The treatment of neurological disorders with large-molecule biotherapeutics requires that the therapeutic drug be transported across the blood–brain barrier (BBB). Recombinant biotherapeutics, such as neurotrophins, enzymes, decoy receptors, and monoclonal antibodies (MAb), do not cross the BBB. These biotherapeutics can be re-engineered as brain-penetrating bifunctional IgG fusion proteins. These recombinant proteins comprise two domains, the transport domain and the therapeutic domain, respectively. The transport domain is an MAb that acts as a molecular Trojan horse by targeting a BBB-specific endogenous receptor that induces receptor-mediated transcytosis into the brain, such as the human insulin receptor (HIR) or the transferrin receptor (TfR).
1. Introduction
The hematoencephalic or blood–brain barrier (BBB) is the anatomical and molecular barrier that separates in vivo the brain from the blood. This barrier evolved to prevent the transport to the brain of peripheral neurotransmitters, cytokines, and microorganisms, which may produce deleterious, if not lethal, effects in the central nervous system (CNS). The characteristics of this barrier have been extensively reviewed, and it is basically only permeable to lipophilic molecules of <400 Da
[1][2][3][4]. Thus, histamine, a small polar molecule of 110 Da does not cross the BBB
[2]. Hydrophobic nutrients of low molecular weight gain access to the brain through the BBB via facilitated transporters, as in the case of GLUT1 for glucose and LAT1 for large neutral amino acids
[5][6]. Proteins, in general, do not cross the BBB; however, there are a few exceptions where proteins produced in peripheral organs gain access to the brain via receptor-mediated transcytosis, as in the case of insulin, transferrin, leptin, and insulin-like growth factor
[7][8][9][10]. Targeting these BBB endogenous transporters with monoclonal antibodies gained attention in the early 1990s, and an in vivo demonstration of the efficacy of a brain-penetrating construct was published using vasopressin intestinal peptide (VIP) conjugated to the OX26 monoclonal antibody to the rat transferrin receptor using the avidin–biotin technology
[11]. The administration of the OX26-avidin-biotinylated-VIP produced a marked increase in the brain blood flow. On the contrary, the biotinylated-VIP had no effect in the brain, as it does not cross the BBB
[11]. The construction and efficacy of chemical conjugates targeting either the transferrin or the insulin receptor in rodents and non-human primates have been reported
[12][13][14][15].
With the cloning of monoclonal antibodies to the mouse transferrin (TfRMAb) and human insulin (HIRMAb) BBB receptors, respectively
[16][17], the engineering of bifunctional IgG fusion proteins was possible
[18][19][20]. These fusion proteins comprise a transport domain, i.e., TfRMAb or HIRMAb, and a therapeutic domain fused to the C-terminus of either the heavy or light chain of the transporting MAb. Thus, any potential protein therapeutic can be transported through the BBB into the brain in the form of a fusion protein targeting a BBB-receptor-mediated transport (
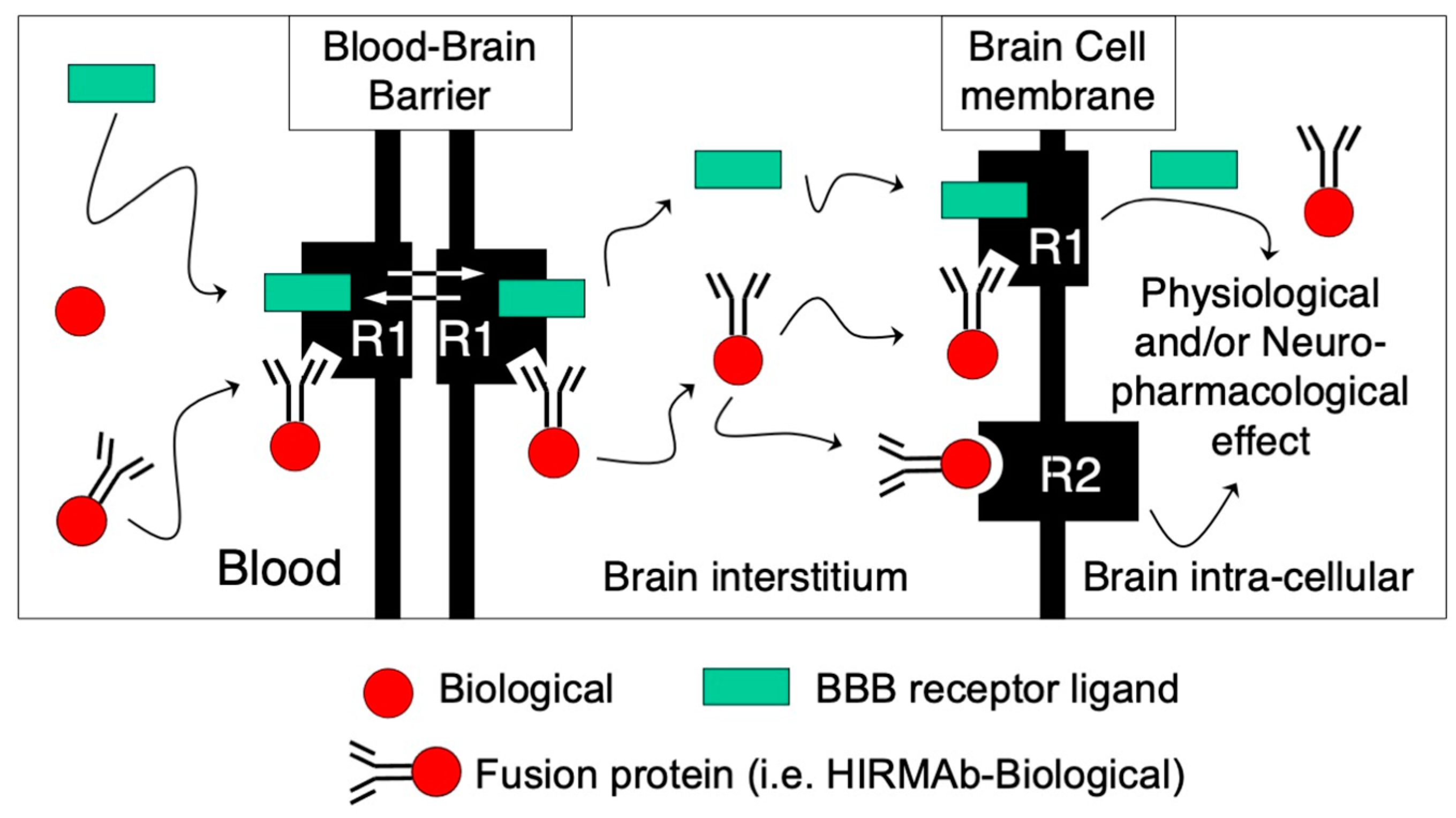
Figure 1). In this schematic representation, a protein therapeutic in fused to the C-terminus of the transporting MAb, which binds to a BBB receptor, inducing the transport of the fusion protein through the BBB. The binding of the MAb fusion protein to its BBB receptor does interfere with the binding of its endogenous ligand, so both the ligand and the MAb fusion are transported through the BBB and released into the brain interstitial fluid. Depending on the characteristics of the therapeutic domain, the fusion protein can (i) target a receptor on the surface of brain cells, as in the case of neurotrophic factors; (ii) bind and inactivate a target molecule, as in the case of decoy receptors and bispecific MAbs; and (iii) be internalized in brain cells via receptor-mediated endocytosis through the same transport systems used to cross the BBB, as in the case of enzymes for the treatment of lysosomal storage disorders (LSD) and/or bispecific MAbs. A detailed mathematical model of receptor-mediated transport across the BBB was recently published
[21]. A generation of IgG fusion proteins targeting both the human and mouse BBB transport systems has been engineered (
Table 1 and
Table 2).
Figure 1. Receptor-mediated transport of IgG fusion proteins across the BBB. Biologicals (red circle) do not cross the BBB and stay in circulation following IV administration, as in the case of enzymes, MAbs, decoy receptors, and/or neurotrophic factors. These potential therapeutic agents for the CNS can be re-engineered as fusion proteins with an MAb targeting a BBB receptor that induces receptor-mediated transcytosis (R1), such as the human BBB insulin receptor (HIR) or the transferrin receptor (TfR). The transport domain of the IgG fusion protein targets the BBB R1 endogenous receptor to gain access to the brain. The transport MAb binds to an exofacial epitope of the receptor without interfering with the normal transport of its endogenous ligand (green rectangle) to gain access to the brain. Depending on the therapeutic domain of the IgG fusion protein, it can (i) bind to its ligand in the brain interstitial compartment, as in the case of bispecific MAbs or decoy receptors; (ii) target a brain cell membrane receptor (R2), such as neurotrophic factors; or (iii) be endocytosed via the same targeted R1 receptor in brain cells as lysosomal enzymes to produce physiological and/or neuropharmacological effect.
Table 1. Brain-penetrating human IgG fusion proteins.
1 The transport domain of these human fusion proteins is a monoclonal antibody directed to the human BBB insulin receptor (HIRMAb) or the transferrin receptor (TfRMAb). The therapeutic domain of the fusion protein and its application are listed for the corresponding IgG fusion protein. * Indication has a primary CNS disease burden.
Table 2. Brain-penetrating mouse IgG fusion proteins.
| IgG Fusion Protein 1 |
Therapeutic Domain |
Indication |
Reference |
| TfRMAb-IDUA |
Iduronidase (IDUA) |
Hurler syndrome (MPS I) |
[35] |
| TfRMAb-IDS |
Iduronate-2-sulfatase (IDS) |
Hunter syndrome (MPS II) |
[36] |
| TfRMAb-SGSH |
Sulfamidase (SGSH) |
Sanfilippo A (MPSIIIA) * |
[37] |
| TfRMAb-Aβ bispecific antibody |
Anti-Aβ amyloid single-chain Fv antibody (scFv) |
Alzheimer’s * |
[38] |
| TfRMAb-TNFR |
Tumor necrosis factor decoy receptor (TNFR) |
Parkinson’s, Alzheimer’s, and/or stroke * |
[39] |
| TfRMAb-EPO |
Erythropoietin (EPO) |
Parkinson’s, Alzheimer’s, and/or stroke * |
[40] |
| TfRMAb-GDNF |
Glial-cell-derived neurotrophic factor (GDNF)) |
Parkinson’s, and/or stroke * |
[41] |
| TfRMAb-Avidin |
Any mono-biotinylated therapeutic |
Various |
[42] |
1 The transport domain of these mouse fusion proteins is a monoclonal antibody directed to the mouse BBB-transferrin receptor (TfRMAb). The therapeutic domain of the fusion protein and its experimental application are listed for the corresponding IgG fusion protein. * Indication has a primary CNS disease burden.
2. Genetic Engineering of IgG Fusion Proteins
The genetic engineering of IgG fusion proteins has been performed using either individual expression vectors for light- and heavy-chain expression genes or tandem vectors carrying both light- and heavy-chain expression genes
[22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42]. The cDNA corresponding to the mature therapeutic domain (without the signal peptide) is ligated into the C-terminus of the appropriate expression gene via designed restriction endonuclease sites, which provides a short linker composed of 2–4 serine residues. The therapeutic gene can be inserted in either the heavy or light chain of the transport MAb, and a few examples are shown in
Figure 2. In particular cases, the short linker approach produces suboptimal levels of enzyme activity and/or production, which may be restored by the introduction of a long 31-amino-acid linker corresponding to the IgG3 hinge region
[27][28]. The engineering of IgG fusion proteins may be performed by fusing the therapeutic domain on the N-terminus of the transport MAb, i.e., the heavy chain of the MAb. However, it was demonstrated using a glucuronidase (GUSB) fusion protein that this construct had a marked reduction in the affinity for the target receptor, to levels that would abolish its transport through the BBB
[43]. For studies in rodents, the engineering of IgG fusion proteins has been performed targeting the mouse or rat transferrin receptor
[17][19][20][44]. For studies in humans and non-human primates, the IgG fusion proteins were initially produced with an MAbs directed to the human insulin receptor, and lately the MAbs have been directed to the human transferrin receptor as well
[16][24]. The anti-human insulin receptor MAb cross-reacts with the BBB insulin receptor of old-world primates, such as the rhesus monkey
[45]. Targeting the rodent transferrin receptor or the human insulin or transferrin receptors at the BBB resulted in a comparable brain uptake of 1–3% of the injected dose. This relates to the abundance of these receptors at the BBB, which is comparable in humans
[46]. However, the abundance of the mouse BBB transferrin receptor is approximately 7-fold higher than that of the mouse BBB insulin receptor
[46][47]. Therefore, targeting the mouse BBB insulin receptor would produce lower levels of brain uptake. The manufacturing of IgG fusion proteins presents advantages compared to chemical conjugation, including simplified downstream purification due to protein-A capture
[48]. IgG fusion proteins were engineered targeting both transferrin and insulin BBB receptors with high affinities in the low nM range (
Table 1 and
Table 2). The extensive number of peer-reviewed publications discussed below validated the high-affinity approach for the transport across the BBB, targeting either the insulin or the transferrin receptor.
Figure 2. Genetic engineering of IgG fusion proteins. The therapeutic domain of the IgG bifunctional fusion protein can be fused to the C-terminus of either the heavy or light chain of the transport monoclonal antibody (MAb), in this case targeting the BBB human insulin receptor (HIR). The indication for these IgG fusion proteins is: HIRMAb-PPT1, Batten disease type 1; HIRMAb-GLB1, GM1-gangliosidosis; HIRMAb-HEXA, Tay–Sachs disease; and HIRMAb-ASM, Niemann–Pick disease types A and B. From reference
[28].
There are, however, few publications postulating that a low-affinity monovalent MAb directed to the BBB TfR transport system may result in improved brain uptake
[49][50]. This is based on the hypothesis that bivalent TfRMAbs cause TfR clustering and selective triage of the antibody-TfR complex to the lysosome and degradation of TfR on the cell membrane, whereas this is avoided with monovalent TfRMAbs
[49][50]. However, this was based on tissue culture experiments with TfRMAb-avidin fusion proteins, which are known to form tetrameric structures from the association of avidin monomers
[51][52]. No toxic effects of other high-affinity TfRMAb fusion proteins were reported in in vitro or in vivo studies. Chronic treatment with intravenous (IV) 2 mg/kg BW TfRMAb-GDNF twice weekly for 12 weeks produced no downregulation of the BBB TfR, as the terminal pharmacokinetics and brain uptake were comparable to those obtained prior to the chronic treatment
[53]. Moreover, no evidence of BBB TfR downregulation was reported in a chronic study performed in the cynomolgus monkey with pabinafusp alfa, the high-affinity human TfRMAb-IDS fusion protein, with doses up to 30 mg/kg/week for 26 weeks
[54]. Kinetics modeling of the receptor-mediated transport across the BBB showed that the optimal receptor-binding properties would be an MAb with a KD of 0.5–5 nM and an association rate constant (kon) of 10
5–10
6 M
−1 s
−1, which would produce a dissociation T
1/2 of ~10–120 min
[55]. Targeting MAbs, i.e., TfRMAb and/or HIRMAb, with these kinetic properties produced therapeutic brain delivery at a low injection dose of 1–3 mg/kg BW in the various CNS models discussed below, including clinical trials in LSD.
The brain uptake via a BBB receptor-mediated transport is a function of the antibody affinity for the receptor, the injection dose, and the plasma area under the curve (AUC), which may be affected by the therapeutic domain of the fusion protein, as in the case of LSD enzymes targeting peripheral M6P receptors. For example, the fusion of IDUA to the transport MAb reduces the brain AUC of the fusion protein compared to the MAb alone
[56]. Kinetics modeling showed that the lower the affinity of the antibody for the TfR, the greater the ID required to maintain a given brain AUC
[55]. For example, the brain AUC of a TfRMAb-IDUA fusion protein with a moderate affinity for the TfR, KD = 36 nM, would require an injected dose of 30 mg/kg BW to produce a brain AUC comparable to the one of a TfRMAb-IDUA fusion protein with high affinity (KD = 0.36–3.6 nM) at a 10-fold lower injected dose of 3 mg/kg BW
[55]. A lower therapeutic dose is also preferred to reduce potential adverse effects, as in the case of IgG-neurotrophic factor fusion proteins
[31][57].