Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cynthia Amaning Danquah | -- | 4248 | 2022-07-27 18:04:24 | | | |
| 2 | Rita Xu | Meta information modification | 4248 | 2022-07-28 03:37:45 | | | | |
| 3 | Rita Xu | + 2 word(s) | 4250 | 2022-07-29 10:51:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Danquah, C.A.; Minkah, P.A.B.; Agana, T.A.; Moyo, P.; Ofori, M.; Doe, P.; Rali, S.; Junior, I.O.D.; Amankwah, K.B.; Somuah, S.O.; et al. The Genus Tulbaghia. Encyclopedia. Available online: https://encyclopedia.pub/entry/25592 (accessed on 07 February 2026).
Danquah CA, Minkah PAB, Agana TA, Moyo P, Ofori M, Doe P, et al. The Genus Tulbaghia. Encyclopedia. Available at: https://encyclopedia.pub/entry/25592. Accessed February 07, 2026.
Danquah, Cynthia Amaning, Prince Amankwah Baffour Minkah, Theresa A. Agana, Phanankosi Moyo, Michael Ofori, Peace Doe, Sibusiso Rali, Isaiah Osei Duah Junior, Kofi Bonsu Amankwah, Samuel Owusu Somuah, et al. "The Genus Tulbaghia" Encyclopedia, https://encyclopedia.pub/entry/25592 (accessed February 07, 2026).
Danquah, C.A., Minkah, P.A.B., Agana, T.A., Moyo, P., Ofori, M., Doe, P., Rali, S., Junior, I.O.D., Amankwah, K.B., Somuah, S.O., Nugbemado, I.N., Maharaj, V.J., Bhakta, S., & Gibbons, S. (2022, July 27). The Genus Tulbaghia. In Encyclopedia. https://encyclopedia.pub/entry/25592
Danquah, Cynthia Amaning, et al. "The Genus Tulbaghia." Encyclopedia. Web. 27 July, 2022.
Copy Citation
Amaryllidaceae is a significant source of bioactive phytochemicals with a strong propensity to develop new drugs. The genera Allium, Tulbaghia, Cyrtanthus and Crinum biosynthesize novel alkaloids and other phytochemicals with traditional and pharmacological uses. Amaryllidaceae biomolecules exhibit multiple pharmacological activities such as antioxidant, antimicrobial, and immunomodulatory effects.
Amaryllidaceae
Tulbaghia
natural products
1. Introduction
Amaryllidaceae belongs to the order Asparagales and consists of bulbous flowering plants separated into three infrageneric ranks: Agapanthoideae, Allioideae and Amaryllidoideae, as delineated by the Angiosperm Phylogeny Group [1]. The term “Amaryllidaceae” is frequently used in either phytochemical or pharmacological literature to refer to plants or alkaloids originating from the subfamily Amaryllidoideae [2][3]. Monocotyledonous plants constitute seventy-nine genera (including Allium, Crinum, Cyrtanthus, and Tulbaghia) with over 1000 species [4]. Aside from their broad pantropical distribution, Amaryllidaceae are located in Africa, the Mediterranean Coast and South America, and have high adaptation and speciation [5]. The genus Allium is distributed in temperate, arid, semi-arid and subtropical areas such as the Mediterranean region, central Asia, Africa and parts of Europe. As herbaceous geophyte perennials, Allium comprises a plethora of species with pungent linear leaves that may or may not arise from a bulb or rhizome [6][7]. The Tulbaghia genus, popularly called “sweet garlic”, “wild garlic”, or “pink agapanthus”, is crown shaped with outgrowth or appendages of the perianth and predominantly colonizes the Eastern cape belt of South Africa, and is adapted for growth in areas such as Europe and America [8][9]. The genus Crinum encompasses 104 species and appear as showy flowers on leafless stems, which thrive in the tropics and warm temperate parts, specifically Asia, Africa, America, and Australia [10]. Cyrtanthus is popularly known as “fire lily” due to its unique rapidly flowering response to natural bush fires. Most species are found in South Africa and play an important role in South African traditional medicine [11].
Amaryllidaceous plants are known for their ornamental, nutritional, and medicinal value. Given their attractive flowering plant-like features, Crinum species are prized for their umbel lily-like blossoms in China and Japan [3]. Concurrently, Amaryllidaceae are known for their longstanding exploitation in medicinal therapy owing to their inherent biosynthesis of chemically diverse bioactive compounds with peculiar biological properties. The use of proximate and mineral composition analysis enabled the identification of phytoconstituents [10][12], while in vitro, in vivo, and in silico model systems have permitted the unravelling of intrinsic pharmacological activities of the natural products and other alkaloids isolated from this source [13][14][15]. Of note, bioactive compounds from Amaryllidaceae possesses a wide range of bioactivities ranging from antioxidant [16][17], anti-inflammatory [16][18], antimicrobial [17], antifungal [19], antiviral [20][21], antiplasmodial [22][23][24], anticarcinogenic [18][25][26], antispasmodic [1][27], antiplatelet [28], antiasthmatic [29], antithrombotic [30][31], antitumor [25], antihyperlipidemic [25], antihyperglycemic [25][32][33], antiarthritic [25], antimutagenic [16], immunomodulatory [16] and several others [34].
Given the aforementioned biological activities, Allium, Tulbaghia, Cyrtanthus and Crinum are utilized in traditional medicinal therapy for varying diseases and conditions [35][36][37][38][39][40][41]. For example, Allium is used as concoctions, decoctions, extracts, and herbal preparations to treat angina, amoebic dysentery, arthritis, cardiovascular diseases, cholera, catarrh, dysmenorrhea, fever, headaches, hepatitis, stomach disorders, throat infections, and prostatic hypertrophy [30][31][35][36][37][38]. The genus Tulbaghia has unique pharmacotherapeutic properties and is utilized to manage ailments such as earache, pyrexia, tuberculosis, and rheumatism [9][42]. Crinum species are used to treat haemorrhoids, malaria, osteoarthritis, varicosities, wounds, urinary tract infections, and gynaecological remedies [40][41]. Cyrtanthus are also employed in the management of ailments associated with pregnancy, as well as cystitis, age-related dementia, leprosy, scrofula, headaches, chronic coughs, among others [43][44]. In modern clinical practice, galanthamine from Amaryllidaceae is a primary choice of drug in managing symptomatic neurological disorders such as Alzheimer’s disease due to its selective inhibitory action on the acetylcholine biosynthetic enzyme, acetylcholinesterase [45]. The pancratistatin phenanthridone class of alkaloids are also promising chemotherapeutic drug candidates with unique cell line-specific antiproliferative properties, conferring a selective advantage for clinical development [46].
Although Amaryllidaceae represents a source of valuable bioactive compounds, developing promising drug candidates into clinically relevant therapeutics has been slow. Similarly, other genera in this family, including Cyrtanthus, Crinum and Tulbaghia, are untapped reservoirs and could serve as an alternative window for novel drug targets and warrant further investigation.
2. The Genus Tulbaghia
2.1. Botanical Description
Tulbaghia is made up of monocotyledonous species with herbaceous perennial bulbs covered by brown scales and are mostly found in Africa [8]. South African species possess bulb-like corms or rhizomes which are swollen, irregularly shaped and wrapped in dry, fibrous leaves [8]. Members of this genus usually possess a raised crown-like structure or ring at the center of their flower tube [8]. Their seeds are black, flat and elongated with the mature ones having embryos [8]. Examples of species of this genus are Tulbaghia violacea (T. violacea), Tulbaghia acutiloba Harv. (T. acutiloba), Tulbaghia capensis L. (T. capensis) and Tulbaghia cepacea L.f (T. cepacea) [8].
2.2. Geographical Distribution and Traditional Uses of Tulbaghia Species
With approximately 66 species (https://www.kew.org/science accessed on 22 February 2022) [47], Tulbaghia is the second-most species-rich genus within Amaryllidaceae. Tulbaghia is a monocotyledonous genus comprised morphologically of herbaceous perennial bulbous species, which produce a variety of volatile sulfur compounds, hence resulting in a distinct pungent garlic odor released by bruised plants [8][48]. The genus was named by Carl Linnaeus after Ryk Tulbagh (1699–1771), a former governor of the Cape of Good Hope in South Africa, where most of the native species are to be found, particularly in the Eastern Cape Province [49]. In addition to South Africa, the genus is widely distributed across southern African countries including Botswana, Lesotho, Swaziland, and Zimbabwe, where the plant is revered in folk medicine being used for the treatment of a plethora of infectious and non-infectious diseases [9] as highlighted in Table 1.
Table 1. Geographical distribution and traditional uses of Tulbaghia species.
| Plant Species | Geographical Distribution | Traditional Uses | References |
|---|---|---|---|
| T. violacea | Indigenous to the Eastern Cape, KwaZulu-Natal, Gauteng, Free State and Mpumalanga Provinces of South Africa. | The leaves and bulbs are used in the management of fever and colds, tuberculosis, asthma, and stomach problems. The leaves are eaten as vegetables and for the management of oesophageal cancer. It is also used as a snake repellent. | [8][50] |
| T. alliacea | Native to South Africa and grows mostly in the Eastern Cape and southern KwaZulu-Natal Provinces of South Africa. | Its bruised rhizome is used locally in bathwater to relieve fever, rheumatism, and paralysis, and in small doses as a laxative. T. alliacea is used for the management of stomach problems, asthma, and pulmonary tuberculosis. Its rhizome infusion is administered as an enema. | [8][51] |
| T. simmleri | Native to the South African Drakensberg mountains growing as isolated plants on rocky ledges. | Bulbs and leaves are used as a remedy for gastrointestinal ailments, enemas, high blood pressure, heart problems, chest complaints, high cholesterol, constipation, rheumatism, asthma, fever, pulmonary tuberculosis, earache, human immunodeficiency virus (HIV), paralysis, and cardiovascular diseases. | [50][52] |
| T. acutiloba | Found in the rainfall regions of southern Africa, occurring in the Eastern Cape, KwaZulu-Natal, Limpopo, Free State, Gauteng, North West, and Mpumalanga Provinces of South Africa, as well as in Lesotho, Swaziland and Botswana. | T. acutiloba leaves are used as a culinary herb and snake repellent. It is used to treat barrenness, flu, bad breath, and as an aphrodisiac. It is also cultivated to keep snakes away from the homestead. | [8] |
| T. natalensis | Although native to South Africa, but is now grown worldwide. | It is used as a culinary herb and snake repellent. | [53] |
| T. cernua | Commonly found in the Eastern Cape, Free State, Gauteng, KwaZulu-Natal, Limpopo, Mpumalanga, North West and Western Cape Provinces of South Africa. | It is used for ornamental purposes. | [8] |
| T. leucantha | Widely distributed in southern Africa including Botswana, Lesotho, South Africa, Swaziland, Zambia, and Zimbabwe. | Its rhizome is scraped clean and boiled in stews or roasted as a vegetable. Its leaves and stems are used as a culinary herb and protective charm. | [53] |
| T. ludwigiana | Commonly found in the Eastern Cape, KwaZulu-Natal, Northern Provinces of South Africa and in Swaziland. | It is traditionally used as a love charm. | [53] |
2.3. Phytochemistry of Tulbaghia
Tulbaghia produces many different classes of compounds with diverse chemical structures dominated by sulfur-containing natural products (Figure 1).
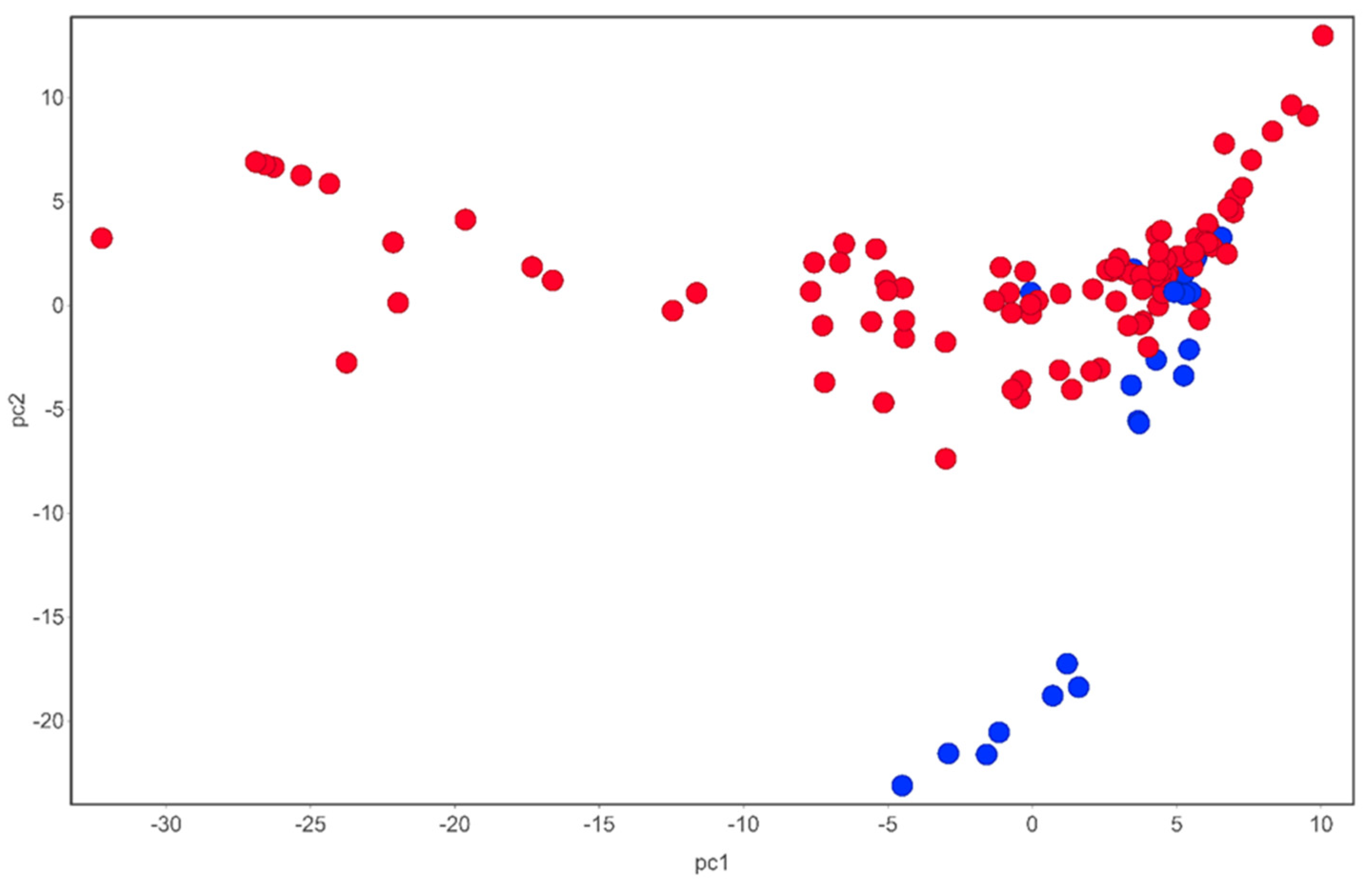
Figure 1. Chemical space of compounds identified from T. violacea. Blue circles are sulfur-containing compounds while red circles are compounds devoid of sulfur in their chemical structures. PCA analysis carried out using DataWarrior [54].
Most compounds reported have a small molecular weight (<500) and are of a broad lipophilicity (Figure 2).
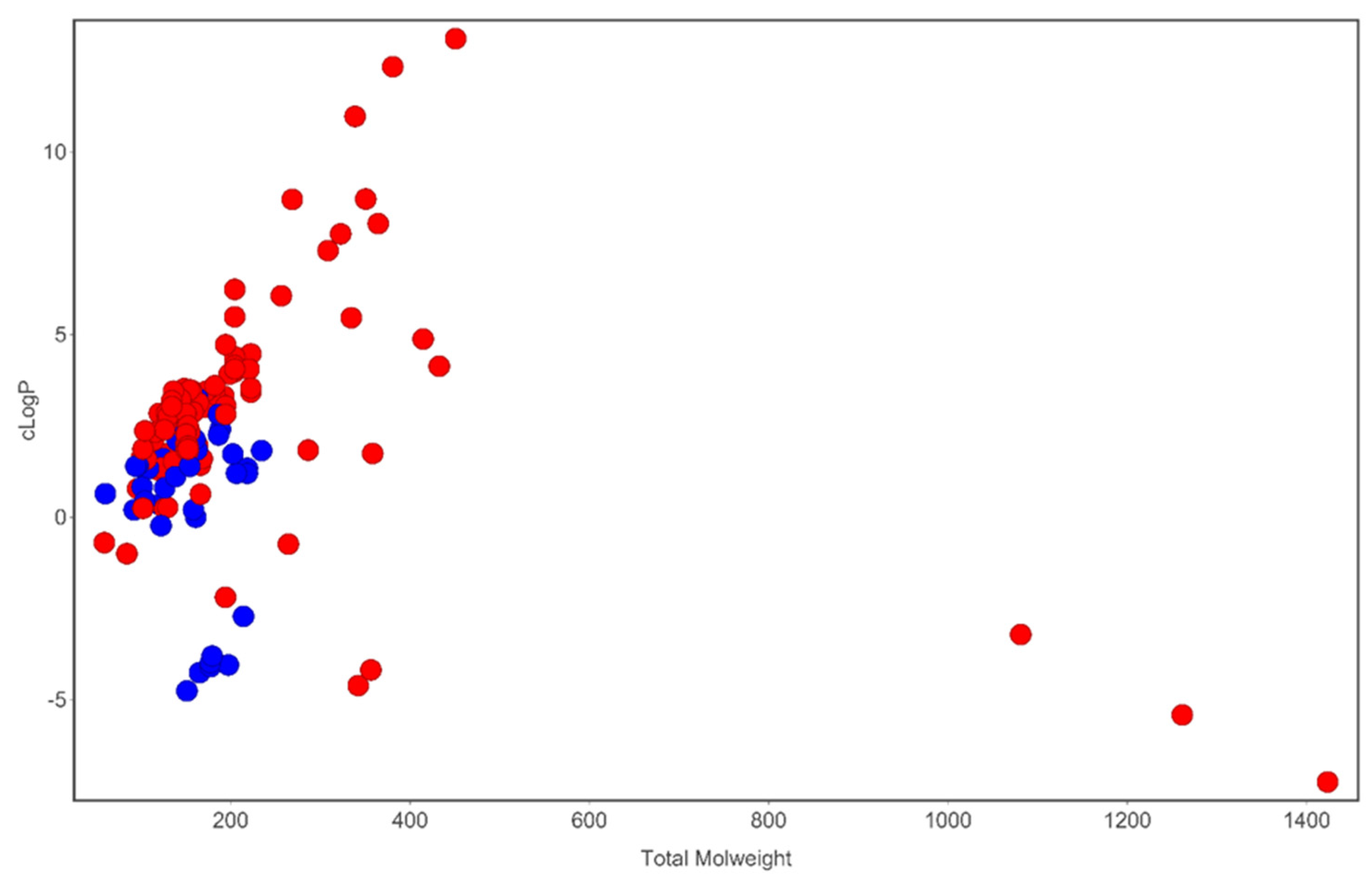
Figure 2. Analysis of cLogP and molecular weight space occupied by compounds identified in T. violacea. Blue circles are sulfur-containing compounds while red circles are compounds devoid of sulfur in their chemical structures. Plot generated using DataWarrior [54].
Tulbaghia violacea has been the most widely investigated for its phytochemistry and pharmacological properties. To date, close to 100 compounds have been tentatively identified, largely using gas chromatography techniques, from different parts of this species [55]. Most prominent are the sulfur compounds with reported broad-spectrum pharmacological activity. The thiosulfinate marasmicin (1) is the most prolific antimicrobial compound reported thus far from this genus [56]. This compound is formed from its precursor compound marasmin (2), by the enzyme c-lyase. Marasmicin is responsible for the characteristic garlic odor generated by damaged plants [48]. Other notable compounds produced by this species include phenols, tannins and flavonoids [55], which are also responsible for several observed biological activities. Phytochemical characterization has been carried out, albeit minimally for other Tulbaghia species particularly T. alliacea and T. acutiloba. Unlike other genera in Amaryllidaceae, Tulbaghia is so far devoid of any alkaloids [57][58]. Despite the extensive in vitro pharmacological screening of extracts of Tulbaghia, it is possible that less effort has been made to isolate and identify their active principles. Hence, the phytochemistry of the genus Tulbaghia largely remains understudied. The chemicals structurers of noteworthy compounds isolated from T. violacea have been represented in Figure 3.
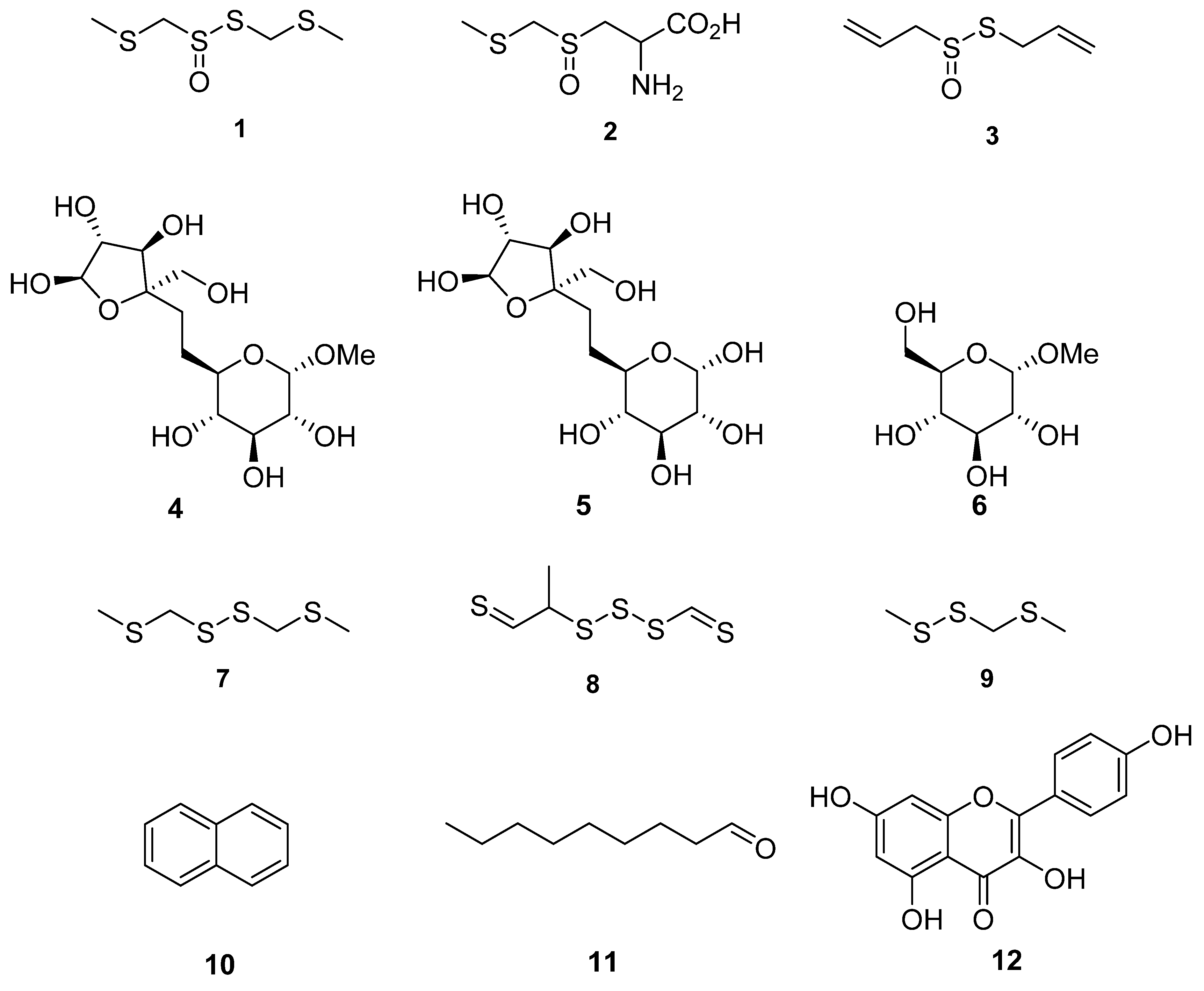
Figure 3. Chemical structures of compounds identified in T. violacea. (1) Marasmicin (1), (2) marasmin (2), allicin (3)—possesses antibacterial and antifungal activity, d-fructofuranosyl-β(2→6)-methyl-α-d-glucopyranoside (4), β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside (5), methyl-α-d-glucopyranoside (6), bis(methylthiomethyl) disulfide (7)—found to constitute 48% of volatiles in aerial parts of T. violacea [55], methyl-2-thioethyl thiomethyl trisulfide (8)—found to constitute 16% of volatile compounds in aerial parts of T. violacea [55], methyl (methylthio)methyl disulfide (9)—found to constitute 10 % of volatile compounds in aerial parts of T. violacea [55], naphthalene (10)—interestingly observed to significantly increase in concentration in plants infected by the fungus Beauveria bassiana in comparison to untreated controls [59], nonanal (11)—also observed to significantly decrease in concentration in plants infected by the fungi Beauveria bassiana in comparison to untreated controls [59] and finally kaempferol (12)—which possesses multiple biological activities including antioxidant, anticancer and anti-inflammatory properties [60][61][62].
2.4. Pharmacological Studies of Tulbaghia Species
Because of its perceived medicinal value, Tulbaghia has received marked interest within the scientific community which has meticulously subjected it to various in vitro and in vivo studies experimentally evaluating its pharmacological activities. The volume of published studies generated from these investigations mirror the distribution of the genus with most articles on Tulbaghia having emerged from South Africa (Table 2), a country highly rich in this genus both in terms of species diversity and abundance.
Table 2. Published documents on the genus Tulbaghia per country.
| Country | No. of Documents * |
|---|---|
| South Africa | 99 |
| United Kingdom | 15 |
| United States | 12 |
| Czech Republic | 8 |
| Italy | 7 |
| India | 6 |
| Germany | 5 |
| Australia | 3 |
| China | 3 |
| Belgium | 2 |
* Data retrieved following query of the Scopus database (https://www.scopus.com/, accessed on 22 February 2022) using the keyword “Tulbaghia”. The search was carried out on 22 February 2022.
The greatest numbers of pharmacological screens have been on interrogating the antimicrobial properties of this genus. This is closely followed by cardiovascular, antioxidants and cancer investigations as shown in Table 3. T. violacea prominently features, being the most studied species, with T. alliacea and T. aticulata having received minimal attention.
Table 3. Number of published studies per specific disease or pharmacological area.
| Disease | No. of Published Studies # |
|---|---|
| Antimicrobial | 26 |
| Cancer | 11 |
| Antioxidant | 13 |
| Diabetes | 2 |
| Cardiovascular | 12 |
| Antithrombogenic | 2 |
| Miscellaneous | 17 |
# Studies considered are those published from 1997 to 2022. A number of these, published before 2013, have been succinctly discussed by Aremu and Van Staden [8].
2.4.1. Antimicrobial and Antiparasitic Activity
As antimicrobial resistance continues to be a global health threat, the need to find therapeutic alternatives has never been more urgent [63]. This has encouraged scientists to search for novel alternatives with natural products having drawn marked interest as a potential oasis of new antimicrobial agents [64][65][66]. Tulbaghia has received significant relevance in this regard, with multiple studies providing ample evidence substantiating its use as an antimicrobial agent. Extracts of T. violacea have potency against many microbial species including those designated as priority by the World Health Organization. These include Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Klebsiella pneumoniae (K. pneumoniae) with MIC values ranging between 20 and 300 µg/mL [67]. This activity was confirmed in another study where the disc diffusion method was used [68]. In addition to bacteriostatic activity, extracts of T. violacea have shown noteworthy potency against yeasts including Candida albicans (C. albicans) and Candida parapsilosis (C. parapsilosis) with MIC and MMC values ranging between 20 and 40 µg/mL [68]. Beyond human pathogens, extracts of T. violacea have activity against microorganisms of agricultural significance, for example against the fungus Aspergillus flavus (A. flavus), which is responsible for significant agricultural produce loss at a global scale due to production of aflatoxins [69]. Extracts of T. violacea compromised cell wall synthesis by significantly reducing β-glucan and chitin synthesis in A. flavus corresponding to a dose-dependent inhibition of the enzymes β-glucan and chitin synthase, respectively [70]. Further studies suggested an alternative mode of action (MoA) via reduction of ergosterol production in fungi [71]. Interestingly, related to value in agriculture, a patent has been filed on the use of extracts of T. violacea as a plant protecting remedy as a substitute for chemical agents [72]. Some thought-provoking studies have shown that growth conditions including light intensities, watering frequency and pH, substantially impact both growth and biological potency of T. violacea extracts against Fusarium oxysporum (F. oxysporum) [73][74]. Likewise, storage conditions of dried plant material also affect the antimicrobial potency of extracts [56]. In addition to antimicrobial activity, T. violacea has shown good antiparasitic activity against the parasitic worm Meloidogyne incognita (M. incognita) on tomato roots and in soil [75]. Antiparasitic activity has also been observed against Trypanosoma brucei (T. brucei) (IC50 = 2.83 µg/mL) and Leishmania tarentolae (L. tarentolae) (IC50 = 6.29 µg/mL) [67]. Table 4 highlights the antimicrobial activity of Tulbaghia species.
Table 4. Antimicrobial activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Dichloromethane | Bulbs | MIC ranging from 20 to 300 µg/mL against Bacillus subtilis, methicillin-resistant S. aureus, S. epidermidis, E. coli, K. pneumoniae, P. aeruginosa, C. albicans and C. parapsilosis. | [67] |
| T. violacea | Hexane and ethanol | Flowers and callus cultures | Moderate to strong broad-antimicrobial (E. coli, P. aeruginosa, S. aureus, Aspergillus niger and C. albicans) activity observed by zone of inhibition in the agar well disc diffusion method. | [68] |
| T. violacea | Water | Bulbs | Significant reduction in A. flavus β-glucan and chitin synthesis corresponding to a dose-dependent inhibition of the enzymes β-glucan and chitin synthase, respectively.This results in inhibition of ergosterol production in the fungus. | [70][71] |
| T. violacea | Acetone | Bulbs | Varied light intensities, pH and watering frequencies substantially impacted both growth and potency of plant extracts against the fungi F. oxysporum. | [73][74] |
| T. violacea | Water | Roots, bulbs, leaves and flowers | Significantly compromised population densities of the nematode M. incognita race 2 on tomato roots and in the soil. | [75] |
| T. violacea | Dichloromethane | Bulbs | Antiparasitic activity against T. brucei (IC50 = 2.83 µg/mL) and L. tarentolae (IC50 = 6.29 µg/mL). | [67] |
2.4.2. Anticancer Activity
Owing to the need for novel anticancer agents [76] and motivated by the success of cancer drug discovery projects from natural products [77], Mthembu and Motadi in (2014), evaluated the in vitro anticancer properties of crude methanol extracts of T. violacea using an MTT assay [78]. Extracts displayed time- and concentration-dependent antiproliferative properties against cervical cancer cell lines with an IC50 of 150 µg/mL. The MoA was deciphered to be induction of apoptosis by a p53-independent pathway [78]. However, in contrast to this finding, continued work showed a proportional increase in the activity of caspase 3/7, and the expression of p53 genes strongly suggests apoptosis was triggered by a p53-dependent pathway [79]. This latter finding has been partly substantiated by data emerging from a study examining the antineoplastic properties of T. violacea against ovarian tumor cells. These extracts were shown to partially induce both apoptosis and necrosis with the most pronounced activity due to induction of autophagy [80].
Triple-negative breast cancer remains one of the most challenging cancers, being highly aggressive [81]. T. violacea extracts have demonstrated good cytotoxic activity against MDA-MB-231, with an IC50 of 300 µg/mL [82]. Additionally, extracts inhibited migration of the cancer cell lines (metastasis), an important physiological process in the progression of this cancer [83]. In addition to the gynecological cancers, antineoplastic properties of T. violacea were further observed against pancreatic cancer with 63% inhibition of cell proliferation at a concentration of 250 µg/mL [68]. Against a non-sex-specific cancer, T. violacea showed noticeable activity against oral cancer with an IC50 of 0.2 and 1 mg/mL for acetone and water-soluble extracts, respectively. Extracts activated caspase activity in a dose-dependent manner leading to induction of apoptosis in the human oral cancer cell line [84]. Using a bioassay guided approach, the active anticancer compounds in T. violacea have been identified to be glucopyranosides d-fructofuranosyl-β (2→6)-methyl-α-d-glucopyranoside and β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside. Both compounds act by mediating induction of apoptosis in Chinese hamster cells by targeting the mitochondrial (intrinsic) pathway [85][86]. A summary of the anticancer activity of Tulbaghia species is shown in Table 5.
Table 5. Anticancer activity of Tulbaghia.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Methanol | Leaves and roots | Marked time- and dose-dependent cytotoxic effect on cancer cell lines. Induced apoptosis using p53-independent pathway. | [78] |
| T. violacea | Methanol, butanol, and hexane | Leaves | Methanol extract was prolific against multiple cell lines. Hela and ME-180 cell lines treated with methanol and hexane extracts showed an increase in caspase 3/7 activity. Both methanol and hexane extracts induced a 10-fold increase in expression of p53 gene in Hela cells. | [79] |
| T. violacea | Methanol:water:formic acid (80:20:0.1, v/v/v) | Flowers | Demonstrated activity against ovarian tumor cells. | [80] |
| T. violacea | Water and methanol | Leaves | Water-soluble extract emerged as the most cytotoxic (IC50 = 314 µg/mL), compared to the methanol extract (IC50 = 780 µg/mL), against the MDA-MB-231 triple-negative breast cancer cell line. Water-soluble extract prevented cell migration completely for 13 h at 300 µg/mL. | [82] |
| T. violacea | Hexane and ethanol | Flowers and callus cultures | Extracts showed marked cytotoxicity (60–74% growth inhibition at 250 µg/mL) against three different cell lines (Hep G2, PC-3 and MCF-7). | [68] |
| T. violacea | Acetone and water | Leaves | Anticancer activity against oral cancer with an IC50 (acetone extract) of 0.2 mg/mL; IC50 (water extract) of 1 mg/mL. | [84] |
| T. violacea | Methanol:water (1:1) | Whole plants | Two pro-apoptotic glucopyranosides d-fructofuranosyl-β (2→6)-methyl-α-d-glucopyranoside and β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside isolated and identified as active anticancer agents in the plant. | [85] |
| T. violacea | Water | Whole plants | MoA of the three compounds, namely methyl-α-d-glucopyranoside, d-fructofuranosyl-β (2→6)-methyl-α-d-glucopyranoside and β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside isolated from the water extract, deciphered to be through induction of apoptosis by targeting the mitochondrial (intrinsic) pathway | [86] |
2.4.3. Antioxidant Activity
The imbalance of reactive oxygen species (ROS) and antioxidants in the body can lead to oxidative stress [87]. This physiological condition can result in cellular and tissue damage [88]. Oxidative stress is associated with pathologies including cancer, cardiovascular disease, diabetes, and neurodegenerative diseases amongst others [88][89]. To avert the development of oxidative stress, attenuation of ROS has been identified as a viable target, with natural products seen as a potential source capable of neutralizing it [88]. Tulbaghia has generated some interest on this front particularly as it is rich in compounds with proven antioxidant activity including phenols, tannins and flavonoids. Multiple studies have demonstrated that extracts of Tulbaghia have marked antioxidant activity as assessed using different assays in vitro including Trolox equivalent antioxidant capacity (TEAC; also commonly referred to as the ABTS assay), ferric-reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) (Table 6) [58][80][90][91]. Furthermore, using an in vivo model of Caenorhabditis elegans, T. violacea extracts attenuated oxidative stress produced by a free radical generator, (2,2′-azobis-2-amidinopropane dihydrochloride; AAPH), in the roundworm [80]. Data from these studies strongly suggested continued investigation of other species in the search for more potent antioxidant agents from Tulbaghia. The antioxidant activity of Tulbaghia species is highlighted in Table 6.
Table 6. Antioxidant activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Water | Leaves | Dose-dependent antioxidant activity measured using the DPPH (Log IC50 = 0.49 mg/mL) and ABTS (Log IC50 = 0.24 mg/mL) assays | [92] |
| T. violacea | Methanol/water/formic acid (80:20:0.1, v/v/v) | Flowers | Marked antioxidant activity was observed using 3 different types of assays, namely DPPH, FRAP and TREC | [80] |
| T. acutiloba | Hydro-methanolic extracts | Roots, rhizomes, leaves and flowers | Dose-dependent antioxidant activity observed with the rhizome extract emerging as the most active plant part (IC50 DPPH = 0.202 mg/mL and peak scavenging activity of 95) | [91] |
| T. violacea | Hexane and ethanol | Flowers and callus cultures | Dose-dependent antioxidant activity with IC50 ranging from 1.933 to 7.350 mg/mL in the DPPH assay | [68] |
| T. violacea | Acetone | Leaves | IC50 DPPH = 0.08 mg/mL; IC50 ABTS = 0.03 mg/mL | [84] |
| T. acutiloba | Acetone | Leaves | IC50 DPPH = 0.16 mg/mL; IC50 ABTS = 0.07 mg/mL | [84] |
| T. alliacea | Acetone | Leaves | IC50 DPPH = 0.06 mg/mL; IC50 ABTS = 0.06 mg/mL | [84] |
| T. cernua | Acetone | Leaves | IC50 DPPH = 0.21 mg/mL; IC50 ABTS = 2.34 mg/mL | [84] |
| T. leucantha | Acetone | Leaves | IC50 DPPH = 0.39 mg/mL; IC50 ABTS = 0.03 mg/mL | [84] |
| T. ludwigiana | Acetone | Leaves | IC50 DPPH = 0.26 mg/mL; IC50 ABTS = 0.09 mg/mL | [84] |
| T. natalensis | Acetone | Leaves | IC50 DPPH = 2.70 mg/mL; IC50 ABTS = 0.04 mg/mL | [84] |
2.4.4. Antidiabetic, Anticardiovascular and Antithrombogenic Activity
The incidence of diabetes and cardiovascular diseases continues to grow substantially across the globe, with both conditions combined accounting for the highest global morbidity and mortality [93][94]. Both of these chronic conditions are closely linked with cardiovascular disease being responsible for high morbidity and mortality in diabetic patients [95]. Tulbaghia has been documented in ethnopharmacological studies for the treatment of these ailments with emerging scientific data strongly validating its use. In streptozotocin diabetes-induced rat models, T. violacea attenuated diabetes-associated physiological conditions resulting in improved body weights, reduced fasting blood glucose levels, enhanced glucose tolerance and significantly elevated plasma insulin and liver glycogen content [96]. These data were corroborated in another study in which T. violacea noticeably reduced blood glucose and serum lipid (triglyceride (TG), total cholesterol (TC), and very low-density lipoprotein (VLDL)) levels while raising plasma insulin in a streptozotocin-induced diabetic rat model [97]. In an assessment for negating cardiovascular associated conditions, T. violacea in in vivo models markedly reduced systolic blood pressure (BP), diastolic BP, mean arterial pressure (MAP) and the heart rate in both age-induced and spontaneous hypertensive rats [98]. Furthermore, dosing rats with extracts of T. violacea led to improved kidney function [99]. This is an essential pharmacological property as kidney function is impaired in hypertension leading to high morbidity and mortality in people suffering from cardiovascular diseases [100].
One of the multiple factors strongly associated with cardiovascular disease is atherothrombotic vascular disease (AVD). Platelet aggregation plays a role in development of AVD and subsequent cardiovascular events [90][101]. Against this background, platelet aggregation has been identified as a key process to target to prevent AVD. Encouragingly, T. violacea demonstrated marked potency being able to significantly inhibit platelet adhesion 15 min post-exposure (Table 7) [90][92].
Table 7. Antidiabetic, anticardiovascular and antithrombogenic activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| Diabetes | ||||
| T. violacea | Methanol | Rhizome | Attenuated diabetes associated physiological complications in streptozotocin-induced diabetic rats. | [96] |
| T. violacea | Methanol | Rhizome | Noticeably reduced blood glucose and serum lipid (TG, TC, and VLDL) levels while raising plasma insulin in a streptozotocin-induced diabetic rat model. | [97] |
| Cardiovascular | ||||
| T. violacea | Methanol | Leaves | Markedly reduced systolic BP, diastolic BP, mean arterial pressure and the heart rate in both age-induced and spontaneous hypertensive rats. | [98] |
| T. violacea | Methanol | Rhizome | 50 mg/kg significantly improved kidney function in vivo. | [99] |
| T. acutiloba | Hydro-methanolic extracts | Roots, rhizomes, leaves and flowers | All extracts inhibited the Angiotensin-1-Converting Enzyme in vitro (> 50 % inhibition at a concentration range of 125–1000 μg/mL). Extracts of leaves demonstrated activity comparable to that of the control drug ramipril. | [91] |
| Antithrombogenic | ||||
| T. violacea | Water | Leaves | Noticeable inhibition of platelet adhesion by a novel scaffold consisting of polycaprolactone incorporated with 10 % (w/w) plant extracts. | [90] |
| T. violacea | Water | Leaves | Marked inhibition of platelet adhesion (70% inhibition at 0.1 mg/mL within 15 min post-exposure). | [92] |
2.4.5. Miscellaneous Pharmacological Activity
In addition to diabetes and cardiovascular diseases, T. violacea has shown activity against another chronic condition, Alzheimer’s disease. In an in vivo Alzheimer’s disease transgenic C. elegans strain model, T. violacea significantly reduced 1-42 β-amyloid peptide formation (Table 8) [80]. T. violacea exhibited in vivo anticonvulsant activity by attenuating tonic convulsions induced by either pentylenetetrazole, bicuculline, picrotoxin, strychnine or NMDLA [102] and validating its traditional use for the treatment of epilepsy. T. violacea displayed marked tick repellence properties of fungus-exposed plants at low treatment concentrations (5% w/v and 10% w/v) [59], further enhancing its credentials as a potential agricultural product. Somewhat concerning is that, extracts of T. violacea also induced genotoxic effects albeit at high test concentrations (250, 500 and 1000 µg/mL) in the Allium cepa assay [103]. Furthermore, broad murine macrophage antiproliferative and cytotoxicity activity, influenced by extract test concentrations, type of solvent and plant part used, have been observed (Table 8) [104]. There is consequently a need for rigorous assessment of safety of extracts of this and other species of the genus Tulbaghia.
Table 8. Miscellaneous biological properties of extracts of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Methanol/water/formic acid (80:20:0.1, v/v/v) | Flowers | Reduced 1-42 β-amyloid peptide formation and arrested oxidative stress in vivo. | [80] |
| T. violacea | Methanol | Leaves | Demonstrated in vivo anticonvulsant activity by attenuating tonic convulsions induced by either pentylenetetrazole, bicuculline, picrotoxin, strychnine or NMDLA. | [102] |
| T. violacea | Acetone | Mixture of leaves and bulbs | Marked tick repellence properties of fungus-exposed plants at low treatment concentrations (5 % w/v and 10 % w/v). | [59] |
| T. violacea | Water | Leaves, stems, and roots | Induced conspicuous genotoxicity effects at high test concentrations (250, 500 and 1000 µg/mL) in the A. cepa assay. | [103] |
| T. violacea | Water and ethanol | Leaves, stems, and roots | Broad murine macrophage antiproliferative and cytotoxicity activity influenced by both extract test concentrations, type of solvent and plant part used. | [104] |
References
- Barile, E.; Capasso, R.; Izzo, A.A.; Lanzotti, V.; Sajjadi, S.E.; Zolfaghari, B. Structure-activity relationships for saponins from Allium hirtifolium and Allium elburzense and their antispasmodic activity. Planta Med. 2005, 71, 1010–1018.
- Chase, M.W.; Reveal, J.L.; Fay, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2009, 161, 132–136.
- Takos, A.M.; Rook, F. Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use. Int. J. Mol. Sci. 2013, 14, 11713–11741.
- Vosa, C.G.; Siebert, S.J.; Van Wyk, A.E.B. Micromorphology and cytology of Prototulbaghia siebertii, with notes on its taxonomic significance. Upsp. Inst. Repos. 2011, 41, 311–314.
- Elgorashi, E.E.; van Staden, J. Bioactivity and Bioactive Compounds of African Amaryllidaceae; ACS Publications: Washington, DC, USA, 2009; ISBN 1947-5918.
- Fenwick, G.R.; Hanley, A.B.; Whitaker, J.R. The genus allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271.
- Fenwick, G.R.; Hanley, A.B. The genus allium—Part 2. Crit. Rev. Food Sci. Nutr. 1985, 22, 273–377.
- Aremu, A.O.; Van Staden, J. The genus Tulbaghia (Alliaceae)—A review of its ethnobotany, pharmacology, phytochemistry and conservation needs. J. Ethnopharmacol. 2013, 149, 387–400.
- Styger, G.; Aboyade, O.M.; Gibson, D.; Hughes, G. Tulbaghia—A Southern African Phytomedicine. J. Altern. Complement. Med. 2016, 22, 255–261.
- Jagtap, U.B.; Lekhak, M.M.; Fulzele, D.P.; Yadav, S.R.; Bapat, V.A. Analysis of selected Crinum species for galanthamine alkaloid: An anti-Alzheimer drug. Curr. Sci. 2014, 107, 2008–2010.
- Nair, J.J.; Aremu, A.O.; Van Staden, J. Isolation of narciprimine from Cyrtanthus contractus (Amaryllidaceae) and evaluation of its acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2011, 137, 1102–1106.
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind. Crops Prod. 2013, 43, 237–244.
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246.
- Sharma, D.; Rani, R.; Chaturvedi, M.; Rohilla, P.; Yadav, J.P. In silico and in vitro approach of Allium cepa and isolated quercetin against MDR bacterial strains and Mycobacterium smegmatis. S. Afr. J. Bot. 2019, 124, 29–35.
- Stoica, F.; Aprodu, I.; Enachi, E.; Stănciuc, N.; Condurache, N.N.; Duță, D.E.; Bahrim, G.E.; Râpeanu, G. Bioactive’s Characterization, Biological Activities, and In Silico Studies of Red Onion (Allium cepa L.) Skin Extracts. Plants 2021, 10, 2330.
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods 2013, 5, 930–939.
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.-L. Anti-microbial and anti-oxidant activities of Illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengeineered 2013, 4, 244–248.
- Elberry, A.A.; Mufti, S.; Al-Maghrabi, J.; Abdel Sattar, E.; Ghareib, S.A.; Mosli, H.A.; Gabr, S.A. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediat. Inflamm. 2014, 2014, 640746.
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. 2006, 1112, 3–22.
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234.
- Harazem, R.; El Rahman, S.A.; El-Kenawy, A. Evaluation of Antiviral Activity of Allium cepa and Allium sativum Extracts Against Newcastle Disease Virus. Alex. J. Vet. Sci. 2019, 61, 108–118.
- Elmi, T.; Hajialiani, F.; Asadi, M.R.; Sadeghi, S.; Namazi, M.J.; Tabatabaie, F.; Zamani, Z. Antimalarial effects of the hydroalcoholic extract of Allium paradoxum in vitro and in vivo. J. Parasit. Dis. 2021, 45, 1055–1064.
- Ruslan, M.S.; Baba, M.S. In vivo antimalarial assessment and toxicity evaluation of garlic (Allium sativum) in plasmodium berghei NK65-induced mice. Malays. Appl. Biol. 2018, 47, 17–24.
- Syaban, M.F.R.; Rachman, H.A.; Arrahman, A.D.; Hudayana, N.; Khamid, J.P.; Pratama, F.A. Allium sativum as antimalaria agent via falciapin protease-2 inhibitor mechanism: Molecular docking perspective. Clin. Res. J. Intern. Med. 2021, 2, 130–135.
- Upadhyay, R.K. Nutritional and therapeutic potential of Allium vegetables. J. Nutr. Ther. 2017, 6, 18–37.
- Thomson, M.; Ali, M. Garlic : A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81.
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Capasso, R.; Izzo, A.A. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. J. Agric. Food Chem. 2005, 53, 935–940.
- Galmarini, C.R.; Goldman, I.L.; Havey, M.J. Genomics Genetic analyses of correlated solids, flavor, and health-enhancing traits in onion (Allium cepa L.). Mol. Genet. Genom. 2001, 265, 543–551.
- Takahashi, M.; Shibamoto, T. Chemical compositions and antioxidant/anti-inflammatory activities of steam distillate from freeze-dried onion (Allium cepa L.) sprout. J. Agric. Food Chem. 2008, 56, 10462–10467.
- Nishimura, H.; Wijaya, C.H.; Mizutani, J. Volatile flavor components and antithrombotic agents: Vinyldithiins from Allium victorialis L. J. Agric. Food Chem. 1988, 36, 563–566.
- Brace, L.D. Cardiovascular benefits of garlic (Allium sativum L.). J. Cardiovasc. Nurs. 2002, 16, 33–49.
- Ali, M.; Thomson, M.; Afzal, M. Garlic and onions: Their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 55–73.
- Sabiu, S.; Madende, M.; Ajao, A.A.; Aladodo, R.A.; Nurain, I.O.; Ahmad, J.B. The Genus Allium (Amaryllidaceae: Alloideae): Features, Phytoconstituents, and Mechanisms of Antidiabetic Potential of Allium cepa and Allium sativum, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128138229.
- Muñoz-Torrero López-Ibarra, D. Recent Advances in Pharmaceutical Sciences I; Transworld Research Network: Trivandrum, India, 2011; ISBN 8178955288.
- Akash, M.S.H.; Rehman, K.; Chen, S. Spice plant Allium cepa: Dietary supplement for treatment of type 2 diabetes mellitus. Nutrition 2014, 30, 1128–1137.
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625.
- Kumar, K.P.S.; Debjit, B.; Pankaj, T. Allium cepa: A traditional medicinal herb and its health benefits. J. Chem. Pharm. Res. 2010, 2, 283–291.
- Shri, R.; Bora, K.S. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia 2008, 79, 86–96.
- Kongkwamcharoen, C.; Itharat, A.; Pipatrattanaseree, W.; Ooraikul, B. Effects of Various Preextraction Treatments of Crinum asiaticum Leaf on Its Anti-Inflammatory Activity and Chemical Properties. Evid. Based. Complement. Alternat. Med. 2021, 2021, 8850744.
- Fennell, C.W.; Van Staden, J. Crinum species in traditional and modern medicine. J. Ethnopharmacol. 2001, 78, 15–26.
- Maroyi, A. Ethnobotanical, phytochemical and pharmacological properties of Crinum bulbispermum (Burm f) Milne-Redh and Schweick (Amaryllidaceae). Trop. J. Pharm. Res. 2016, 15, 2497–2506.
- Takaidza, S.; Pillay, M.; Mtunzi, F.M. Biological activities of species in the genus Tulbaghia: A review. Afr. J. Biotechnol. 2015, 14, 3037–3043.
- Herrera, M.R.; Machocho, A.K.; Nair, J.J.; Campbell, W.E.; Brun, R.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Cyrtanthus elatus. Fitoterapia 2001, 72, 444–448.
- Nair, J.J.; van Staden, J. Chemical and biological studies of the South African Amaryllidaceae genera Crinum, Ammocharis, Amaryllis, Cyrtanthus and Brunsvigia. S. Afr. J. Bot. 2021, 142, 467–476.
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162.
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275.
- Govaerts, R. World Checklist of Selected Plant Species. Facilitated by the Royal Botanic Gardens, Kew. 2015. Available online: https://wcsp.science.kew.org/about.do (accessed on 25 March 2022).
- Kubec, R.; Velíšek, J.; Musah, R.A. The amino acid precursors and odor formation in society garlic (Tulbaghia violacea Harv.). Phytochemistry 2002, 60, 21–25.
- Dillon, H.; Nelson, E.C. Tulbaghia leucantha: Alliaceae. Kew Mag. 1991, 8, 12–15.
- Makunga, N.P. Medicinal Plants of South Africa; Briza Publications: Pretoria, South Africa, 2010; Volume 105.
- Van Wyk, B.E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868.
- Raji, I.A.; Obikeze, K.; Mugabo, P.E. Potential beneficial effects of tulbaghia violacea william henry harvey (Alliaceae) on cardiovascular system—A Review. Trop. J. Pharm. Res. 2015, 14, 1111–1117.
- Pooley, E. A Field Guide to the Wild Flowers of KwaZulu-Natal and the Eastern Region. Natal Flora Publ. Trust. Pg 2005, 93, 630.
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473.
- Pino, J.A.; Quijano-Celís, C.E.; Fuentes, V. Volatile compounds of tulbaghia violacea harv. J. Essent. Oil-Bear. Plants 2008, 11, 203–207.
- Ranglová, K.; Krejčová, P.; Kubec, R. The effect of storage and processing on antimicrobial activity of Tulbaghia violacea. S. Afr. J. Bot. 2015, 97, 159–164.
- Smith, S.; Stansbie, J. Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administration; CRC Press: Boca Raton, FL, USA, 2003; p. 230.
- Takaidza, S.; Mtunzi, F.; Pillay, M. Analysis of the phytochemical contents and antioxidant activities of crude extracts from Tulbaghia species. J. Tradit. Chin. Med. 2018, 38, 272–279.
- Staffa, P.; Nyangiwe, N.; Msalya, G.; Nagagi, Y.P.; Nchu, F. The effect of Beauveria bassiana inoculation on plant growth, volatile constituents, and tick (Rhipicephalus appendiculatus) repellency of acetone extracts of Tulbaghia violacea. Vet. World 2020, 13, 1159–1166.
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10.
- Teffo, L.S.; Aderogba, M.A.; Eloff, J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010, 76, 25–29.
- Yang, C.; Yang, W.; He, Z.; Guo, J.; Yang, X.; Wang, R.; Li, H. Kaempferol Alleviates Oxidative Stress and Apoptosis Through Mitochondria-dependent Pathway During Lung Ischemia-Reperfusion Injury. Front. Pharmacol. 2021, 12, 11.
- Murray, C.J. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 388, 629–655.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258.
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2018, 26, 5501–5541.
- Salam, A.M.; Quave, C.L. Opportunities for plant natural products in infection control. Curr. Opin. Microbiol. 2018, 45, 189–194.
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum extracts exhibit anti-parasitic and antimicrobial activities. Molecules 2018, 23, 313.
- Eid, H.H.; Metwally, G.F. Phytochemical and biological study of callus cultures of Tulbaghia violacea Harv. Cultivated in Egypt. Nat. Prod. Res. 2017, 31, 1717–1724.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170.
- Belewa, V.; Baijnath, H.; Frost, C.; Somai, B.M. Tulbaghia violacea Harv. plant extract affects cell wall synthesis in Aspergillus flavus. J. Appl. Microbiol. 2017, 122, 921–931.
- Somai, B.M.; Belewa, V.; Frost, C. Tulbaghia violacea (Harv) Exerts its Antifungal Activity by Reducing Ergosterol Production in Aspergillus flavus. Curr. Microbiol. 2021, 78, 2989–2997.
- Pretorius, J.C. Extracts and Compounds from Tulbaghia Violacea and Their Use as Biological Plant Protecting Agents 2014. Google Patents US8697149B2, 15 April 2014.
- Ncise, W.; Daniels, C.W.; Etsassala, N.G.E.R.; Nchu, F. Interactive effects of light intensity and ph on growth parameters of a bulbous species (Tulbaghia violacea l.) in hydroponic cultivation and its antifungal activities. Med. Plants 2021, 13, 442–451.
- Ncise, W.; Daniels, C.W.; Nchu, F. Effects of light intensities and varying watering intervals on growth, tissue nutrient content and antifungal activity of hydroponic cultivated Tulbaghia violacea L. under greenhouse conditions. Heliyon 2020, 6, 3906.
- Malungane, M.M.F.; Florah, M.M. Effect of Crude Extracts of Tulbaghia violacea (Wild Garlic) on Growth of Tomato and Supression of Meloidogyne Species; University of Limpopo: Mankweng, South Africa, 2014.
- Kaushik, I.; Ramachandran, S.; Prasad, S.; Srivastava, S.K. Drug rechanneling: A novel paradigm for cancer treatment. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 68, pp. 279–290.
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043.
- Mthembu, N.N.; Motadi, L.R. Apoptotic potential role of Agave palmeri and Tulbaghia violacea extracts in cervical cancer cells. Mol. Biol. Rep. 2014, 41, 6143–6155.
- Motadi, L.R.; Choene, M.S.; Mthembu, N.N. Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci. Rep. 2020, 10, 12924.
- Rivas-García, L.; Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; Quiles, J.L.; Llopis, J.; Sánchez-González, C. Unravelling potential biomedical applications of the edible flower Tulbaghia violacea. Food Chem. 2022, 381, 132096.
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690.
- Dlamini, Z.; Alouna, M.; Hull, R.; Penny, C. Abstract 2843: The effects of extracts of the indigenous South African plant, Tulbaghia violacea, on triple negative breast cancer cells. In Proceedings of the NCRI Cancer Conference, London, UK, 8–12 November 2021.
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683.
- Takaidza, S.; Kumar, A.M.; Ssemakalu, C.C.; Natesh, N.S.; Karanam, G.; Pillay, M. Anticancer activity of crude acetone and water extracts of Tulbaghia violacea on human oral cancer cells. Asian Pac. J. Trop. Biomed. 2018, 8, 456–462.
- Lyantagaye, S.L. Two new pro-apoptotic glucopyranosides from Tulbaghia violacea. J. Med. Plants Res. 2013, 7, 2214–2220.
- Lyantagaye, S. Characterization of the Biochemical Pathway of Apoptosis Induced by D-glucopyranoside Derivatives from Tulbaghia violacea. Annu. Res. Rev. Biol. 2014, 4, 962–977.
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175.
- Thorpe, G.W.; Fong, C.S.; Alic, N.; Higgins, V.J.; Dawes, I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: Oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 2004, 101, 6564–6569.
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132.
- Madike, L.N.; Pillay, M.; Popat, K.C. Antithrombogenic properties of Tulbaghia violacea–loaded polycaprolactone nanofibers. J. Bioact. Compat. Polym. 2020, 35, 102–116.
- Arhin, I.; Depika, D.; Ajay, B.; Delon, N.; Irene, M. Biochemical, phytochemical profile and angiotensin-1 converting enzyme inhibitory activity of the hydro-methanolic extracts of Tulbaghia acutiloba harv. J. Nat. Remedies 2019, 19, 221–235.
- Madike, L.N.; Pillay, M.; Popat, K.C. Antithrombogenic properties of: Tulbaghia violacea aqueous leaf extracts: Assessment of platelet activation and whole blood clotting kinetics. RSC Adv. 2021, 11, 30455–30464.
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 1–8.
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053.
- Matheus, A.S.D.M.; Tannus, L.R.M.; Cobas, R.A.; Palma, C.C.S.; Negrato, C.A.; Gomes, M.D.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789.
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 2015, 15, 408.
- Moodley, K.; Mackraj, I. Metabolic effects of tulbaghia violacea harv. In a diabetic model. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 113–122.
- Raji, I.; Obikeze, K.; Mugabo, P. Comparison of the acute effects of Tulbaghia violacea William Henry Harvey (Alliaceae) on blood pressure and heart rate of ageing male normotensive Wistar kyoto rats and adult male spontaneously hypertensive rats. Trop. J. Pharm. Res. 2016, 15, 2429–2434.
- Moodley, K.; Naidoo, Y.; Mackraj, I. Effects of Tulbaghia violacea Harv. (Alliaceae) rhizome methanolic extract on kidney function and morphology in Dahl salt-sensitive rats. J. Ethnopharmacol. 2014, 155, 1194–1203.
- Navar, L.G. The role of the kidneys in hypertension. J. Clin. Hypertens. 2005, 7, 542–549.
- Davì, G.; Patrono, C. Platelet Activation and Atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494.
- Masoud, K.A.A.; Okobi, E.; Ekpo, G.J. Amabeoku Investigation of Some Possible Mechanisms Involved in the Anticonvulsant Activity of Tulbaghia violacea Harv. J. Pharm. Pharmacol. 2017, 5.
- Madike, L.N.; Takaidza, S.; Ssemakalu, C.; Pillay, M. Genotoxicity of aqueous extracts of Tulbaghia violacea as determined through an Allium cepa assay. S. Afr. J. Sci. 2019, 115, 1–6.
- Madike, L.N.; Takaidza, S.; Ssemakalu, C.C.; Pillay, M. The effect of extracts of Tulbaghia violacea on the proliferation of a murine macrophage cell line. S. Afr. J. Bot. 2020, 130, 185–197.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
29 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




