Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Cynthia Amaning Danquah and Version 3 by Rita Xu.
Amaryllidaceae is a significant source of bioactive phytochemicals with a strong propensity to develop new drugs. The genera
Allium
,
Tulbaghia
,
Cyrtanthus
and
Crinum
biosynthesize novel alkaloids and other phytochemicals with traditional and pharmacological uses. Amaryllidaceae biomolecules exhibit multiple pharmacological activities such as antioxidant, antimicrobial, and immunomodulatory effects.
- Amaryllidaceae
- Tulbaghia
- natural products
1. Introduction
Amaryllidaceae belongs to the order Asparagales and consists of bulbous flowering plants separated into three infrageneric ranks: Agapanthoideae, Allioideae and Amaryllidoideae, as delineated by the Angiosperm Phylogeny Group [1]. The term “Amaryllidaceae” is frequently used in either phytochemical or pharmacological literature to refer to plants or alkaloids originating from the subfamily Amaryllidoideae [2][3][2,3]. Monocotyledonous plants constitute seventy-nine genera (including Allium, Crinum, Cyrtanthus, and Tulbaghia) with over 1000 species [4]. Aside from their broad pantropical distribution, Amaryllidaceae are located in Africa, the Mediterranean Coast and South America, and have high adaptation and speciation [5]. The genus Allium is distributed in temperate, arid, semi-arid and subtropical areas such as the Mediterranean region, central Asia, Africa and parts of Europe. As herbaceous geophyte perennials, Allium comprises a plethora of species with pungent linear leaves that may or may not arise from a bulb or rhizome [6][7][6,7]. The Tulbaghia genus, popularly called “sweet garlic”, “wild garlic”, or “pink agapanthus”, is crown shaped with outgrowth or appendages of the perianth and predominantly colonizes the Eastern cape belt of South Africa, and is adapted for growth in areas such as Europe and America [8][9][8,9]. The genus Crinum encompasses 104 species and appear as showy flowers on leafless stems, which thrive in the tropics and warm temperate parts, specifically Asia, Africa, America, and Australia [10]. Cyrtanthus is popularly known as “fire lily” due to its unique rapidly flowering response to natural bush fires. Most species are found in South Africa and play an important role in South African traditional medicine [11].
Amaryllidaceous plants are known for their ornamental, nutritional, and medicinal value. Given their attractive flowering plant-like features, Crinum species are prized for their umbel lily-like blossoms in China and Japan [3]. Concurrently, Amaryllidaceae are known for their longstanding exploitation in medicinal therapy owing to their inherent biosynthesis of chemically diverse bioactive compounds with peculiar biological properties. The use of proximate and mineral composition analysis enabled the identification of phytoconstituents [10][12][10,12], while in vitro, in vivo, and in silico model systems have permitted the unravelling of intrinsic pharmacological activities of the natural products and other alkaloids isolated from this source [13][14][15][13,14,15]. Of note, bioactive compounds from Amaryllidaceae possesses a wide range of bioactivities ranging from antioxidant [16][17][16,17], anti-inflammatory [16][18][16,18], antimicrobial [17], antifungal [19], antiviral [20][21][20,21], antiplasmodial [22][23][24][22,23,24], anticarcinogenic [18][25][26][18,25,26], antispasmodic [1][27][1,27], antiplatelet [28], antiasthmatic [29], antithrombotic [30][31][30,31], antitumor [25], antihyperlipidemic [25], antihyperglycemic [25][32][33][25,32,33], antiarthritic [25], antimutagenic [16], immunomodulatory [16] and several others [34].
Given the aforementioned biological activities, Allium, Tulbaghia, Cyrtanthus and Crinum are utilized in traditional medicinal therapy for varying diseases and conditions [35][36][37][38][39][40][41][35,36,37,38,39,40,41]. For example, Allium is used as concoctions, decoctions, extracts, and herbal preparations to treat angina, amoebic dysentery, arthritis, cardiovascular diseases, cholera, catarrh, dysmenorrhea, fever, headaches, hepatitis, stomach disorders, throat infections, and prostatic hypertrophy [30][31][35][36][37][38][30,31,35,36,37,38]. The genus Tulbaghia has unique pharmacotherapeutic properties and is utilized to manage ailments such as earache, pyrexia, tuberculosis, and rheumatism [9][42][9,42]. Crinum species are used to treat haemorrhoids, malaria, osteoarthritis, varicosities, wounds, urinary tract infections, and gynaecological remedies [40][41][40,41]. Cyrtanthus are also employed in the management of ailments associated with pregnancy, as well as cystitis, age-related dementia, leprosy, scrofula, headaches, chronic coughs, among others [43][44][43,44]. In modern clinical practice, galanthamine from Amaryllidaceae is a primary choice of drug in managing symptomatic neurological disorders such as Alzheimer’s disease due to its selective inhibitory action on the acetylcholine biosynthetic enzyme, acetylcholinesterase [45]. The pancratistatin phenanthridone class of alkaloids are also promising chemotherapeutic drug candidates with unique cell line-specific antiproliferative properties, conferring a selective advantage for clinical development [46].
Although Amaryllidaceae represents a source of valuable bioactive compounds, developing promising drug candidates into clinically relevant therapeutics has been slow. Similarly, other genera in this family, including Cyrtanthus, Crinum and Tulbaghia, are untapped reservoirs and could serve as an alternative window for novel drug targets and warrant further investigation.
2. The Genus Tulbaghia
2.1. Botanical Description
Tulbaghia is made up of monocotyledonous species with herbaceous perennial bulbs covered by brown scales and are mostly found in Africa [8]. South African species possess bulb-like corms or rhizomes which are swollen, irregularly shaped and wrapped in dry, fibrous leaves [8]. Members of this genus usually possess a raised crown-like structure or ring at the center of their flower tube [8]. Their seeds are black, flat and elongated with the mature ones having embryos [8]. Examples of species of this genus are Tulbaghia violacea (T. violacea), Tulbaghia acutiloba Harv. (T. acutiloba), Tulbaghia capensis L. (T. capensis) and Tulbaghia cepacea L.f (T. cepacea) [8].2.2. Geographical Distribution and Traditional Uses of Tulbaghia Species
With approximately 66 species (https://www.kew.org/science accessed on 22 February 2022) [47], Tulbaghia is the second-most species-rich genus within Amaryllidaceae. Tulbaghia is a monocotyledonous genus comprised morphologically of herbaceous perennial bulbous species, which produce a variety of volatile sulfur compounds, hence resulting in a distinct pungent garlic odor released by bruised plants [8][48][8,48]. The genus was named by Carl Linnaeus after Ryk Tulbagh (1699–1771), a former governor of the Cape of Good Hope in South Africa, where most of the native species are to be found, particularly in the Eastern Cape Province [49]. In addition to South Africa, the genus is widely distributed across southern African countries including Botswana, Lesotho, Swaziland, and Zimbabwe, where the plant is revered in folk medicine being used for the treatment of a plethora of infectious and non-infectious diseases [9] as highlighted in Table 1.Table 1. Geographical distribution and traditional uses of Tulbaghia species.
| Plant Species | Geographical Distribution | Traditional Uses | References |
|---|---|---|---|
| T. violacea | Indigenous to the Eastern Cape, KwaZulu-Natal, Gauteng, Free State and Mpumalanga Provinces of South Africa. | The leaves and bulbs are used in the management of fever and colds, tuberculosis, asthma, and stomach problems. The leaves are eaten as vegetables and for the management of oesophageal cancer. It is also used as a snake repellent. | [8][8[50],50] |
| T. alliacea | Native to South Africa and grows mostly in the Eastern Cape and southern KwaZulu-Natal Provinces of South Africa. | Its bruised rhizome is used locally in bathwater to relieve fever, rheumatism, and paralysis, and in small doses as a laxative. T. alliacea is used for the management of stomach problems, asthma, and pulmonary tuberculosis. Its rhizome infusion is administered as an enema. | [8][51][8,51] |
| T. simmleri | Native to the South African Drakensberg mountains growing as isolated plants on rocky ledges. | Bulbs and leaves are used as a remedy for gastrointestinal ailments, enemas, high blood pressure, heart problems, chest complaints, high cholesterol, constipation, rheumatism, asthma, fever, pulmonary tuberculosis, earache, human immunodeficiency virus (HIV), paralysis, and cardiovascular diseases. | [50][52][50,52] |
| T. acutiloba | Found in the rainfall regions of southern Africa, occurring in the Eastern Cape, KwaZulu-Natal, Limpopo, Free State, Gauteng, North West, and Mpumalanga Provinces of South Africa, as well as in Lesotho, Swaziland and Botswana. | T. acutiloba leaves are used as a culinary herb and snake repellent. It is used to treat barrenness, flu, bad breath, and as an aphrodisiac. It is also cultivated to keep snakes away from the homestead. | [8] |
| T. natalensis | Although native to South Africa, but is now grown worldwide. | It is used as a culinary herb and snake repellent. | [53] |
| T. cernua | Commonly found in the Eastern Cape, Free State, Gauteng, KwaZulu-Natal, Limpopo, Mpumalanga, North West and Western Cape Provinces of South Africa. | It is used for ornamental purposes. | [8] |
| T. leucantha | Widely distributed in southern Africa including Botswana, Lesotho, South Africa, Swaziland, Zambia, and Zimbabwe. | Its rhizome is scraped clean and boiled in stews or roasted as a vegetable. Its leaves and stems are used as a culinary herb and protective charm. | [53] |
| T. ludwigiana | Commonly found in the Eastern Cape, KwaZulu-Natal, Northern Provinces of South Africa and in Swaziland. | It is traditionally used as a love charm. | [53] |
2.3. Phytochemistry of Tulbaghia
Tulbaghia produces many different classes of compounds with diverse chemical structures dominated by sulfur-containing natural products (Figure 1).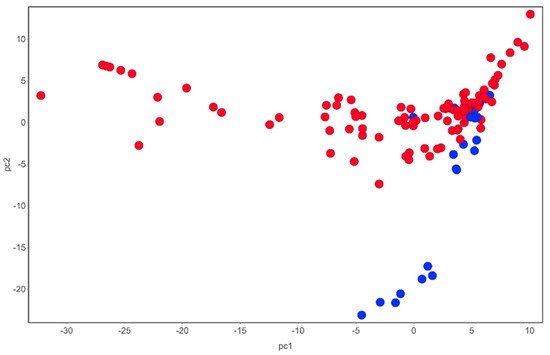
Figure 1. Chemical space of compounds identified from T. violacea. Blue circles are sulfur-containing compounds while red circles are compounds devoid of sulfur in their chemical structures. PCA analysis carried out using DataWarrior [54].
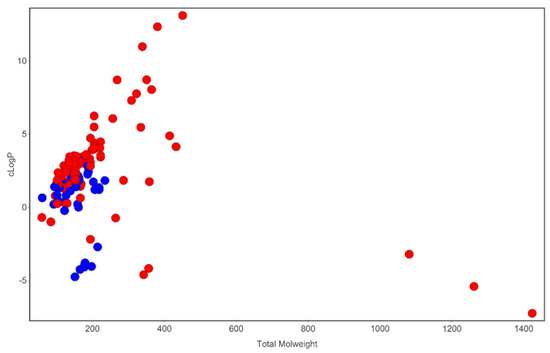
Figure 2. Analysis of cLogP and molecular weight space occupied by compounds identified in T. violacea. Blue circles are sulfur-containing compounds while red circles are compounds devoid of sulfur in their chemical structures. Plot generated using DataWarrior [54].
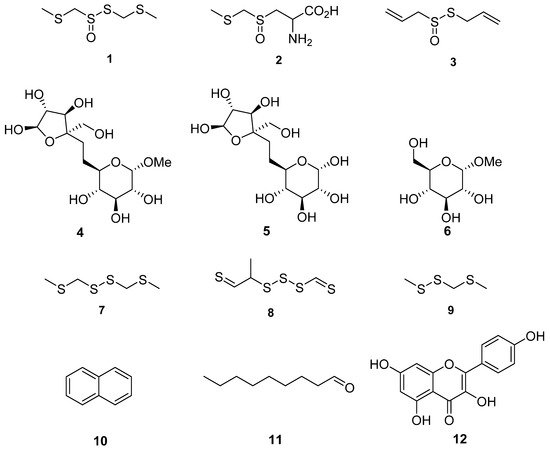
Figure 3. Chemical structures of compounds identified in T. violacea. (1) Marasmicin (1), (2) marasmin (2), allicin (3)—possesses antibacterial and antifungal activity, d-fructofuranosyl-β(2→6)-methyl-α-d-glucopyranoside (4), β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside (5), methyl-α-d-glucopyranoside (6), bis(methylthiomethyl) disulfide (7)—found to constitute 48% of volatiles in aerial parts of T. violacea [55], methyl-2-thioethyl thiomethyl trisulfide (8)—found to constitute 16% of volatile compounds in aerial parts of T. violacea [55], methyl (methylthio)methyl disulfide (9)—found to constitute 10 % of volatile compounds in aerial parts of T. violacea [55], naphthalene (10)—interestingly observed to significantly increase in concentration in plants infected by the fungus Beauveria bassiana in comparison to untreated controls [59], nonanal (11)—also observed to significantly decrease in concentration in plants infected by the fungi Beauveria bassiana in comparison to untreated controls [59] and finally kaempferol (12)—which possesses multiple biological activities including antioxidant, anticancer and anti-inflammatory properties [60][61][62][60,61,62].
2.4. Pharmacological Studies of Tulbaghia Species
Because of its perceived medicinal value, Tulbaghia has received marked interest within the scientific community which has meticulously subjected it to various in vitro and in vivo studies experimentally evaluating its pharmacological activities. The volume of published studies generated from these investigations mirror the distribution of the genus with most articles on Tulbaghia having emerged from South Africa (Table 2), a country highly rich in this genus both in terms of species diversity and abundance.Table 2. Published documents on the genus Tulbaghia per country.
| Country | No. of Documents * | |
|---|---|---|
| South Africa | 99 | |
| United Kingdom | 15 | |
| United States | 12 | |
| 13 | Czech Republic | 8 |
| Diabetes | Italy | 7 |
| India | 6 | |
| Germany |
Table 3. Number of published studies per specific disease or pharmacological area.
| Disease | No. of Published Studies # |
|---|---|
| Antimicrobial | 26 |
| 5 | |
| Australia | |
| Cancer | 112 |
| Cardiovascular | 12 |
| Antithrombogenic | 2 |
| Miscellaneous | 173 |
| China | 3 |
| Belgium | 2 |
| Antioxidant |
# Studies considered are those published from 1997 to 2022. A number of these, published before 2013, have been succinctly discussed by Aremu and Van Staden [8].
2.4.1. Antimicrobial and Antiparasitic Activity
As antimicrobial resistance continues to be a global health threat, the need to find therapeutic alternatives has never been more urgent [63]. This has encouraged scientists to search for novel alternatives with natural products having drawn marked interest as a potential oasis of new antimicrobial agents [64][65][66][64,65,66]. Tulbaghia has received significant relevance in this regard, with multiple studies providing ample evidence substantiating its use as an antimicrobial agent. Extracts of T. violacea have potency against many microbial species including those designated as priority by the World Health Organization. These include Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Klebsiella pneumoniae (K. pneumoniae) with MIC values ranging between 20 and 300 µg/mL [67]. This activity was confirmed in another study where the disc diffusion method was used [68]. In addition to bacteriostatic activity, extracts of T. violacea have shown noteworthy potency against yeasts including Candida albicans (C. albicans) and Candida parapsilosis (C. parapsilosis) with MIC and MMC values ranging between 20 and 40 µg/mL [68]. Beyond human pathogens, extracts of T. violacea have activity against microorganisms of agricultural significance, for example against the fungus Aspergillus flavus (A. flavus), which is responsible for significant agricultural produce loss at a global scale due to production of aflatoxins [69]. Extracts of T. violacea compromised cell wall synthesis by significantly reducing β-glucan and chitin synthesis in A. flavus corresponding to a dose-dependent inhibition of the enzymes β-glucan and chitin synthase, respectively [70]. Further studies suggested an alternative mode of action (MoA) via reduction of ergosterol production in fungi [71]. Interestingly, related to value in agriculture, a patent has been filed on the use of extracts of T. violacea as a plant protecting remedy as a substitute for chemical agents [72]. Some thought-provoking studies have shown that growth conditions including light intensities, watering frequency and pH, substantially impact both growth and biological potency of T. violacea extracts against Fusarium oxysporum (F. oxysporum) [73][74][73,74]. Likewise, storage conditions of dried plant material also affect the antimicrobial potency of extracts [56]. In addition to antimicrobial activity, T. violacea has shown good antiparasitic activity against the parasitic worm Meloidogyne incognita (M. incognita) on tomato roots and in soil [75]. Antiparasitic activity has also been observed against Trypanosoma brucei (T. brucei) (IC50 = 2.83 µg/mL) and Leishmania tarentolae (L. tarentolae) (IC50 = 6.29 µg/mL) [67]. Table 4 highlights the antimicrobial activity of Tulbaghia species.Table 4. Antimicrobial activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Dichloromethane | Bulbs | MIC ranging from 20 to 300 µg/mL against Bacillus subtilis, methicillin-resistant S. aureus, S. epidermidis, E. coli, K. pneumoniae, P. aeruginosa, C. albicans | |
| M. incognita | ||||
| race 2 on tomato roots and in the soil. | ||||
| [ | 75 | ] | ||
| T. violacea | Dichloromethane | Bulbs | Antiparasitic activity against T. brucei (IC50 = 2.83 µg/mL) and L. tarentolae (IC50 = 6.29 µg/mL). | [67] |
2.4.2. Anticancer Activity
Owing to the need for novel anticancer agents [76] and motivated by the success of cancer drug discovery projects from natural products [77], Mthembu and Motadi in (2014), evaluated the in vitro anticancer properties of crude methanol extracts of T. violacea using an MTT assay [78]. Extracts displayed time- and concentration-dependent antiproliferative properties against cervical cancer cell lines with an IC50 of 150 µg/mL. The MoA was deciphered to be induction of apoptosis by a p53-independent pathway [78]. However, in contrast to this finding, continued work showed a proportional increase in the activity of caspase 3/7, and the expression of p53 genes strongly suggests apoptosis was triggered by a p53-dependent pathway [79]. This latter finding has been partly substantiated by data emerging from a study examining the antineoplastic properties of T. violacea against ovarian tumor cells. These extracts were shown to partially induce both apoptosis and necrosis with the most pronounced activity due to induction of autophagy [80]. Triple-negative breast cancer remains one of the most challenging cancers, being highly aggressive [81]. T. violacea extracts have demonstrated good cytotoxic activity against MDA-MB-231, with an IC50 of 300 µg/mL [82]. Additionally, extracts inhibited migration of the cancer cell lines (metastasis), an important physiological process in the progression of this cancer [83]. In addition to the gynecological cancers, antineoplastic properties of T. violacea were further observed against pancreatic cancer with 63% inhibition of cell proliferation at a concentration of 250 µg/mL [68]. Against a non-sex-specific cancer, T. violacea showed noticeable activity against oral cancer with an IC50 of 0.2 and 1 mg/mL for acetone and water-soluble extracts, respectively. Extracts activated caspase activity in a dose-dependent manner leading to induction of apoptosis in the human oral cancer cell line [84]. Using a bioassay guided approach, the active anticancer compounds in T. violacea have been identified to be glucopyranosides d-fructofuranosyl-β (2→6)-methyl-α-d-glucopyranoside and β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside. Both compounds act by mediating induction of apoptosis in Chinese hamster cells by targeting the mitochondrial (intrinsic) pathway [85][86][85,86]. A summary of the anticancer activity of Tulbaghia species is shown in Table 5.Table 5. Anticancer activity of Tulbaghia.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Methanol | Leaves and roots | Marked time- and dose-dependent cytotoxic effect on cancer cell lines. Induced apoptosis using p53-independent pathway. | [78] |
Table 7. Antidiabetic, anticardiovascular and antithrombogenic activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| and | C. parapsilosis | . | [ | 67] |
| Diabetes | ||||
| T. violacea | Methanol, butanol, and hexane | Leaves | Methanol extract was prolific against multiple cell lines. Hela and ME-180 cell lines treated with methanol and hexane extracts showed an increase in caspase 3/7 activity. Both methanol and hexane extracts induced a 10-fold increase in expression of p53 gene in Hela cells. | [79] |
| T. violacea | Methanol:water:formic acid (80:20:0.1, v/v/v) | Flowers | Demonstrated activity against ovarian tumor cells. | [80] |
Table 6. Antioxidant activity of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. violacea | Water | Leaves | Dose-dependent antioxidant activity measured using the DPPH (Log IC50 = 0.49 mg/mL) and ABTS (Log IC50 = 0.24 mg/mL) assays | [92 | ] | |||||||||
| T. violacea | ||||||||||||||
| Hexane and ethanol | T. violaceaFlowers and callus cultures | Methanol/water/formic acid (80:20:0.1, v/v/v)Moderate to strong broad-antimicrobial (E. coli, | ||||||||||||
| T. violacea | Flowers | P. aeruginosa | Methanol, S. aureus, Aspergillus nigerMarked antioxidant activity was observed using 3 different types of assays, namely DPPH, FRAP and TREC | [80] | ||||||||||
| Rhizome | Attenuated diabetes associated physiological complications in streptozotocin-induced diabetic rats. | Leaves | and C. albicans) activity observed by zone of inhibition in the agar well disc diffusion method. | [68] | ||||||||||
| [ | 96 | ] | T. violacea | Water | Bulbs | T. acutiloba | Hydro-methanolic extractsSignificant reduction in | Roots, rhizomes, leaves and flowersA. flavus β-glucan and chitin synthesis corresponding to a dose-dependent inhibition of the enzymes β-glucan and chitin synthase, respectively.This results in inhibition of ergosterol production in the fungus. | Dose-dependent antioxidant activity observed with the rhizome extract emerging as the most active plant part (IC50 DPPH = 0.202 mg/mL and peak scavenging activity of 95) | [91] | T. violacea | Water and methanol | Leaves | |
| Demonstrated in vivo anticonvulsant activity by attenuating tonic convulsions induced by either pentylenetetrazole, bicuculline, picrotoxin, strychnine or NMDLA. | [ | 102 | ] | T. violacea[ | Methanol | Rhizome | Noticeably reduced blood glucose and serum lipid (TG, TC, and VLDL) levels while raising plasma insulin in a streptozotocin-induced diabetic rat model.70 | |||||||
| T. violacea | Acetone | Mixture of leaves and bulbs | Marked tick repellence properties of fungus-exposed plants at low treatment concentrations (5 % w/v and 10 % | ][[9771][70,] | ||||||||||
| w/v | ). | 71 | ] | |||||||||||
| [ | 59 | ] | T. violacea | Acetone | Bulbs | Varied light intensities, pH and watering frequencies substantially impacted both growth and potency of plant extracts against the fungi | Water-soluble extract emerged as the most cytotoxic (IC50 = 314 µg/mL), compared to the methanol extract (IC50 = 780 µg/mL), against the MDA-MB-231 triple-negative breast cancer cell line. Water-soluble extract prevented cell migration completely for 13 h at 300 µg/mL. | [82] | ||||||
| T. violacea | ||||||||||||||
| T. violacea | F. oxysporum | . | Hexane and ethanol | Flowers and callus cultures | Cardiovascular | Dose-dependent antioxidant activity with IC50 ranging from 1.933 to 7.350 mg/mL in the DPPH assay | [68 | [ | ||||||
| T. violacea | Water | Leaves, stems, and roots | Induced conspicuous genotoxicity effects at high test concentrations (250, 500 and 1000 µg/mL) in the A. cepa assay. | 73][74][73,74] | ||||||||||
| ] | [ | Hexane and ethanol | Flowers and callus cultures | Extracts showed marked cytotoxicity (60–74% growth inhibition at 250 µg/mL) against three different cell lines (Hep G2, PC-3 and MCF-7). | [68] | |||||||||
| 103 | ] | T. violacea | Water | Roots, bulbs, leaves and flowers | T. violaceaSignificantly compromised population densities of the nematode | Acetone | Leaves | IC50 DPPH = 0.08 mg/mL; IC | T. violacea50 ABTS = 0.03 mg/mL | [ | Methanol | Leaves | Markedly reduced systolic BP, diastolic BP, mean arterial pressure and the heart rate in both age-induced and spontaneous hypertensive rats.84 | [ |
| T. violacea | ] | 98 | ] | T. violacea | Acetone and water | |||||||||
| T. acutiloba | Leaves | Acetone | Anticancer activity against oral cancer with an IC50 (acetone extract) of 0.2 mg/mL; IC50 (water extract) of 1 mg/mL. | Leaves[84] | ||||||||||
| IC | 50 | DPPH = 0.16 mg/mL; | IC50 ABTS = 0.07 mg/mL | [84] | T. violacea | Methanol:water (1:1) | ||||||||
| Water and ethanol | T. violacea | Methanol | Rhizome | Whole plants | Two pro-apoptotic glucopyranosides d | |||||||||
| 50 mg/kg significantly improved kidney function in vivo. | [ | 99 | ] | T. alliacea | ||||||||||
| T. acutiloba | Acetone | -fructofuranosyl-β (2→6)-methyl-α- | Hydro-methanolic extractsd-glucopyranoside and β-d-fructofuranosyl-(2→6)-α-d-glucopyranoside isolated and identified as active anticancer agents in the plant. | Leaves | IC50 DPPH = 0.06 mg/mL; IC[85] | |||||||||
| 50 | ABTS = 0.06 mg/mL | [ | 84 | ] | Roots, rhizomes, leaves and flowers | All extracts inhibited the Angiotensin-1-Converting Enzyme in vitro (> 50 % inhibition at a concentration range of 125–1000 μg/mL). Extracts of leaves demonstrated activity comparable to that of the control drug ramipril. | [91] | T. violacea | Water | Whole plants | MoA of the three compounds, namely methyl-α-d-glucopyranoside, d-fructofuranosyl-β (2→6)-methyl-α-d | |||
| T. cernua | Acetone | Leaves | -glucopyranoside and β- | ICd-fructofuranosyl-(2→6)-α-50 DPPH = 0.21 mg/mL; ICd-glucopyranoside isolated from the water extract, deciphered to be through induction of apoptosis by targeting the mitochondrial (intrinsic) pathway | 50 ABTS = 2.34 mg/mL[86] |
2.4.3. Antioxidant Activity
The imbalance of reactive oxygen species (ROS) and antioxidants in the body can lead to oxidative stress [87]. This physiological condition can result in cellular and tissue damage [88]| [ | |||||||
| 84 | |||||||
| ] | |||||||
| Antithrombogenic | |||||||
| T. leucantha | Acetone | Leaves | IC50 DPPH = 0.39 mg/mL; IC50 | ||||
| T. violacea | Water | Leaves | ABTS = 0.03 mg/mL | Noticeable inhibition of platelet adhesion by a novel scaffold consisting of polycaprolactone incorporated with 10 % (w/w) plant extracts.[84] | |||
| [ | 90 | ] | T. ludwigiana | Acetone | Leaves | IC50 DPPH = 0.26 mg/mL; IC50 ABTS = 0.09 mg/mL | [84] |
| T. natalensis | Acetone | Leaves | IC50 DPPH = 2.70 mg/mL; IC50 ABTS = 0.04 mg/mL | [84] |
2.4.4. Antidiabetic, Anticardiovascular and Antithrombogenic Activity
The incidence of diabetes and cardiovascular diseases continues to grow substantially across the globe, with both conditions combined accounting for the highest global morbidity and mortality [93][94][93,94]. Both of these chronic conditions are closely linked with cardiovascular disease being responsible for high morbidity and mortality in diabetic patients [95]. Tulbaghia has been documented in ethnopharmacological studies for the treatment of these ailments with emerging scientific data strongly validating its use. In streptozotocin diabetes-induced rat models, T. violacea attenuated diabetes-associated physiological conditions resulting in improved body weights, reduced fasting blood glucose levels, enhanced glucose tolerance and significantly elevated plasma insulin and liver glycogen content [96]| T. violacea | ||
| Water | ||
| Leaves | ||
| Marked inhibition of platelet adhesion (70% inhibition at 0.1 mg/mL within 15 min post-exposure). | ||
| [ | 92 | ] |
2.4.5. Miscellaneous Pharmacological Activity
In addition to diabetes and cardiovascular diseases, T. violacea has shown activity against another chronic condition, Alzheimer’s disease. In an in vivo Alzheimer’s disease transgenic C. elegans strain model, T. violacea significantly reduced 1-42 β-amyloid peptide formation (Table 8) [80]. T. violacea exhibited in vivo anticonvulsant activity by attenuating tonic convulsions induced by either pentylenetetrazole, bicuculline, picrotoxin, strychnine or NMDLA [102] and validating its traditional use for the treatment of epilepsy. T. violacea displayed marked tick repellence properties of fungus-exposed plants at low treatment concentrations (5% w/v and 10% w/v) [59], further enhancing its credentials as a potential agricultural product. Somewhat concerning is that, extracts of T. violacea also induced genotoxic effects albeit at high test concentrations (250, 500 and 1000 µg/mL) in the Allium cepa assay [103]. Furthermore, broad murine macrophage antiproliferative and cytotoxicity activity, influenced by extract test concentrations, type of solvent and plant part used, have been observed (Table 8) [104]. There is consequently a need for rigorous assessment of safety of extracts of this and other species of the genus Tulbaghia.Table 8. Miscellaneous biological properties of extracts of Tulbaghia species.
| Plant Species | Extraction Solvent | Plant Part Used | Biological Activity | References |
|---|---|---|---|---|
| T. violacea | Methanol/water/formic acid (80:20:0.1, v/v/v) | Flowers | Reduced 1-42 β-amyloid peptide formation and arrested oxidative stress in vivo. | [80] |
| T. violacea | Methanol | |||
| Leaves, stems, and roots | ||||
| Broad murine macrophage antiproliferative and cytotoxicity activity influenced by both extract test concentrations, type of solvent and plant part used. | [ | 104 | ] |
