [45], etc.; for tissue engineering and wound healing has been reported in several investigations [46][47][48]. Adequate biocompatibility, higher tensile strength, customizable temperature responsiveness, multi-drug loading capability, targeted drug delivery, and controlled drug delivery are among the unique and/or improved features of composite hydrogels [19]. The inclusion of some other polymer inside the hydrogel matrix to make interpenetrating polymeric network system (IPN) hydrogels for biomedical application [49] is another useful way to alter the functionalities of a hydrogel. The polymeric networks in IPN hydrogels can efficiently adjust mechanical characteristics, swelling/deswelling behavior, and drug loading/release pattern [50].
2. Unique Properties of PNIPAM
Physically crosslinked hydrogels can be assembled via ionic/hydrogen bond formation, Van der Waals forces, hydrophobic interactions, crystalline structure, etc. [51]. The strong hydrogen bonding between the polymeric chains in the hydrogel may promote drug release, which can be further controlled via the type of solvent, degree of sonication, solution temperature, polymer concentration, etc.; in hydrogel formulation. Another method, crosslinking, has been most commonly utilized to overcome biomaterial physicochemical and mechanical limitations via strong interconnections between the reaction molecules. Furthermore, ionic interactions can be initiated at room temperature and physiological pH to overcome constraints [52]. Bifunctional crosslinking agent-initiated hydrogels are generally developed via several techniques, e.g., condensation polymerization, irradiation using high-energy ionizing radiation such as electron beams, gamma rays, or X-rays, chain-growth polymerization, etc. [52].
In biomedical science, PNIPAM-based hydrogels have versatile applications for the effective therapeutic delivery of molecules by modulating substance movement in a medium, altering thermo-controlled dimensions [53]. A monomeric structure of PNIPAM commonly identified by isopropyl and amide moieties maintains LCST at ~32 °C in aqueous conditions. PNIPAM hydrogels are developed by crosslinking themselves or their derivates, exhibiting a reversible and extreme volume phase transition towards LCST through swelling/shrinking [54]. Along with the changes in size during the transition phase, there are also several other changes in many properties, such as hydrophilicity [55][56], transparency [57], and apparent electrostatic permittivity [58]. The mixing of polymer in a solvent leads to polymeric dissolution at a specific temperature (T), which the negative ΔGmix of the Gibbs free energy equation can well indicate. The involvement of thermodynamics in the method forms hydrogen bonds that create significant negative enthalpy change of mixing (ΔHmix < 0), contributing to dissolution. Lowering PNIPAM’s temperature below LCST develops a counterbalance between the hydrogen bonds of hydrophilic amide groups and molecules of water, leading to hydrophobic interactions among isopropyl groups and hence collapsing the PNIPAM chain to avoid water contact [54].
PNIPAM hydrogels exhibit a slow response to environmental temperature variations due to the formation of the thick skin surface, decreasing the outward diffusion of water molecules and causing hydrogel collapse at temperatures above LCST [59][60][61]. Likewise, the swelling rate of hydrogels is relatively slower at temperatures lower than LCST. Therefore, improving the rate of thermoresponsiveness of established PNIPAM hydrogels has become a research subject [62]. Some physical strategies have been involved in enhancing the thermal responsiveness of PNIPAM hydrogels, such as phase separation [63], porogen [64], freezing [59], vacuum-synthesis [65], and interpenetrating polymer networks [66]. Along with these, alternate chemical approaches have also been introduced, which include the addition of hydrophilic moieties [67], grafting freely mobile hydrophilic moieties [68], and reversible addition–fragmentation chain transfer (RAFT) polymerization [69] to attain a rapid response rate. Stimuli-responsive hydrogels require mechanical properties for various applications. Hence, to improve the PNIPAM hydrogel‘s mechanical properties, some techniques have been implemented, such as an interconnected polymer network [70], a hydrogel with two networks [71], a hydraulic ring for slides [72], nanoporous PNIPAM hydrogel [73], and copolymerized PNIPAM hydrogel [74]. Free radical redox polymerization of PNIPAM hydrogels is relatively weak and can even be affected using standard mechanical testing equipment [75].
Takigawa et al. determined PNIPAM hydrogel’s Young’s modulus (E0) for the first time in 1997. They found a stress–strain graph of the collapsed and swelled gel to be linear in a specific hardness strength test, where the E0 was a hundred times more for the collapsed state. The swelled and collapsed conditions were found to have fracture strains of 35% and 75%, respectively [75]. The collapsed state was found to have a higher E0 than the swollen state, as the collapsed gels had an increased number of crosslinks. The change in E0 at the initial stage of the scaffold was probably due to rapid relaxation shown by the macroporous hydrogel, which remains constant for a more extended period during cellular growth [76].
PNIPAM hydrogel has inadequate mechanical performance in the highly swelled state, prominently identified as a disadvantage in drug delivery. Its nonbiodegradable nature has led to surgical excision after the release of the drug. Removing the device by typical surgical operations is also challenging if it is too light, as it might break down during handling. However, PNIPAM hydrogel’s lack of controlled release capability, i.e.; leading to the release of impregnated drugs within 24 h, is a severe reason for the possibility of being restricted as a drug carrier [77][78][79]. A swelled PNIPAM hydrogel with a decreased polymeric mass per unit volume explains its poor mechanical characteristics and increased drug release rate. The decreased polymeric mass per unit volume results in the fast release of impregnated drugs because of the gel’s open pores and low mechanical characteristics. Rapid drug release from the drug reservoir is because of the weak intermolecular bonds of swelled PINPAM hydrogel [66].
In an aqueous phase at a certain temperature range of about 32 °C, for drug delivery, the PNIPAM hydrogel could be combined with bioactive components to form a solution. The introduction of polymers becomes possible in that particular physical condition. The subcutaneous injection of the polymer-loaded gel leads to sustained release and an immediate increase in physiological temperature (to about 36.5–37.5 °C). The biodegradability of the hydrogel leads to the release of encapsulated bioactive compounds, initially in the body via diffusion and later on by a mixture of diffusion and mechanical breakdown [80]. Both physical and chemical properties (melting point, temperature, glass transition storage modulus, crystallinity, etc.) are responsible for the biodegradability of the polymer [81]. The reduced biodegradability of PNIPAM hydrogel has restricted its use in clinical practice. Different crosslinking agents and/or biodegradable polymers or native polymers, including poly(amino acids) [82], polysaccharides [83], proteins [84], and synthetic polymers including poly(esters) [85], poly(caprolactone) [14], and poly(ethylene glycol) [86], have now been analyzed for the development of biodegradable PNIPAM hydrogels [80]. PNIPAM is also highly biocompatible with animal cells [80]. Cao et al. explored the use of PNIPAM–chitosan copolymers for ophthalmic drug delivery. The copolymer was utilized for encapsulation of timolol maleate molecules for around 12 h to effectively lower intraocular pressure (IOP). The in vivo study of PNIPAM–chitosan for thermo-sensitive hydrogels confirmed non-cytotoxicity, hence furnishing new insights into glaucoma therapy along with several other eye illnesses [87].
Biopolymers or artificially degradable compounds help alter the chemical composition of PNIPAM to obtain biodegradability and biocompatibility effectively. In a study by Das et al.; covalently crosslinked PNIPAM hydrogels utilizing NIPAM as the monomer, dextrin as the biopolymer, potassium persulfate (KPS) N,N′-methylene bisacrylamide (MBA) as the promoter, and N,N′-methylene bisacrylamide (MBA) as the crosslinker were effectively synthesized. Ciprofloxacin and ornidazole could be administered in a controlled manner by the novel PNIPAM hydrogel, as it is nontoxic and biodegradable [88].
3. Phase Transition for PNIPAMs
The polyacrylamide structure is mostly crafted with a hydrophilic amide group (almost 90%) and -C-C- hydrophobic portion. However, poly(N-isopropyl-acrylamide) and other N-substituted acrylamide polymers (PNIPAM) have balanced hydrophilic and hydrophobic regions below LCST. The gel-polymer/water system’s total energy is lowered due to hydrophobic polymers enveloped by water molecules below LCST [89]. The solvation and transition capacity of PNIPAM in cold water increases when the temperature is raised off its LCST (LCST ≈ 32–34 °C), leading to the “coil to globule” of the polymeric chain’s (CG) transition. The CG transition is in charge of phase inversion into rich layers. Polymeric/water phases further exhibit volume phase transition (VPT) [90]. A loss in entropy of water molecules enveloping the hydrophilic polymeric chain is counterbalanced by an increase in enthalpy owing to hydrogen bonding between the hydroxyl groups surrounding the polymeric chain’s hydrophobic sections. Hydrophobic hydration is a process that allows a hydrophobic polymer to stay hydrated in an aqueous environment. If the temperature is increased above LCST, water molecules leave the polymer chain and form a globule structure. As a result, the PNIPAM–polymer is hydrated, and a definite volume phase transition is observed. The phase separation initially occurs due to PNIPAM molecule incorporation into larger aggregates [91][92] via several mechanisms and factors, e.g., dewetting caused by solvent fluctuations, cooperative hydration [93][94][95], the aqueous medium’s energy state [96], endothermic heat [97], precipitation polymerization [98], etc. The hydrogen bond between water molecules and PNIPAM is weaker due to the temperature rising above LCST, leading to the formation of an unstable solution. Further, the transitions of the PNIPAM–polymer can be confirmed by FTIR spectroscopy; the hydrodynamic diameter of PNIPAM gel affects the volume phase transition, causing dehydration of the polymeric gel [90].
The dependency of LCST on molecular weight and concentration of PNIPAM polymer in H2O and D2O can be confirmed via dielectric relaxation spectroscopy with small- and wide-angle x-ray scattering (SWAXS) (DRS) to show phase transition. Several investigations have reported a strong effect of interpenetration between the diverse chains of PNIPAM at high concentration decreasing correlation length (ξ). The molecular weight of PNIPAM is entirely independent of ξ at higher concentrations due to PNIPAM chains’ crammed state [92]. Interface formation and interchange aggregation are reported to be higher as a result of lowering the molecular weight of the polymer [92]. Several investigations have studied the phase transition effect due to LCST and confirmed peculiar behavior prevails with hydration [94][99][100][101]. The phase transition of the PNIPAM network can also be modulated by precipitation polymerization at 37−45 °C (near the LCST). Several analytical studies, e.g., atomic force microscopy (AFM) with photon correlation spectroscopy (PCS), etc.; have reported narrow, dispersed, spherical microgel and hydrogel volume phase transition behaviors [102][103]. Moreover, the phase transition property of PNIPAM-based hydrogel systems is advantageous due to its high mechanical strength and encapsulation capability, which has gained more attention in biomedical and tissue engineering applications [104].
4. Preparation of PNIPAM-Based Hydrogel Systems
The ideal hydrophilic polymer-based hydrogel possesses good biocompatibility and cell viability, excellent mechanical strength (especially stiffness for tissue engineering), high adhesion, moisture retention, promotion of cell proliferation, and high absorption capacity of the fluid for tissue engineering and wound healing [105]. Hydrogels are hydrophilic/hydrophobic monomeric unit-based systems that can be crosslinked via several techniques (e.g., physical and chemical crosslinking) to produce an elastic structure that can be affected/modulated via monomer, initiator, and crosslinker selection [106]. The synthesis or preparation of hydrogels with various functional properties can be initiated with physical and chemical crosslinking [105]. Several physical crosslinking methods (as displayed in Figure 1), e.g., ionic interaction, hydrophobic bonds, protein interaction, etc.; may improve the toughness and self-healing ability of the hydrogel system, which is further required for biomedical application. The hydrogels formed by ionic interaction, i.e.; dynamic interaction of the negatively charged groups or metal-ligand interactions, have improved self-healing, ionic conductivity, biological properties, etc. However, various limitations have also been reported, e.g., poor mechanical characteristics, complex/strong bonds between polymers, etc.; limiting the preparation technique’s usage [105]. Several investigations have been reported e.g., alginate/N-isopropylacrylamide (NIPAM) hydrogel [107], PNIPAM/poly(sodium acrylate) hydrogel [108], methacryloylchitosan/PNIPAM hydrogel [109], polyacrylamide/sodium alginate IPN hydrogel [110], etc.; based on ionic interaction of PNIPAM-based hydrogel systems for improving stiffness, cell viability, and mechanical integrity of the hydrogel system. Another physical crosslinking method involves the usage of dynamic hydrogen bond interaction, which is often unstable in an aqueous environment but is possible to rebuild after breaking; it can improve self-healing, cell biocompatibility, biodegradability, etc.; properties [111] via an IPN hydrogel preparation effective in wound-healing dressing. Several investigations, e.g., chitosan-poly (vinyl alcohol) (PVA) DN (double network) hydrogel [111], sodium alginate (SA)/polyacrylamide (PAM) semi-IPN hydrogel [112], hydrazide-functionalized PNIPAM/dialdehyde dextrin thermoresponsive hydrogel [113], etc.; have reported, based on dynamic hydrogen bond mechanistic, PNIPAM hydrogel preparations for biomedical application. Another commonly utilized physical crosslinking method, freeze–thaw, can form ice crystal and fabricate the polymeric chain around the crystals, followed by fabrication of a microporous structure while melting the crystals [114]. The freeze–thaw method can proliferate stem cells and ECM deposition [115], cell compatibility, and biodegradability [116] in the biomedical application based on the modulation of temperature, time, number of cycles, polymer contents, etc. [116][117]. Some recent investigations, e.g., PNIPAM hydrogel [118], PNIPAM/cellulose nanocrystal hybrid hydrogel [119], chitosan-graft-PNIPAM/PVA hydrogel [120], etc.; utilized the freeze–thaw physical crosslinking method and were reported to have high encapsulation efficiency, stimuli-responsive, adsorption capacity, etc. Hydrophilic polymers with hydrophobic end groups/side chains/monomers can be associated via physical crosslinking, resulting in high mechanical strength via strong hydrophobic interaction [105]. Several studies e.g., polyacrylamide (PAAm)/polyacrylic acid (PAAc)/PNIPAM hydrogel [121], poly(N-isopropylacrylamide) hydrogel [122][123], etc.; have been successfully prepared and reported high mechanical strength for biomedical application. The low mechanical stability and strength of physically crosslinked, reversible hydrogels may improve the utilization of chemical crosslinkers connected via covalent interactions. Diverse chemical crosslinking mechanisms have been reported, e.g., conjugation reaction, free radical polymerization, enzymatic reaction, etc.; where the hydrogel was formed via covalent bonds [124]. Conjugation occurred in mild conditions in the presence of Michael addition, Schiff’s base, Diels–Alder addition, etc.; which are green methods in the presence of condensate functional groups to improve biodegradability, transparency, and adhesiveness of the hydrogel system [105][124]. To overcome the limitations associated with the mechanical properties of hydrogel prepared by conjugation, free radical polymerization was reinforced via heating, ultraviolet radiation, energy radiation, electrolysis, etc.; to improve the swelling, porosity, and mechanical strength of the hydrogel system for biomedical application [124]. Several studies, e.g., PNIPAM/magnetite nanoparticles hydrogel [125], poly(N-isopropylacrylamide) hydrogel [126], PNIPAM–Ln(DPA)3 hydrogels [127], PNIPAM/gold nanocluster hydrogel [128], etc.; have utilized free radical polymerization for PNIPAM-based hydrogel preparation to improve mechanical strength and stability. The enzymatic reaction of natural polysaccharides in the presence of several enzymes, e.g., transglutaminase, tyrosinase, urease, horseradish peroxidase, etc.; occurs in very mild conditions and can retain the biological properties and improve the mechanical strength of the polymers utilized for hydrogel preparation. In various reports, e.g., N-isopropylacrylamide (NIPAM) and acrylic acid (AAc) hydrogel [129], vinylimidazole/PNIPAM) hydrogel [130], NaCMC/PNIPAM hydrogels [131], etc.; enzymatic crosslinking was seen to be effective for improving the mechanical stability and strength of PNIPAM-based hydrogels.
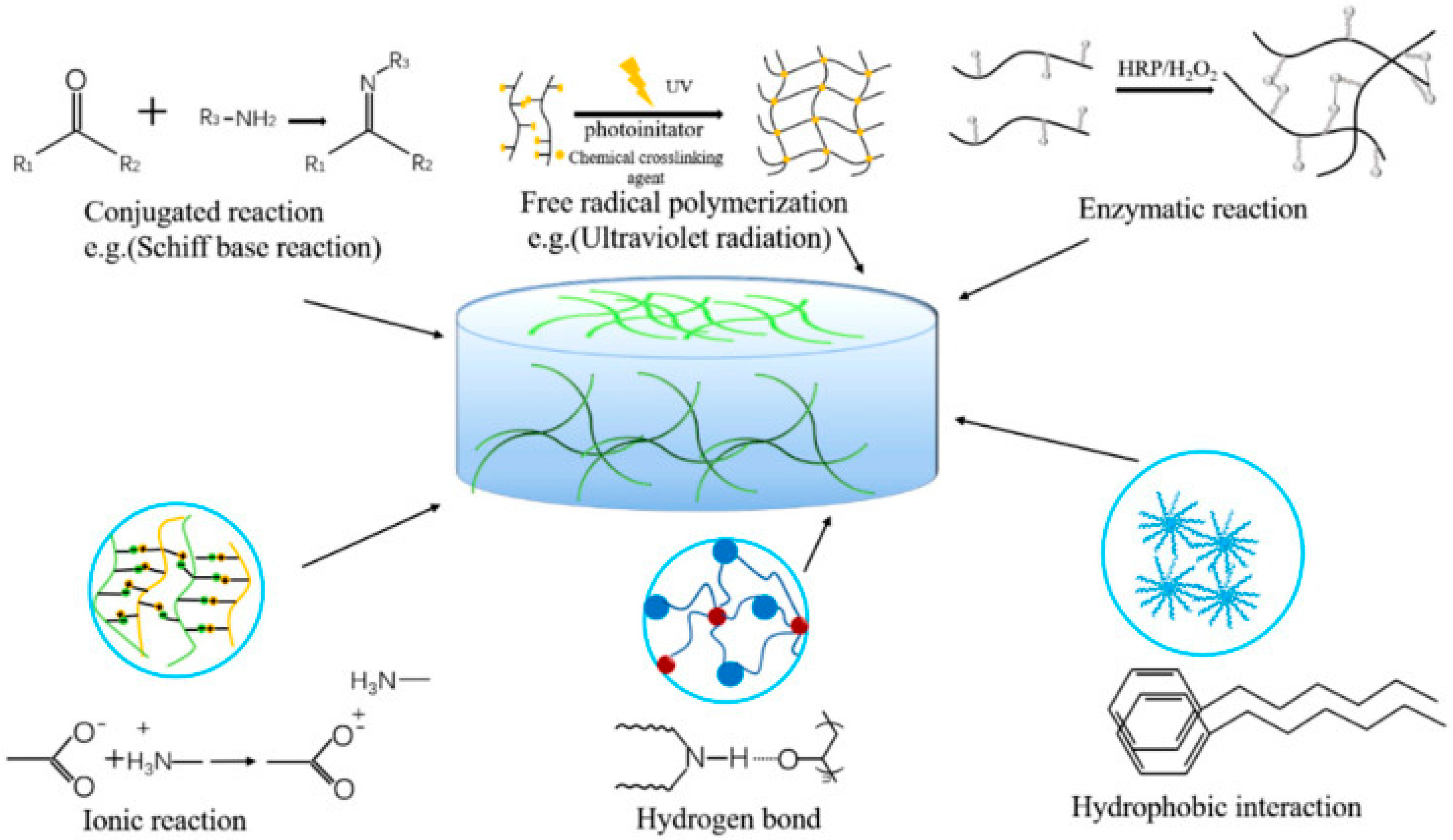 Figure 1. Physical and chemical crosslinking methods to prepare hydrogel systems for biomedical application [104].
Figure 1. Physical and chemical crosslinking methods to prepare hydrogel systems for biomedical application [104].References
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133.
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518.
- Wang, G.; Chen, X.; Liu, S.; Wong, C.; Chu, S. Copolymer Brushes with Temperature-Triggered, Reversibly Switchable Bactericidal and Antifouling Properties for Biomaterial Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 27207–27217.
- Zhao, D.; Ma, W.; Wang, R.; Yang, X.; Li, J.; Qiu, T.; Xiao, X. The preparation of green fluorescence-emissioned carbon dots/poly(N-isopropylacrylamide) temperature-sensitive hydrogels and research on their properties. Polymers 2019, 11, 1171.
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740.
- Kim, S.; Lee, K.; Cha, C. Refined control of thermoresponsive swelling/deswelling and drug release properties of poly (N-isopropylacrylamide) hydrogels using hydrophilic polymer crosslinkers. J. Biomater. Sci. Polym. Ed. 2016, 27, 1698–1711.
- Oak, M.; Mandke, R.; Singh, J. Smart polymers for peptide and protein parenteral sustained delivery. Drug Discov. Today Technol. 2012, 9, e131–e140.
- Turturro, S.B.; Guthrie, M.J.; Appel, A.A.; Drapala, P.W.; Brey, E.M.; Pérez-Luna, V.H.; Mieler, W.F.; Kang-Mieler, J.J. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011, 32, 3620–3626.
- Gupta, M.K.; Martin, J.R.; Dollinger, B.R.; Hattaway, M.E.; Duvall, C.L. Thermogelling, ABC Triblock Copolymer Platform for Resorbable Hydrogels with Tunable, Degradation-Mediated Drug Release. Adv. Funct. Mater. 2017, 27, 1–14.
- McCune, J.A.; Mommer, S.; Parkins, C.C.; Scherman, O.A. Design Principles for Aqueous Interactive Materials: Lessons from Small Molecules and Stimuli-Responsive Systems. Adv. Mater. 2020, 32, 1–14.
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339.
- Zhao, Y.; Shi, C.; Yang, X.; Shen, B.; Sun, Y.; Chen, Y.; Xu, X.; Sun, H.; Yu, K.; Yang, B.; et al. PH- and Temperature-Sensitive Hydrogel Nanoparticles with Dual Photoluminescence for Bioprobes. ACS Nano 2016, 10, 5856–5863.
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; Le Meins, J.F.; Latxague, L. A thermosensitive low molecular weight hydrogel as scaffold for tissue engineering. Eur. Cells Mater. 2012, 23, 147–160.
- Gan, J.; Guan, X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016, 6, 32967–32978.
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855.
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580.
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft. Matter. 2009, 5, 511–524.
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585.
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159.
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342.
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321.
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835.
- Ghavaminejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223.
- Singer, A.J.; Dagum, A.B. Current Management of Acute Cutaneous Wounds. N. Engl. J. Med. 2008, 359, 1037–1046.
- Chang, Y.; Yandi, W.; Chen, W.Y.; Shih, Y.J.; Yang, C.C.; Chang, Y.; Ling, Q.D.; Higuchi, A. Tunable bioadhesive copolymer hydrogels of thermoresponsive poly(N-isopropyl acrylamide) containing zwitterionic polysulfobetaine. Biomacromolecules 2010, 11, 1101–1110.
- Mi, L.; Xue, H.; Li, Y.; Jiang, S. A thermoresponsive antimicrobial wound dressing hydrogel based on a cationic betaine ester. Adv. Funct. Mater. 2011, 21, 4028–4034.
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam); amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064.
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689.
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351.
- Cunliffe, D.; Alarcón, C.d.; Peters, V.; Smith, J.R.; Alexander, C. Thermoresponsive surface-grafted poly(N-isopropylacrylamide) copolymers: Effect of phase transitions on protein and bacterial attachment. Langmuir 2003, 19, 2888–2899.
- Nie, L.; Li, J.; Lu, G.; Wei, X.; Deng, Y.; Liu, S.; Zhong, S.; Shi, Q.; Hou, R.; Sun, Y.; et al. Temperature responsive hydrogel for cells encapsulation based on graphene oxide reinforced poly(N-isopropylacrylamide)/hydroxyethyl-chitosan. Mater. Today Commun. 2022, 31, 103697.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603.
- Zhang, C.L.; Cao, F.H.; Wang, J.L.; Yu, Z.L.; Ge, J.; Lu, Y.; Wang, Z.H.; Yu, S.H. Highly Stimuli-Responsive Au Nanorods/Poly(N-isopropylacrylamide) (PNIPAM) Composite Hydrogel for Smart Switch. ACS Appl. Mater. Interfaces 2017, 9, 24857–24863.
- Wang, C.; Flynn, N.T.; Langer, R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv. Mater. 2004, 16, 1074–1079.
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453.
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207.
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227.
- Wu, S.W.; Liu, X.; Miller, A.L.; Cheng, Y.S.; Yeh, M.L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316.
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853.
- Dadoo, N.; Gramlich, W.M. Spatiotemporal Modification of Stimuli-Responsive Hyaluronic Acid/Poly(N-isopropylacrylamide) Hydrogels. ACS Biomater. Sci. Eng. 2016, 2, 1341–1350.
- Zhang, J.; Huang, Q.; Du, C.; Peng, R.; Hua, Y.; Li, Q.; Hu, A.; Zhou, J. Preparation and Anti-Mold Properties of Nano-ZnO/Poly(N-isopropylacrylamide) Composite Hydrogels. Molecules 2020, 25, 4135.
- Bajpai, S.K.; Bajpai, M.; Sharma, L. In Situ Formation of Silver Nanoparticles in Poly(N-isopropyl Acrylamide) Hydrogel for Antibacterial Applications. Des. Monomers Polym. 2011, 14, 383–394.
- Wei, J.; He, P.; Liu, A.; Chen, X.; Wang, X.; Jing, X. Surface Modification of Hydroxyapatite Nanoparticles with Thermal-Responsive PNIPAM by ATRP. Macromol. Biosci. 2009, 9, 1237–1246.
- Liu, X.; Song, T.; Chang, M.; Meng, L.; Wang, X.; Sun, R.; Ren, J. Carbon Nanotubes Reinforced Maleic Anhydride-Modified Xylan-g-Poly(N-isopropylacrylamide) Hydrogel with Multifunctional Properties. Materials 2018, 11, 354.
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130.
- Han, X.; Xu, H.; Che, L.; Sha, D.; Huang, C.; Meng, T.; Song, D. Application of Inorganic Nanocomposite Hydrogels in Bone Tissue Engineering. iScience 2020, 23, 101845.
- Wahid, F.; Zhao, X.-J.; Jia, S.-R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208.
- Matricardi, P.; di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187.
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63.
- Sosnik, A.; Seremeta, K.P. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25.
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369.
- Hoffman, A.S. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Control. Release 1987, 6, 297–305.
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702.
- Ju, G.; Cheng, M.; Xiao, M.; Xu, J.; Pan, K.; Wang, X.; Zhang, Y.; Shi, F. Smart Transportation Between Three Phases Through a Stimulus-Responsive Functionally Cooperating Device. Adv. Mater. 2013, 25, 2915–2919.
- Sun, T.; Song, W.; Jiang, L. Control over the responsive wettability of poly(N-isopropylacrylamide) film in a large extent by introducing an irresponsive molecule. Chem. Commun. 2005, 13, 1723–1725.
- Füllbrandt, M.; Ermilova, E.; Asadujjaman, A.; Hölzel, R.; Bier, F.F.; von Klitzing, R.; Schönhals, A. Dynamics of Linear Poly(N-isopropylacrylamide) in Water around the Phase Transition Investigated by Dielectric Relaxation Spectroscopy. J. Phys. Chem. B 2014, 118, 3750–3759.
- Wang, M.; Gao, Y.; Cao, C.; Chen, K.; Wen, Y.; Fang, D.; Li, L.; Guo, X. Binary Solvent Colloids of Thermosensitive Poly(N-isopropylacrylamide) Microgel for Smart Windows. Ind. Eng. Chem. Res. 2014, 53, 18462–18472.
- Zhang, X.Z.; Yang, Y.Y.; Chung, T.S. The influence of cold treatment on properties of temperature-sensitive poly(N-isopropylacrylamide) hydrogels. J. Colloid Interface Sci. 2002, 246, 105–111.
- Matsuo, E.S.; Tanaka, T. Kinetics of discontinuous volume-phase transition of gels. J. Chem. Phys. 1988, 89, 1695–1703.
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371.
- Zhang, X.Z.; Xu, X.D.; Cheng, S.X.; Zhuo, R.X. Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft. Matter. 2008, 4, 385–391.
- Sayil, C.; Okay, O. Macroporous poly (N-isopropylacrylamide) networks. Polym. Bull. 2002, 506, 499–506.
- Zhang, J.T.; Cheng, S.X.; Huang, S.W.; Zhuo, R.X. Temperature-sensitive poly (N-isopropylacrylamide) hydrogels with macroporous structure and fast response rate. Macromol. Rapid Commun. 2003, 24, 447–451.
- Zhang, X.Z.; Wang, F.J.; Chu, C.C. Thermoresponsive hydrogel with rapid response dynamics. J. Mater. Sci. Mater. Med. 2003, 14, 451–455.
- Zhang, X.Z.; Wu, D.Q.; Chu, C.C. Synthesis, characterization and controlled drug release of thermosensitive IPN-PNIPAAm hydrogels. Biomaterials 2004, 25, 3793–3805.
- Zhang, X.Z.; Yang, Y.Y.; Wang, F.J.; Chung, T.S. Thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with expanded network structures and improved oscillating swelling-deswelling properties. Langmuir 2002, 18, 2013–2018.
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 2001, 42, 6851–6857.
- Vázquez-Dorbatt, V.; Tolstyka, Z.P.; Maynard, H.D. Synthesis of aminooxy end-functionalized pnipaam by raft polymerization for protein and polysaccharide conjugation. Macromolecules 2009, 42, 7650–7656.
- Zhang, J.T.; Cheng, S.X.; Zhuo, R.X. Poly(vinyl alcohol)/poly(N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels with rapid response to temperature changes. Colloid Polym. Sci. 2003, 281, 580–583.
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957.
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 1–8.
- Ma, X.; Li, Y.; Wang, W.; Ji, Q.; Xia, Y. Temperature-sensitive poly(N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels by in situ polymerization with improved swelling capability and mechanical behavior. Eur. Polym. J. 2013, 49, 389–396.
- Lencina, M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44.
- Takigawa, T.; Yamawaki, T.; Takahashi, K.; Masuda, T. Change in Young’s modulus of poly(N-isopropylacrylamide) gels by volume phase transition. Polym. Gels Netw. 1998, 5, 585–589.
- Rivero, R.E.; Capella, V.; Liaudat, A.C.; Bosch, P.; Barbero, C.A.; Rodríguez, N.; Rivarola, C.R. Mechanical and physicochemical behavior of a 3D hydrogel scaffold during cell growth and proliferation. RSC Adv. 2020, 10, 5827–5837.
- Zhang, X.Z.; Zhuo, R.X.; Cui, J.Z.; Zhang, J.T. A novel thermo-responsive drug delivery system with positive controlled release. Int. J. Pharm. 2002, 235, 43–50.
- Gutowska, A.; Bark, J.S.; Kwon, I.C.; Bae, Y.H.; Cha, Y.; Kim, S.W. Squeezing hydrogels for controlled oral drug delivery. J. Control. Release 1997, 48, 141–148.
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible adsorption by a pH- and temperature-sensitive acrylic hydrogel. J. Control. Release 2002, 80, 247–257.
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36.
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742.
- Boere, K.W.M.; Soliman, B.G.; Rijkers, D.T.S.; Hennink, W.E.; Vermonden, T. Thermoresponsive injectable hydrogels cross-linked by native chemical ligation. Macromolecules 2014, 47, 2430–2438.
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197.
- Charan, H.; Kinzel, J.; Glebe, U.; Anand, D.; Garakani, T.M.; Zhu, L.; Bocola, M.; Schwaneberg, U.; Böker, A. Grafting PNIPAAm from β-barrel shaped transmembrane nanopores. Biomaterials 2016, 107, 115–123.
- Li, Z.; Guo, X.; Matsushita, S.; Guan, J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 2011, 32, 3220–3232.
- Yang, J.; van Lith, R.; Baler, K.; Hoshi, R.A.; Ameer, G.A. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules 2014, 15, 3942–3952.
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194.
- Das, D.; Ghosh, P.; Ghosh, A.; Haldar, C.; Dhara, S.; Panda, A.B.; Pal, S. Stimulus-Responsive, Biodegradable, Biocompatible, Covalently Cross-Linked Hydrogel Based on Dextrin and Poly(N -isopropylacrylamide) for in Vitro/in Vivo Controlled Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 14338–14351.
- Ono, Y.; Shikata, T. Hydration and dynamic behavior of poly (N-isopropylacrylamide) s in aqueous solution: A sharp phase transition at the lower critical solution temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031.
- Yanase, K.; Buchner, R.; Sato, T. Microscopic insights into the phase transition of poly (N-isopropylacrylamide) in aqueous media: Effects of molecular weight and polymer concentration. J. Mol. Liq. 2020, 302, 112025.
- P.R. ten Wolde; Chandler, D. Drying-induced hydrophobic polymer collapse. Proc. Natl. Acad. Sci. USA 2002, 99, 6539–6543.
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012.
- Hou, L.; Wu, P. LCST transition of PNIPAM-b-PVCL in water: Cooperative aggregation of two distinct thermally responsive segments. Soft. Matter. 2014, 10, 3578–3586.
- Kojima, H.; Tanaka, F. Cooperative Hydration Induces Discontinuous Volume Phase Transition of Cross-Linked Poly(N-isopropylacrylamide) Gels in Water. Macromolecules 2010, 43, 5103–5113.
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Moskalets, A.P.; Dubovik, A.S.; Plashchina, I.G.; Khokhlov, A.R. Energetics and Mechanisms of poly(N-isopropylacrylamide) Phase Transitions in Water–Methanol Solutions. Macromolecules 2020, 53, 10765–10772.
- Shan, J.; Chen, J.; Nuopponen, M.; Tenhu, H. Two phase transitions of poly(N-isopropylacrylamide) brushes bound to gold nanoparticles. Langmuir ACS J. Surf. Colloids 2004, 20, 4671–4676.
- Rey, M.; Hou, X.; Tang, J.S.J.; Vogel, N. Interfacial arrangement and phase transitions of PNiPAm microgels with different crosslinking densities. Soft. Matter. 2017, 13, 8717–8727.
- Okada, Y.; Tanaka, F.; Kujawa, P.; Winnik, F.M. Unified model of association-induced lower critical solution temperature phase separation and its application to solutions of telechelic poly(ethylene oxide) and of telechelic poly(N-isopropylacrylamide) in water. J. Chem. Phys. 2006, 125, 244902.
- Pham, Q.-T.; Yao, Z.-H.; Chang, Y.-T.; Wang, F.-M.; Chern, C.-S. LCST phase transition kinetics of aqueous poly(N-isopropylacrylamide) solution. J. Taiwan Inst. Chem. Eng. 2018, 93, 63–69.
- Bischofberger, I.; Trappe, V. New aspects in the phase behaviour of poly-N-isopropyl acrylamide: Systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Sci. Rep. 2015, 5, 15520.
- Werner, P.; Münzberg, M.; Hass, R.; Reich, O. Process analytical approaches for the coil-to-globule transition of poly(N-isopropylacrylamide) in a concentrated aqueous suspension. Anal. Bioanal. Chem. 2017, 409, 807–819.
- Hu, X.; Tong, Z.; Lyon, L.A. Control of poly(N-isopropylacrylamide) microgel network structure by precipitation polymerization near the lower critical solution temperature. Langmuir ACS J. Surf. Colloids 2011, 27, 4142–4148.
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304.
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Choi, E.J.; Ha, S.; Lee, J.; Premkumar, T.; Song, C. UV-mediated synthesis of pNIPAM-crosslinked double-network alginate hydrogels: Enhanced mechanical and shape-memory properties by metal ions and temperature. Polymer 2018, 149, 206–212.
- Zarzyka, I.; Pyda, M.; di Lorenzo, M.L. Influence of crosslinker and ionic comonomer concentration on glass transition and demixing/mixing transition of copolymers poly(N-isopropylacrylamide) and poly(sodium acrylate) hydrogels. Colloid Polym. Sci. 2014, 292, 485–492.
- Ma, X.M.; Li, R.; Ren, J.; Lv, X.C.; Zhao, X.H.; Ji, Q.; Xia, Y.Z. Restorable high-strength poly(N-isopropylacrylamide) hydrogels constructed through chitosan-based dual macro-cross-linkers with rapid response to temperature jumps. RSC Adv. 2017, 7, 47767–47774.
- Li, Y.; Wang, C.; Zhang, W.; Yin, Y.; Rao, Q. Preparation and characterization of PAM/SA tough hydrogels reinforced by IPN technique based on covalent/ionic crosslinking. J. Appl. Polym. Sci. 2015, 132.
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109.
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580.
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-Bonding Reinforced Injectable Hydrogels: Application as a Thermo-Triggered Drug Controlled-Release System. ACS Appl. Polym. Mater. 2020, 2, 1587–1596.
- Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2001591.
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31.
- Genevro, G.M.; de Moraes, M.A.; Beppu, M.M. Freezing influence on physical properties of glucomannan hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405.
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485.
- Liu, J.; Fan, X.; Tao, Y.; Deng, C.; Yu, K.; Zhang, W.; Deng, L.; Xiong, W. Two-Step Freezing Polymerization Method for Efficient Synthesis of High-Performance Stimuli-Responsive Hydrogels. ACS Omega 2020, 5, 5921–5930.
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119.
- Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Kose, F.A.; Bibire, N. Chitosan-Graft-Poly(N-isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers 2019, 11, 1432.
- Su, Q.; Duan, L.; Zou, M.; Chen, X.; Gao, G.H. The tough allograft adhesive behavior between polyacrylamide and poly(acrylic acid) hydrophobic association hydrogels. Mater. Chem. Phys. 2017, 193, 57–62.
- Cho, E.C.; Lee, J.; Cho, K. Role of Bound Water and Hydrophobic Interaction in Phase Transition of Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules 2003, 36, 9929–9934.
- Custodio, K.K.S.; Claudio, G.C.; Nellas, R.B. Structural Dynamics of Neighboring Water Molecules of N-isopropylacrylamide Pentamer. ACS Omega 2020, 5, 1408–1413.
- Ullah, A.; Lim, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharm. 2022, 612, 121368.
- Lee, E.; Kim, D.; Kim, H.; Yoon, J. Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci. Rep. 2015, 5, 15124.
- Rana, M.M.; Rajeev, A.; Natale, G.; Siegler, H.D. Effects of synthesis-solvent polarity on the physicochemical and rheological properties of poly(N-isopropylacrylamide) (PNIPAm) hydrogels. J. Mater. Res. Technol. 2021, 13, 769–786.
- Li, Q.-F.; Du, X.; Jin, L.; Hou, M.; Wang, Z.; Hao, J. Highly luminescent hydrogels synthesized by covalent grafting of lanthanide complexes onto PNIPAM via one-pot free radical polymerization. J. Mater. Chem. C 2016, 4, 3195–3201.
- Zhu, S.; Wang, X.; Cong, Y.; Liu, L.; Li, L. Free Radical Polymerization of Gold Nanoclusters and Hydrogels for Cell Capture and Light-Controlled Release. ACS Appl. Mater. Interfaces 2021, 13, 19360–19368.
- Kim, S.; Healy, K.E. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide-co-acrylic acid) Hydrogels with Proteolytically Degradable Cross-Links. Biomacromolecules 2003, 4, 1214–1223.
- Schachschal, S.; Adler, H.-J.; Pich, A.; Wetzel, S.; Matura, A.; van Pee, K.-H. Encapsulation of enzymes in microgels by polymerization/cross-linking in aqueous droplets. Colloid Polym. Sci. 2011, 289, 693–698.
- Yi, G.; Huang, Y.; Xiong, F.; Liao, B.; Yang, J.; Chen, X. Preparation and swelling behaviors of rapid responsive semi-IPN NaCMC/PNIPAm hydrogels. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 1073–1078.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.8K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
29 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





