Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sheersha Pramanik and Version 2 by Camila Xu.
Poly(N-isopropylacrylamide) (PNIPAM) is a widely utilized negative thermosensitive polymer (as it has increased solubility with lowering of temperature, causing volume phase transition by forming hydrogen bonds) that has currently sparkled a lot of scientific inquisitiveness. Smart hydrogels based on PNIPAM demonstrate distinct thermoresponsive features close to a lower critical solution temperature (LCST) that enhance their capability in various biomedical applications such as drug delivery, tissue engineering, and wound dressings.
- PNIPAM
- hydrogels
- drug delivery
- tissue engineering
1. Brief on Poly(N-isopropylacrylamide) (PNIPAM)-Based Hydrogel
Poly(N-isopropylacrylamide) (PNIPAM) is a widely utilized negative thermosensitive polymer (as it has increased solubility with lowering of temperature, causing volume phase transition by forming hydrogen bonds) that has currently sparkled a lot of scientific inquisitiveness [1][2][25,26]. In the aqueous medium, PNIPAM assembles a stretched spiral elastic shape; polymer molecules form hydrogen bonds with each other due to hydrogen bonding with molecules of water. It has both hydrophobic isopropyl (–CH(CH3)2) side groups and hydrophilic amide (–CONH–) groups in the structure. PNIPAM has an LCST of 32 °C, which is somewhat lower than human body temperature of 37 °C, and it can be modified by copolymerizing with some distinct hydrophobic or hydrophilic polymers to develop thermo-sensitive, in situ hydrogels. [3][4][5][27,28,29]. The temperature-responsive behaviors are primarily due to connections between these groups and solvent molecules. As a result, PNIPAM converts from solution to gel state as the LCST reaches human body temperature [6][7][8][9][10][30,31,32,33,34]. Additional hydrophilic monomers increase the polymer’s hydrophilicity and lead to more significant interactions with water, resulting in a higher LCST, whereas copolymerization with more hydrophobic monomers reduces LCST [11][35]. This property makes PNIPAM suitable for biomedical applications, e.g., controlled wound dressings, tissue engineering scaffolds, and drug delivery systems [12][13][36,37]. Notwithstanding these appealing benefits, PNIPAM-based hydrogels nevertheless have certain drawbacks, including limited biodegradability [14][38], weak mechanical strength [15][39], insufficient drug loading capacity [16][40], and immediate release [15][17][18][39,41,42], etc.; limiting the feasibility in drug delivery.
Several drugs can be efficiently encapsulated into the hydrophilic polymers by using a lower temperature to dissolve the drug in the solvent to ensure efficient delivery, raising the temperature slightly above the LCST for hydrogel appearance. Furthermore, maintaining precise temperature control permits site-specific drug delivery and delayed release. The PNIPAM solution can be rapidly applied to the skin and activated by utilizing body temperature to create a 3D hydrogel that can help restore tissues by encouraging cell proliferation and differentiation while preserving the homeostatic activity of cells. The temperature-dependent modulation of gel coating and reversible sol-to-gel can be utilized to develop adherence to the skin and its dissociation for wound treatment. Other features of PNIPAM, in conjunction with thermosensitivity, include adjustable geometries and nontoxicity, both of which are advantageous for biological applications [19][43]. Wound dressings play a crucial part in lesion care, and their effectiveness impacts how quickly a wound heals [20][44]. That a warm, moist environment promotes faster healing has been widely demonstrated in several empirical investigations in both in vivo and in vitro wound healing [21][22][45,46]. Various medical studies also recommend that dressings should be translucent to allow easy observation, and that they have the ability to retain fluids [23][24][25][47,48,49]. Aside from the properties described above, zwitterionic hydrogels have lately gained popularity as nonadherent wound dressings due to their high resistance to cell attachment, protein adsorption, and microbial adherence [26][27][50,51]. For wound dressing, PNIPAM can be utilized to retain the skin adhesion and differentiation of gel coatings. Its thermosensitivity is complemented by LCST adjustability, low toxicity, and [24][48] variable structure. [28][52], making it suitable for biomedical applications [19][43].
Many contemporary tissue engineering techniques rely on the use of a material scaffold. These scaffolds act as an artificial extracellular matrix (ECM), allowing cells to be organized into a three-dimensional framework and stimulating the growth and creation of desirable tissue [29][53]. The scaffold material and qualities required vary greatly depending on the tissue of concern and the individual application [30][54]. Thermoresponsive polymers are extensively employed in tissue engineering in two ways: platforms for cell proliferation and injectable solutions for in situ scaffolding [31][55].
The primary disadvantage of PNIPAM-based hydrogels in drug delivery is the incorporation of hydrophobic drugs in the water-loving polymeric core [32][56], since mostly hydrophobic drugs are typically and effectively used in disease treatment. Along with this, the tensile strength [15][39] of these hydrogels is weak, which may sometimes lead to early drug release before arrival at the specific site [33][57]. Several researchers are working painstakingly to improve the mechanical characteristics of PNIPAM hydrogels to fix the reported shortcomings. Hybridization of PNIPAM-based composite hydrogels with appropriate materials such as inorganic/metal nanoparticles [34][58], organic self-assemblies [16][40], and several other polymeric material incorporations/composite formations are some reported viable techniques for achieving this goal [35][36][59,60]. A novel polymeric block could regulate the thermal sensitivity of the resulting hydrogel by adjusting the hydrophilic/hydrophobic equilibrium of PNIPAM. Alternatively, a novel polymeric component can affect the hydrogel network’s chemical rigidity and morphology, modulating the copolymeric hydrogel’s mechanical properties [37][38][39][61,62,63]. The successful composite formation of PNIPAM with various materials, e.g., hyaluronic acid (HA) [40][41][64,65], zinc oxide (ZnO) nanoparticles [42][66], silver nanoparticles [43][67], hydroxyapatite (HAp) nanoparticles [44][68], carbon nanoparticles [45][69], etc.; for tissue engineering and wound healing has been reported in several investigations [46][47][48][70,71,72]. Adequate biocompatibility, higher tensile strength, customizable temperature responsiveness, multi-drug loading capability, targeted drug delivery, and controlled drug delivery are among the unique and/or improved features of composite hydrogels [19][43]. The inclusion of some other polymer inside the hydrogel matrix to make interpenetrating polymeric network system (IPN) hydrogels for biomedical application [49][73] is another useful way to alter the functionalities of a hydrogel. The polymeric networks in IPN hydrogels can efficiently adjust mechanical characteristics, swelling/deswelling behavior, and drug loading/release pattern [50][74].
2. Unique Properties of PNIPAM
Physically crosslinked hydrogels can be assembled via ionic/hydrogen bond formation, Van der Waals forces, hydrophobic interactions, crystalline structure, etc. [51][75]. The strong hydrogen bonding between the polymeric chains in the hydrogel may promote drug release, which can be further controlled via the type of solvent, degree of sonication, solution temperature, polymer concentration, etc.; in hydrogel formulation. Another method, crosslinking, has been most commonly utilized to overcome biomaterial physicochemical and mechanical limitations via strong interconnections between the reaction molecules. Furthermore, ionic interactions can be initiated at room temperature and physiological pH to overcome constraints [52][76]. Bifunctional crosslinking agent-initiated hydrogels are generally developed via several techniques, e.g., condensation polymerization, irradiation using high-energy ionizing radiation such as electron beams, gamma rays, or X-rays, chain-growth polymerization, etc. [52][76].
In biomedical science, PNIPAM-based hydrogels have versatile applications for the effective therapeutic delivery of molecules by modulating substance movement in a medium, altering thermo-controlled dimensions [53][77]. A monomeric structure of PNIPAM commonly identified by isopropyl and amide moieties maintains LCST at ~32 °C in aqueous conditions. PNIPAM hydrogels are developed by crosslinking themselves or their derivates, exhibiting a reversible and extreme volume phase transition towards LCST through swelling/shrinking [54][78]. Along with the changes in size during the transition phase, there are also several other changes in many properties, such as hydrophilicity [55][56][79,80], transparency [57][81], and apparent electrostatic permittivity [58][82]. The mixing of polymer in a solvent leads to polymeric dissolution at a specific temperature (T), which the negative ΔGmix of the Gibbs free energy equation can well indicate. The involvement of thermodynamics in the method forms hydrogen bonds that create significant negative enthalpy change of mixing (ΔHmix < 0), contributing to dissolution. Lowering PNIPAM’s temperature below LCST develops a counterbalance between the hydrogen bonds of hydrophilic amide groups and molecules of water, leading to hydrophobic interactions among isopropyl groups and hence collapsing the PNIPAM chain to avoid water contact [54][78].
PNIPAM hydrogels exhibit a slow response to environmental temperature variations due to the formation of the thick skin surface, decreasing the outward diffusion of water molecules and causing hydrogel collapse at temperatures above LCST [59][60][61][83,84,85]. Likewise, the swelling rate of hydrogels is relatively slower at temperatures lower than LCST. Therefore, improving the rate of thermoresponsiveness of established PNIPAM hydrogels has become a research subject [62][86]. Some physical strategies have been involved in enhancing the thermal responsiveness of PNIPAM hydrogels, such as phase separation [63][87], porogen [64][88], freezing [59][83], vacuum-synthesis [65][89], and interpenetrating polymer networks [66][90]. Along with these, alternate chemical approaches have also been introduced, which include the addition of hydrophilic moieties [67][91], grafting freely mobile hydrophilic moieties [68][92], and reversible addition–fragmentation chain transfer (RAFT) polymerization [69][93] to attain a rapid response rate. Stimuli-responsive hydrogels require mechanical properties for various applications. Hence, to improve the PNIPAM hydrogel‘s mechanical properties, some techniques have been implemented, such as an interconnected polymer network [70][94], a hydrogel with two networks [71][95], a hydraulic ring for slides [72][96], nanoporous PNIPAM hydrogel [73][97], and copolymerized PNIPAM hydrogel [74][98]. Free radical redox polymerization of PNIPAM hydrogels is relatively weak and can even be affected using standard mechanical testing equipment [75][99].
Takigawa et al. determined PNIPAM hydrogel’s Young’s modulus (E0) for the first time in 1997. They found a stress–strain graph of the collapsed and swelled gel to be linear in a specific hardness strength test, where the E0 was a hundred times more for the collapsed state. The swelled and collapsed conditions were found to have fracture strains of 35% and 75%, respectively [75][99]. The collapsed state was found to have a higher E0 than the swollen state, as the collapsed gels had an increased number of crosslinks. The change in E0 at the initial stage of the scaffold was probably due to rapid relaxation shown by the macroporous hydrogel, which remains constant for a more extended period during cellular growth [76][100].
PNIPAM hydrogel has inadequate mechanical performance in the highly swelled state, prominently identified as a disadvantage in drug delivery. Its nonbiodegradable nature has led to surgical excision after the release of the drug. Removing the device by typical surgical operations is also challenging if it is too light, as it might break down during handling. However, PNIPAM hydrogel’s lack of controlled release capability, i.e.; leading to the release of impregnated drugs within 24 h, is a severe reason for the possibility of being restricted as a drug carrier [77][78][79][101,102,103]. A swelled PNIPAM hydrogel with a decreased polymeric mass per unit volume explains its poor mechanical characteristics and increased drug release rate. The decreased polymeric mass per unit volume results in the fast release of impregnated drugs because of the gel’s open pores and low mechanical characteristics. Rapid drug release from the drug reservoir is because of the weak intermolecular bonds of swelled PINPAM hydrogel [66][90].
In an aqueous phase at a certain temperature range of about 32 °C, for drug delivery, the PNIPAM hydrogel could be combined with bioactive components to form a solution. The introduction of polymers becomes possible in that particular physical condition. The subcutaneous injection of the polymer-loaded gel leads to sustained release and an immediate increase in physiological temperature (to about 36.5–37.5 °C). The biodegradability of the hydrogel leads to the release of encapsulated bioactive compounds, initially in the body via diffusion and later on by a mixture of diffusion and mechanical breakdown [80][104]. Both physical and chemical properties (melting point, temperature, glass transition storage modulus, crystallinity, etc.) are responsible for the biodegradability of the polymer [81][105]. The reduced biodegradability of PNIPAM hydrogel has restricted its use in clinical practice. Different crosslinking agents and/or biodegradable polymers or native polymers, including poly(amino acids) [82][106], polysaccharides [83][107], proteins [84][108], and synthetic polymers including poly(esters) [85][109], poly(caprolactone) [14][38], and poly(ethylene glycol) [86][110], have now been analyzed for the development of biodegradable PNIPAM hydrogels [80][104]. PNIPAM is also highly biocompatible with animal cells [80][104]. Cao et al. explored the use of PNIPAM–chitosan copolymers for ophthalmic drug delivery. The copolymer was utilized for encapsulation of timolol maleate molecules for around 12 h to effectively lower intraocular pressure (IOP). The in vivo study of PNIPAM–chitosan for thermo-sensitive hydrogels confirmed non-cytotoxicity, hence furnishing new insights into glaucoma therapy along with several other eye illnesses [87][111].
Biopolymers or artificially degradable compounds help alter the chemical composition of PNIPAM to obtain biodegradability and biocompatibility effectively. In a study by Das et al.; covalently crosslinked PNIPAM hydrogels utilizing NIPAM as the monomer, dextrin as the biopolymer, potassium persulfate (KPS) N,N′-methylene bisacrylamide (MBA) as the promoter, and N,N′-methylene bisacrylamide (MBA) as the crosslinker were effectively synthesized. Ciprofloxacin and ornidazole could be administered in a controlled manner by the novel PNIPAM hydrogel, as it is nontoxic and biodegradable [88][112].
3. Phase Transition for PNIPAMs
The polyacrylamide structure is mostly crafted with a hydrophilic amide group (almost 90%) and -C-C- hydrophobic portion. However, poly(N-isopropyl-acrylamide) and other N-substituted acrylamide polymers (PNIPAM) have balanced hydrophilic and hydrophobic regions below LCST. The gel-polymer/water system’s total energy is lowered due to hydrophobic polymers enveloped by water molecules below LCST [89][113]. The solvation and transition capacity of PNIPAM in cold water increases when the temperature is raised off its LCST (LCST ≈ 32–34 °C), leading to the “coil to globule” of the polymeric chain’s (CG) transition. The CG transition is in charge of phase inversion into rich layers. Polymeric/water phases further exhibit volume phase transition (VPT) [90][114]. A loss in entropy of water molecules enveloping the hydrophilic polymeric chain is counterbalanced by an increase in enthalpy owing to hydrogen bonding between the hydroxyl groups surrounding the polymeric chain’s hydrophobic sections. Hydrophobic hydration is a process that allows a hydrophobic polymer to stay hydrated in an aqueous environment. If the temperature is increased above LCST, water molecules leave the polymer chain and form a globule structure. As a result, the PNIPAM–polymer is hydrated, and a definite volume phase transition is observed. The phase separation initially occurs due to PNIPAM molecule incorporation into larger aggregates [91][92][115,116] via several mechanisms and factors, e.g., dewetting caused by solvent fluctuations, cooperative hydration [93][94][95][117,118,119], the aqueous medium’s energy state [96][120], endothermic heat [97][121], precipitation polymerization [98][122], etc. The hydrogen bond between water molecules and PNIPAM is weaker due to the temperature rising above LCST, leading to the formation of an unstable solution. Further, the transitions of the PNIPAM–polymer can be confirmed by FTIR spectroscopy; the hydrodynamic diameter of PNIPAM gel affects the volume phase transition, causing dehydration of the polymeric gel [90][114].
The dependency of LCST on molecular weight and concentration of PNIPAM polymer in H2O and D2O can be confirmed via dielectric relaxation spectroscopy with small- and wide-angle x-ray scattering (SWAXS) (DRS) to show phase transition. Several investigations have reported a strong effect of interpenetration between the diverse chains of PNIPAM at high concentration decreasing correlation length (ξ). The molecular weight of PNIPAM is entirely independent of ξ at higher concentrations due to PNIPAM chains’ crammed state [92][116]. Interface formation and interchange aggregation are reported to be higher as a result of lowering the molecular weight of the polymer [92][116]. Several investigations have studied the phase transition effect due to LCST and confirmed peculiar behavior prevails with hydration [94][99][100][101][118,123,124,125]. The phase transition of the PNIPAM network can also be modulated by precipitation polymerization at 37−45 °C (near the LCST). Several analytical studies, e.g., atomic force microscopy (AFM) with photon correlation spectroscopy (PCS), etc.; have reported narrow, dispersed, spherical microgel and hydrogel volume phase transition behaviors [102][103][126,127]. Moreover, the phase transition property of PNIPAM-based hydrogel systems is advantageous due to its high mechanical strength and encapsulation capability, which has gained more attention in biomedical and tissue engineering applications [104][128].
4. Preparation of PNIPAM-Based Hydrogel Systems
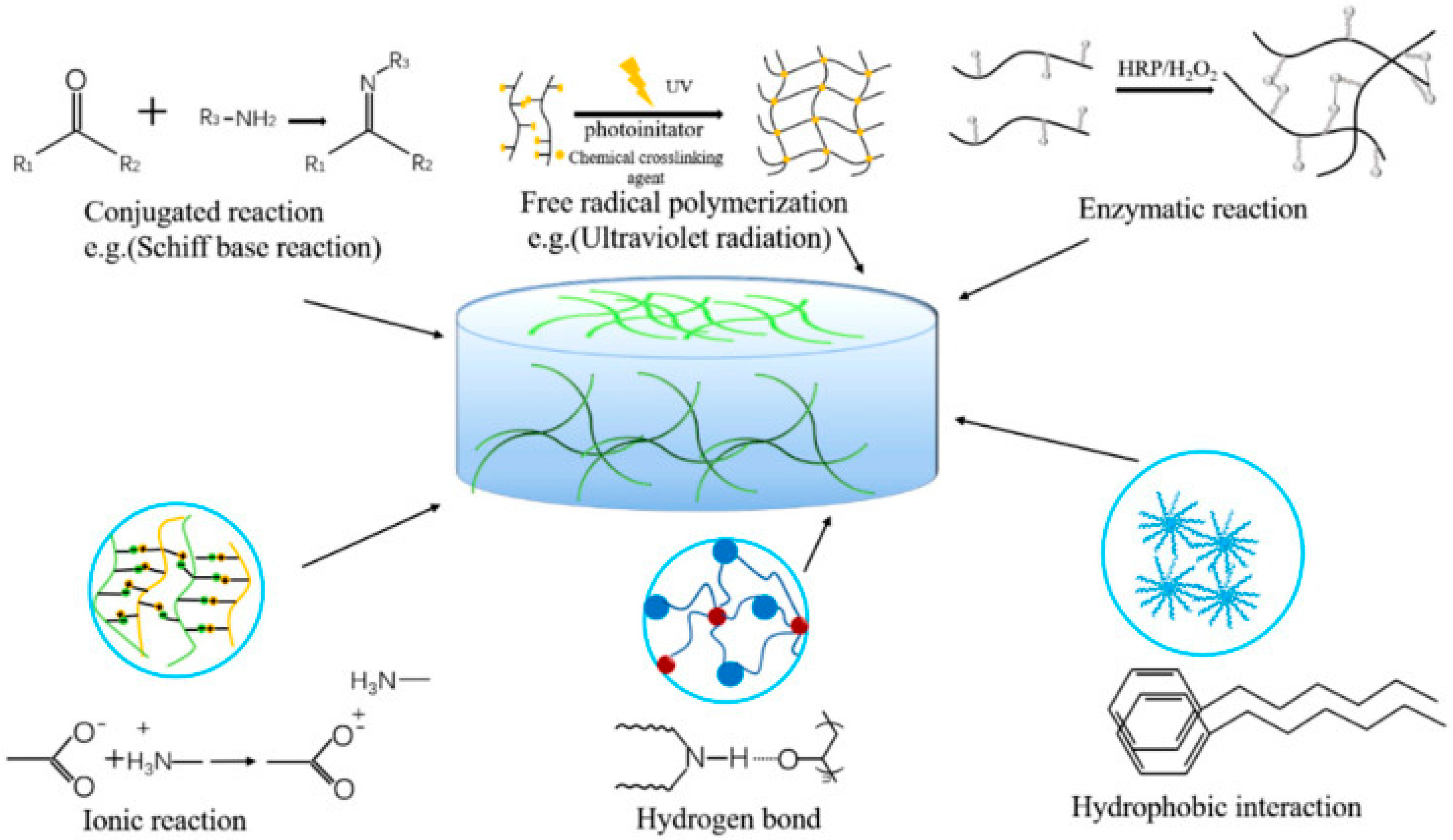
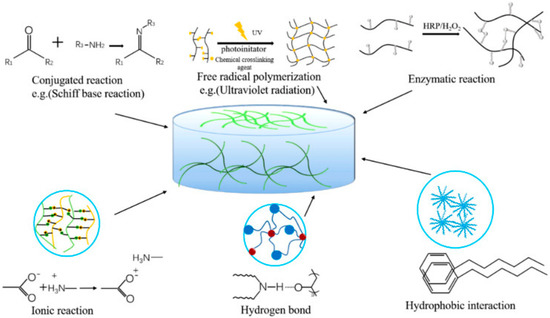
The ideal hydrophilic polymer-based hydrogel possesses good biocompatibility and cell viability, excellent mechanical strength (especially stiffness for tissue engineering), high adhesion, moisture retention, promotion of cell proliferation, and high absorption capacity of the fluid for tissue engineering and wound healing [105][129]. Hydrogels are hydrophilic/hydrophobic monomeric unit-based systems that can be crosslinked via several techniques (e.g., physical and chemical crosslinking) to produce an elastic structure that can be affected/modulated via monomer, initiator, and crosslinker selection [106][130]. The synthesis or preparation of hydrogels with various functional properties can be initiated with physical and chemical crosslinking [105][129]. Several physical crosslinking methods (as displayed in Figure 1), e.g., ionic interaction, hydrophobic bonds, protein interaction, etc.; may improve the toughness and self-healing ability of the hydrogel system, which is further required for biomedical application. The hydrogels formed by ionic interaction, i.e.; dynamic interaction of the negatively charged groups or metal-ligand interactions, have improved self-healing, ionic conductivity, biological properties, etc. However, various limitations have also been reported, e.g., poor mechanical characteristics, complex/strong bonds between polymers, etc.; limiting the preparation technique’s usage [105][129]. Several investigations have been reported e.g., alginate/N-isopropylacrylamide (NIPAM) hydrogel [107][131], PNIPAM/poly(sodium acrylate) hydrogel [108][132], methacryloylchitosan/PNIPAM hydrogel [109][133], polyacrylamide/sodium alginate IPN hydrogel [110][134], etc.; based on ionic interaction of PNIPAM-based hydrogel systems for improving stiffness, cell viability, and mechanical integrity of the hydrogel system. Another physical crosslinking method involves the usage of dynamic hydrogen bond interaction, which is often unstable in an aqueous environment but is possible to rebuild after breaking; it can improve self-healing, cell biocompatibility, biodegradability, etc.; properties [111][135] via an IPN hydrogel preparation effective in wound-healing dressing. Several investigations, e.g., chitosan-poly (vinyl alcohol) (PVA) DN (double network) hydrogel [111][135], sodium alginate (SA)/polyacrylamide (PAM) semi-IPN hydrogel [112][136], hydrazide-functionalized PNIPAM/dialdehyde dextrin thermoresponsive hydrogel [113][137], etc.; have reported, based on dynamic hydrogen bond mechanistic, PNIPAM hydrogel preparations for biomedical application. Another commonly utilized physical crosslinking method, freeze–thaw, can form ice crystal and fabricate the polymeric chain around the crystals, followed by fabrication of a microporous structure while melting the crystals [114][138]. The freeze–thaw method can proliferate stem cells and ECM deposition [115][139], cell compatibility, and biodegradability [116][140] in the biomedical application based on the modulation of temperature, time, number of cycles, polymer contents, etc. [116][117][140,141]. Some recent investigations, e.g., PNIPAM hydrogel [118][142], PNIPAM/cellulose nanocrystal hybrid hydrogel [119][143], chitosan-graft-PNIPAM/PVA hydrogel [120][144], etc.; utilized the freeze–thaw physical crosslinking method and were reported to have high encapsulation efficiency, stimuli-responsive, adsorption capacity, etc. Hydrophilic polymers with hydrophobic end groups/side chains/monomers can be associated via physical crosslinking, resulting in high mechanical strength via strong hydrophobic interaction [105][129]. Several studies e.g., polyacrylamide (PAAm)/polyacrylic acid (PAAc)/PNIPAM hydrogel [121][145], poly(N-isopropylacrylamide) hydrogel [122][123][146,147], etc.; have been successfully prepared and reported high mechanical strength for biomedical application. The low mechanical stability and strength of physically crosslinked, reversible hydrogels may improve the utilization of chemical crosslinkers connected via covalent interactions. Diverse chemical crosslinking mechanisms have been reported, e.g., conjugation reaction, free radical polymerization, enzymatic reaction, etc.; where the hydrogel was formed via covalent bonds [124][148]. Conjugation occurred in mild conditions in the presence of Michael addition, Schiff’s base, Diels–Alder addition, etc.; which are green methods in the presence of condensate functional groups to improve biodegradability, transparency, and adhesiveness of the hydrogel system [105][124][129,148]. To overcome the limitations associated with the mechanical properties of hydrogel prepared by conjugation, free radical polymerization was reinforced via heating, ultraviolet radiation, energy radiation, electrolysis, etc.; to improve the swelling, porosity, and mechanical strength of the hydrogel system for biomedical application [124][148]. Several studies, e.g., PNIPAM/magnetite nanoparticles hydrogel [125][149], poly(N-isopropylacrylamide) hydrogel [126][150], PNIPAM–Ln(DPA)3 hydrogels [127][151], PNIPAM/gold nanocluster hydrogel [128][152], etc.; have utilized free radical polymerization for PNIPAM-based hydrogel preparation to improve mechanical strength and stability. The enzymatic reaction of natural polysaccharides in the presence of several enzymes, e.g., transglutaminase, tyrosinase, urease, horseradish peroxidase, etc.; occurs in very mild conditions and can retain the biological properties and improve the mechanical strength of the polymers utilized for hydrogel preparation. In various reports, e.g., N-isopropylacrylamide (NIPAM) and acrylic acid (AAc) hydrogel [129][153], vinylimidazole/PNIPAM) hydrogel [130][154], NaCMC/PNIPAM hydrogels [131][155], etc.; enzymatic crosslinking was seen to be effective for improving the mechanical stability and strength of PNIPAM-based hydrogels.