Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Philippe Gasque | -- | 3071 | 2022-07-27 05:45:56 | | | |
| 2 | Conner Chen | + 14 word(s) | 3085 | 2022-07-28 04:22:50 | | | | |
| 3 | Conner Chen | + 6 word(s) | 3091 | 2022-07-28 06:19:55 | | | | |
| 4 | Conner Chen | Meta information modification | 3091 | 2022-07-28 11:29:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lebeau, G.; Ah-Pine, F.; Daniel, M.; Bedoui, Y.; Vagner, D.; Frumence, E.; Gasque, P. Cell Biology and Immune Functions of the MSC. Encyclopedia. Available online: https://encyclopedia.pub/entry/25561 (accessed on 08 February 2026).
Lebeau G, Ah-Pine F, Daniel M, Bedoui Y, Vagner D, Frumence E, et al. Cell Biology and Immune Functions of the MSC. Encyclopedia. Available at: https://encyclopedia.pub/entry/25561. Accessed February 08, 2026.
Lebeau, Grégorie, Franck Ah-Pine, Matthieu Daniel, Yosra Bedoui, Damien Vagner, Etienne Frumence, Philippe Gasque. "Cell Biology and Immune Functions of the MSC" Encyclopedia, https://encyclopedia.pub/entry/25561 (accessed February 08, 2026).
Lebeau, G., Ah-Pine, F., Daniel, M., Bedoui, Y., Vagner, D., Frumence, E., & Gasque, P. (2022, July 27). Cell Biology and Immune Functions of the MSC. In Encyclopedia. https://encyclopedia.pub/entry/25561
Lebeau, Grégorie, et al. "Cell Biology and Immune Functions of the MSC." Encyclopedia. Web. 27 July, 2022.
Copy Citation
The acronym mesenchymal stem cells (MSCs) refers either to: Mesenchymal Stem Cell, a term popularized by Caplan in the 1990s and broadly used after that, or Multipotent Mesenchymal Stromal Cell, which is the terminology promoted by Mesenchymal and Tissue Stem Cell Committee of the International Society of Cell Therapy. Mesenchymal stem cells (MSCs) are a subset of non-hematopoietic stem cells found at low frequency, mainly located around vessels (hence also named pericytes) in resting conditions but with high proliferation and multilineage differentiation capacities to orchestrate tissue repair mechanisms.
immunity
mesenchymal stem cells
stromal cells
1. Definition of MSC
The acronym MSC refers either to: Mesenchymal Stem Cell, a term popularized by Caplan in the 1990s [1] and broadly used in the past decades, or Multipotent Mesenchymal Stromal Cell, which is the terminology promoted by Mesenchymal and Tissue Stem Cell Committee of the International Society of Cell Therapy [2].
The adjective “mesenchymal” (“middle” in Greek) suggests a putative mesodermal origin of MSCs, implying that MSCs may derive from the “middle” layer during the embryonic development [1]. A neuroectodermal NC origin of MSC has been highlighted in many tissues by several investigators [3][4][5][6][7][8][9][10]. Neural crest (NC) cells have the capacity to migrate and to participate in the organization of ectodermal and endodermal tissues [9]. This migration ability may be retained during adulthood, by homing to injured tissues [1][11][12][13][14]. Even if MSCs terminology and definition have been a matter of debate (notably about their “stemness”), there is a consensus on certain elements.
First, MSCs of the bone marrow (BM) are distinguishable from hematopoietic stem cells, because of their ability to adhere to plastic vessel and to grow [1][2][15][16][17]. Morphologically, MSCs are spindle-shaped cells that after several passages bear a more homogeneously fibroblastic phenotype [18].
Second, MSCs are characterized by a singular expression of surface proteins. Among the classical markers of MSCs, some literatures aforementioned CD90 (or Thy-1), CD105 (or endoglin, TGF beta receptor), and CD73 (or ecto-5′-nucleotidase) [15][16][19][20]. Other markers have been described for MSC such as CD140b (or PDGFR beta), CD271 (or low-affinity NGFR) [19][21][22], CD146 (MUC18), and CD248 (or endosialin/tumor endothelium marker 1) [23][24][25]. However, these markers are not specific to MSCs and can be found on other cell types. Additionally, MSCs are negative for CD45 (pan-leukocyte marker) and CD34 [15][16], even if some investigators evoked that CD34 may be expressed but lost in ex vivo culture expanded MSC [19][26]. Actually, it is important to note that the in vitro expression of some markers does not always correlate with their expression patterns in vivo [18].
Third, MSCs are multipotent progenitor cells, able to differentiate at least in vitro into three different subsets: adipocyte, chondrocyte and osteoblast [11][15][16]. In addition to these cell types, other possibilities of differentiation have been evoked [27]. Pericytes in the arteries can acquire a macrophage-like phenotype even with phagocytic properties [28][29]. This phenotype switching involves the transcription factor Krüppel-like factor 4 (KLF4) and has been associated with development of atherosclerosis. Some culture conditions can promote smooth muscle and striated muscle gene expression, whereas others promote cardiac or liver gene expression [30]. Finally, glial/neuronal differentiation potential has also been reported [18][20] and which may be due to the NC origin of specific subsets of MSC [4][5][6][7][23][31][32].
MSCs have been isolated from various tissues [3][11][18][33] and including: bone-marrow [34], adipose tissue [35][36], lungs [37], synovial membrane [38], kidney [11], liver [39], dental tissue [40][41][42][43][44], cord blood [45][46], and amniotic fluid notably [47]. Different names have been attributed to these cells depending on their tissue locations (Table 1).
Table 1. The different names given to Multipotent Mesenchymal Stem/Stromal Cells (MSC) and their tissue localization.
| Tissue | Human | Reference |
|---|---|---|
| Bone Marrow | Bone Marrow-Mesenchymal Stem Cell or Adventitial Reticular Cell or Myoid Cell |
[7][48][49][50] |
| Adipose tissue | Adipose Stem Cell | [35][36] |
| Intervertebral disc | Skeletal Progenitor Cell | [51] |
| Synovial membrane | Synovial Membrane-derived MSCs Fibroblast-like synoviocytes (FLS) |
[38][52] |
| Amniotic fluid | Amniotic Stem Cell | [47] |
| Cord blood Placenta |
Umbilical Cord Blood Stem Cell or Wharton’s Jelly derived MSC |
[45][46] |
| Dental tissue | Dental Pulp Stem Cell or Stem cells from Human Exfoliated Deciduous teeth or Periodontal Ligament MSC or Stem Cell from Apical Papilla or Dental Follicle Precursor Cell |
[40][41][42][43][44] |
| Kidney | Mesangial Cell | [3] |
| Brain | Pericytes, perivascular fibroblasts | [53][54] |
| Liver | Hepatic Stellate Cell (HSC) or Perisinusoidal Cell or Ito Cell |
[39][55] |
| Lung | Human Bronchial Fibroblasts or Lung-resident MSC |
[37] |
2. Origins of Pericytes/Perivascular MSC-Fibroblasts Derived from the Neural Crest and/or Mesoderm Embryonic Tissues
The ontogeny of MSC before they rich their final position in adult tissues is still a matter of debate [3]. The identification of MSC relies on the characterization of genetic and protein markers (e.g., tyrosine kinase PDGF α or β receptors, Schwann cell myelin P zero, glioma-associated transcription factor Gli1, collagen, Acta2/alpha SMA, Cspg4/Neuronglial 2-NG2, CD146/Melanoma cell adhesion molecule, CD248/Tumor Endothelial marker 1-TEM1) not restricted to MSC but also shared with pericytes (first identified by the French scientist Charles Rouget in 1873), vascular smooth muscle cells (VSMC), and perivascular fibroblasts [3][11][24][25][31][33][56]. Of note, the latter cell subset do not really fit the definition of pericyte because they are not embedded in the vascular basement membrane [57].
It is now generally accepted that a large pool of MSC is found essentially at the perivascular level and with morphology and marker expression profile similar to pericytes [33][58]. Several studies have shown that post-capillary venule pericytes from the bone marrow are able to differentiate into differentiated MSCs such as osteoblasts and chondrocytes in vivo [59]. More recent genetic lineage-tracing experiments and single-cell RNA sequencing data has reinforced a close link between pericytes and MSC phenotypes, particularly in the Central Nervous System (CNS), a tissue where the highest density of pericytes has been found in the body. RNA profiling of mouse brain vasculature revealed a rather unique pool of perivascular cells made of a two pericyte clusters and three subsets of perivascular fibroblasts [54][60]. Pathway and gene ontology enrichment analyses revealed that fibulin+ type I fibroblasts are the main subtype involved in extracellular matrix proteins (ECM) production and fibrosis. The type III (cell migration-inducing protein-CEMIP+ perivascular fibroblasts) showed robust expression of various growth factors, including Vascular Endothelial Growth Factor (VEGF)-A. Interestingly, the type I to type II (potassium calcium-activated channel subfamily M alpha 1-KCNMA1+ fibroblasts) trajectory was continuous with pericyte type 2 suggesting a lineage from type I to type II to pericytes and consistent with a study in zebrafish demonstrating the stem cell potential of perivascular fibroblasts to differentiate into pericytes [61]. It was estimated that ten perivascular fibroblasts were present per intersegmented vessel but only less than 10% of these cells could differentiate into pericytes. Garcia et al. further discussed the possibility that type II perivascular fibroblasts in the brain probably represent an intermediate state exhibiting a transitional mural cell transcriptional phenotype [54].
MSC have several different developmental origins as reviewed by Majeski [62]. The majority of the MSC/pericytes in the head region, including the CNS, are neural crest (NC) derived, as demonstrated in chick-quail chimeras carried out initially by the French scientist Nicole le Douarin and colleagues [63]. In the peripheral nerves, NC will give rise to perineurial fibroblasts and Schwann cells [64]. More recently, two independent studies published in 2017 have suggested that brain pericytes could also be derived from mesoderm-derived myeloid progenitor cells [65][66]. Studies on the thymus demonstrated that perivascular MSC/pericytes are derived from the NC [8][67][68]. The origins of pericytes in the gut [69], lung [70] and liver [71][72] have been mapped to the mesothelium although NC can give rise to MSC-like cells in the gut [73].
In the kidney, the metanephric mesenchyme of the intermediate mesoderm will give rise to nephrons (from the distal convoluted tubule to the podocytes) and also to all major stromal interstitial cells, including the pericytes, perivascular fibroblasts, VSMCs, and mesangial cells [74]. a NC origin of a subset of perivascular fibroblasts of the kidney and contributing to fibrosis has been proposed from genetic lineage tracing experiments using the Schwann cell Myelin P Zero promoter-GFP/LacZ mice [4]. In the aorta, MSC may have at least four different developmental origins, secondary heart field, NC, somites, and splanchnic mesoderm. This invasion of mesothelial cells occurs at about the same time as the appearance of primitive endothelial and hematopoietic progenitors within the splanchnopleura. The primitive endothelial cells (EC) within the splanchnopleura colonize the floor of the aorta and differentiate in situ to produce the vasculature of the body wall, kidney, visceral organs, and limbs [62]. This process of vasculogenesis involving PDGF high expression by EC is consistent with the notion that mesothelial-derived MSCs are localized to BM via the invasion of the vasculature. Coronary vessels in the heart appear to have a similar development [75]. Mesothelial cells are known to undergo epithelial-to-mesenchymal transition (EMT) to delaminate and to migrate into the organs to produce their mesenchymal components. Interestingly, recent studies also point to a close ontogenic relationship between pericytes/VSMC and perivascular fibroblasts in many organs and supporting the current paradigm of such relationships in pathological settings for instance in the brain and lungs. The recent studies preach for the existence of a continuum of pericytes/perivascular MSC-fibroblasts cell phenotypes observed along vessels (and possibly nerves) and which suggest that these cells can (trans)differentiate into each other in conjunction with vessel/axonal/tissue remodeling. However, this interesting and promising paradigm requires further investigation.
The close relationship of MSC and progenitors with the vasculature will endow them as a possible source of new cells for physiological turnover for the repair or regeneration of local lesions. The canonical and current scenario is that damage to any tissue would release the MSC from its perivascular niche, they will divide and secrete immunoregulatory and trophic factors. Different signaling mechanisms may govern MSC mobilization from the perivascular niche, detaching from the endothelial cues and invading the parenchyma in response to injuries. This is exemplified by the importance of PDGF-B/PDGFRβ which has been demonstrated in many organs such heart, lung, and gut [76].
To date, people have a better idea into the embryonic origin of pericytes/perivascular MSC-fibroblasts in different organs but it is still critical to decipher the mechanisms governing their proliferation spreading along (as well evading) growing vessels in conjunction with angiogenesis (Figure 1). The capacity of these cells to circulate in the blood in various disease settings is of great and emerging importance from a clinical standpoint and including the identification of novel predictive soluble biomarkers of an ongoing pathological process in the tissues. Indeed, CD45−CD31−PDPN+ proinflammatory mesenchymal, or PRIME, cells have been identified in the blood from patients with rheumatoid arthritis, and these cells shared features of inflammatory synovial fibroblasts and predicting inflammatory flares [77]. This line of future studies is of great importance in cancer and other chronic inflammatory diseases associated with infectious diseases. In the context of cancer, some studies relate to the capacity of NG2+ pericytes to give rise to mesenchymal tumors (i.e., osteosarcoma) [78].
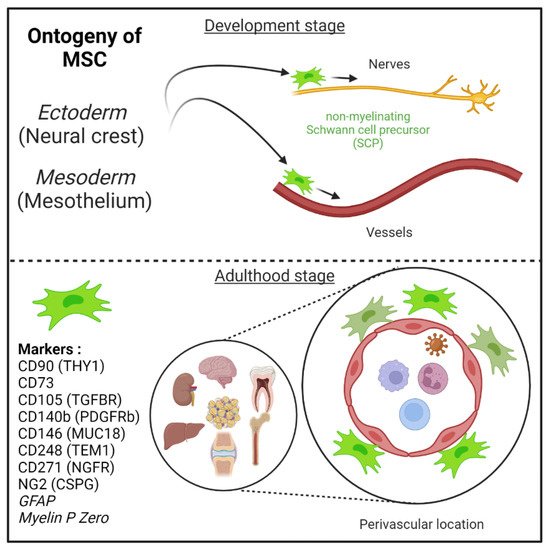
Figure 1. Mesenchymal stem cells (MSC) are derived from either he embryonic ectoderm (neural crest) or the mesoderm. MSCs can migrate along nerves and vessels during development and reside in virtually all post-natal organs and tissues. Along the nerves, MSCs are also known as non-myelinating precursor Schwann cells. Their location around vessels to form perivascular immune privileged niches has been demonstrated by several teams. MSC express several canonical markers which are differentially expressed in all major organs. CD271, glial fibrillary acidic protein (GFAP), and myelin P zero (MPZ) are canonical neuroglial markers.
MSCs can migrate along nerves and vessels during development and reside in virtually all post-natal organs and tissues [5][6][11] (Figure 1). Along the nerves, MSCs are also known as non-myelinating precursor Schwann cells [32][79]. Their location around vessels to form perivascular immune privileged niches has been demonstrated by several teams [3][11][13][14][24][33][80][81][82]. Moreover, perivascular MSC may be able to sense and respond to an event (e.g., virus) in the local environment, via their ability to promote tissue immunoregulatory activities (see below) [14][26][83][84]. Perivascular MSC may therefore represent ideal tissue sanctuaries for viruses remaining dormant while protected from immune attack and rebounding particularly in the context of immunosuppressive drug treatments.
3. Role of MSC in Health (Immunoregulatory Activities and Tissue Repair) and Diseases (Fibrosis)
As described in the model of da Silva Meirelles et al. proposed in 2006, the MSCs in the vascular wall of tissue contribute to tissue maintenance (Figure 2) [11].
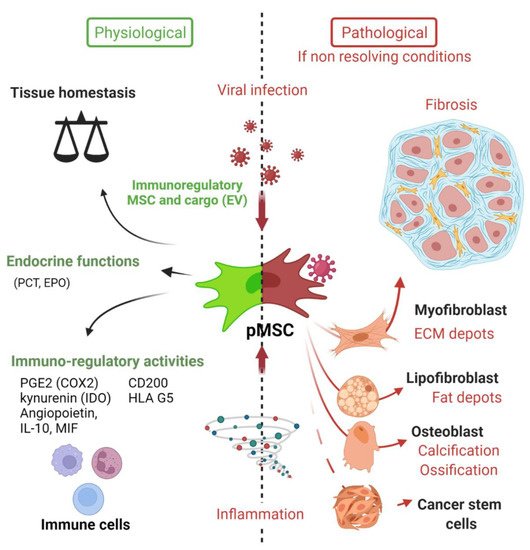
Figure 2. Deciphering the role of Mesenchymal stem cells in the context of viral infection. In physiological conditions, MSC have important immune functions to control viral infection as glatekeepers around vessels (perivascular MSC) and capable of mounting an innate immune antiviral response. MSC will also promote release of cytokines and chemokines to recruit immune cells to clear pathogens. Equally important is the expression of many immune regulatory factors to terminate the adaptive immune response to limit further cell injuries and promote tissue repair. Many viruses may infect directly MSC in tissues, thus remaining in an immunoprivileged niche favoring virus persistence, spreading and possible virus rebound in immunocompromised patients. Viruses associated to chronic inflammation (non-resolving) may also affect MSC differentiation (e.g., into myofibroblast) leading to excess of extracellular cell matrix production and contributing to organ dysfunction. Importantly, allogenic MSC and derived extracellular vesicles (EV) are nowadays important immunoregulatory cargo injected to patients for the treatment of inflammatory-infectious diseases such as COVID-19. Safety issues are nevertheless highly warranted. PCT (pro-calcitonin); EPO (erythropoietin) hormones.
Their motility ability allow them to migrate to the site of inflammation or injury to establish an appropriate response [1][11][12][13][14]. This response indubitably involves their immunoregulatory activities, that have already been comprehensively reviewed by several teams [14][26][84][85][86][87][88]. Among these well-known activities, MSCs modulate innate immune response by different mechanisms: decreasing Dendritic Cells (DCs)’ antigen presentation capacity, maturation and cytokine secretion [83][89]; reducing neutrophils burst respiration [90] and apoptosis; inhibiting natural killer (NK) cell proliferation, cytotoxicity and cytokine production [91][92][93][94]; inhibiting pro-inflammatory factor secretion by activated macrophages [83][95][96][97]. MSCs also exert immunoregulatory activities on adaptive immunity by: reducing T cell proliferation [26][98]; promoting T cell shift from pro-inflammatory (Th1) to anti-inflammatory (Th2) states [83], inhibiting cytotoxic CD8+ T cells [99][100]; inhibiting B cell proliferation through direct effect and T cell-mediated inhibition [101][102][103]; inducing the proliferation of regulatory T cells (Treg) [104][105][106][107].
These actions on the immune system involve both contact-dependent and contact-independent mechanisms. Indeed, MSCs have been shown to have effects on cell survival, function and proliferation of various immune cells by directly interacting with cell surface molecules and receptors [84]. For example, MSCs were shown to inhibit both T cell and B cell proliferation by the interaction between programmed cell death protein 1 (PD1), expressed by lymphocytes, and its ligand PDL1 expressed by MSCs [98]. Additionally, Fas (CD95)—Fas ligand (CD95L) axis is engaged and can induce inflammatory T cell apoptosis [108]. MSCs have also been shown to express the molecule CD200 (named OX2 in rodents) and regulating DC as well as macrophage/microglia immune cell activation via CD200R [109][110][111][112]. Liu and colleagues provided recently a very comprehensive review on the role of many more immunoregulatory cell surface ligands and receptors (e.g., Galectin1, 3, 9) allowing MSC to control directly innate and adaptive immune cells [111].
Contact-independent mechanisms involve the release of soluble immunoactive substances and extracellular vesicles (EVs), which form the MSC-derived secretome. Prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO) (or nitric oxide, NO in mouse) expressed by MSC have been largely described for their immunosuppressive properties, for comprehensive review [85]. The chemokine—iNOS—IDO axis mediated by MSCs leads to T cell inhibition (Shi et al. [85]). Additionally, PGE2 production by MSCs leads to IL-10 secretion by M2-polarized macrophages [97], inhibition of DCs [83], reduction of NK cell activity [92], inhibition of Th17 cells and induction of Tregs [113]. Of note, other immunomodulatory factors have been reported to be secreted by MSCs: TGF-beta 1 (TGF-β1), hepatic growth factor (HGF), metalloproteinase-modified CCL2 (mpCCL2), leukemia inhibitory factor (LIF), and human leukocyte antigen-G5 (HLA-G5) [26].
Moreover, mounting evidence in recent years suggests an important role of EVs containing non-coding RNAs, including miRNAs, in the regulation of the immune system by MSCs [114][115].
The recruitment of immune cells to the injury site is granted by the release of soluble factors that leads to cell chemotaxis. Of note, MSCs can participate to this phenomenon by secreting a broad range of chemokines: CCL2, CCL3, CCL4, CCL5, CCL7, CCL20, CCL26, CXC3CL1, CXCL5, CXCL11, CXCL1, CXCL2, CXCL8, CCL10, and CXCL12 [26][85]. It is important to note that immunoregulatory activities of MSC will be markedly upregulated in response to the canonical Interferon (IFN) -gamma produced by T and NK cells [85].
Of further note, MSCs were initially thought to be important in regenerative medicine due to their ability to differentiate into multiple cell lineages, thereby supporting tissue repair. However, studies of past decades have found that regenerative activities on injured tissue were more likely associated with the MSC-derived secretome rather than the differentiation potential of engrafted MSCs [20][87][116][117]. Indeed, during a lesion, diverse mechanisms governed by MSCs-derived secretome participate in tissue regeneration and homeostasis [14][20][26]. Such as : (1) anti-apoptosis effects mediated either by VEGF, HGF, insulin-like growth factor-1 (IGF-I), stanniocalcin-1, TGF-β, basic fibroblast growth factor (bFGF) or granulocyte-macrophage colony-stimulating factor (GM-CSF); (2) anti-scar with bFGF and HGF; (3) support and growth of tissue progenitor cells mediated by stem cell factor (SCF), LIF, SDF1 alpha/CXCL12, macrophage colony-stimulating factor (M-CSF) and angiopoietin-1; (4) angiogenesis stimulated by bFGF, VEGF, placental growth factor (PIGF), CCL2, interleukin 6 and ECM [26]. Finally, as aforementioned MSCs are able to mediate tissue regeneration by EVs release [118][119][120][121][122]. These EVs contain notably micro-RNAs that target pathways involved in angiogenesis and tissue remodeling [117].
4. MSCs’ Pathological Contributions (Fibrosis and Vessel Calcification)
Despite the beneficial activities that would turn MSCs into an obvious therapeutic approach [123], MSCs are also involved in pathological processes such as cancer [124][125][126] or fibrogenesis (Figure 2) [24][25][127]. In order to maintain the integrity of an organ, a fibrous scar composed of collagen is formed, leading (if uncontrolled) to chronic inflammation, fibrosis and to a loss of organ function [128]. Scar-forming cells are myofibroblasts, which were at length thought to be from an epithelial origin, after epithelial-mesenchymal transition (EMT) [129][130][131]. However, mounting evidence is pointing out the role of resident perivascular MSCs in myofibroblast differentiation and expansion, for comprehensive review, [24][132]. Perivascular MSC (also named pericytes by some authors) are nowadays more and more widely evoked as the main source of collagen-producing cells in fibrosis [31][80][133][134][135][136][137], notably thanks to genetic fate tracing experiment [4][132][134][138][139]. The role of MSCs in giving rise to myofibroblasts has been validated in several models of tissue fibrosis as reviewed by El Agha and colleagues in 2017. For instance, resident perivascular MSCs (genetically traced using hedgehog transcriptional activator glioma-associated oncogene homolog 1 (Gli1)+ or MPZ+ promoters to drive fluorescent protein expression, e.g., GFP) and its profibrotic activity have been described in murine model of fibrosis in either the kidney [4][138], lungs [138][140], heart [138][141], liver [138][139] or bone marrow [142].
Blood vessel remodeling can occur across a variety of pathologic conditions, including osteogenesis-like calcification (arteriosclerosis) and atheroma plaque formation (atherosclerosis) [143][144]. Although some reports have suggested that adventitial fibroblasts can contribute to pathologic changes within the vessel intima and media [145], it has now been shown, using GLI-1 line tracing experiments, that MSC can contribute to vessel calcification through a process of transdifferentiation into osteoblast-like cells [146].
References
- Caplan, A. Mesenchymal Stem-Cells. J. Orthop. Res. 1991, 9, 641–650.
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395.
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In Search of the In Vivo Identity of Mesenchymal Stem Cells. Stem Cells 2008, 26, 2287–2299.
- Asada, N.; Takase, M.; Nakamura, J.; Oguchi, A.; Asada, M.; Suzuki, N.; Yamarnura, K.; Nagoshi, N.; Shibata, S.; Rao, T.N.; et al. Dysfunction of Fibroblasts of Extrarenal Origin Underlies Renal Fibrosis and Renal Anemia in Mice. J. Clin. Investig. 2011, 121, 3981–3990.
- Dupin, E.; Sommer, L. Neural Crest Progenitors and Stem Cells: From Early Development to Adulthood. Dev. Biol. 2012, 366, 83–95.
- Furlan, A.; Adameyko, I. Schwann Cell Precursor: A Neural Crest Cell in Disguise? Dev. Biol. 2018, 444, S25–S35.
- Isern, J.; Garcia-Garcia, A.; Martin, A.M.; Arranz, L.; Martin-Perez, D.; Torroja, C.; Sanchez-Csabo, F.; Mendez-Ferrer, S. The Neural Crest Is a Source of Mesenchymal Stem Cells with Specialized Hematopoietic Stem-Cell-Niche Function. eLife 2014, 3, e03696.
- Foster, K.; Sheridan, J.; Veiga-Fernandes, H.; Roderick, K.; Pachnis, V.; Adams, R.; Blackburn, C.; Kioussis, D.; Coles, M. Contribution of Neural Crest-Derived Cells in the Embryonic and Adult Thymus. J. Immunol. 2008, 180, 3183–3189.
- Le Douarin, N.; Kalcheim, C. The Neural Crest (Developmental and Cell Biology Series), 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; ISBN 978-0-521-62010-9.
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388.
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213.
- Caplan, A.I. New MSC: MSCs as Pericytes Are Sentinels and Gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159.
- Corselli, M.; Chen, C.-W.; Crisan, M.; Lazzari, L.; Péault, B. Perivascular Ancestors of Adult Multipotent Stem Cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1104–1109.
- Singer, N.G.; Caplan, A.I. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317.
- Gazit, Z.; Pelled, G.; Sheyn, D.; Kimelman, N.; Gazit, D. Mesenchymal Stem Cells. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2011; pp. 285–304. ISBN 978-0-12-381422-7.
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749.
- Deans, R.J.; Moseley, A.B. Mesenchymal Stem Cells: Biology and Potential Clinical Uses. Exp. Hematol. 2000, 28, 875–884.
- Phinney, D.G.; Prockop, D.J. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair—Current Views. Stem Cells 2007, 25, 2896–2902.
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of Bone Marrow Mesenchymal Stem Cells by Anti-Nerve Growth Factor Receptor Antibodies. Exp. Hematol. 2002, 30, 783–791.
- Álvarez-Viejo, M.; Menéndez-Menéndez, Y.; Otero-Hernández, J. CD271 as a Marker to Identify Mesenchymal Stem Cells from Diverse Sources before Culture. World J. Stem Cells 2015, 7, 470–476.
- Bedoui, Y.; Lebeau, G.; Guillot, X.; Dargai, F.; Guiraud, P.; Neal, J.W.; Ralandison, S.; Gasque, P. Emerging Roles of Perivascular Mesenchymal Stem Cells in Synovial Joint Inflammation. J. Neuroimmune Pharmacol. 2020, 15, 838–851.
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177.
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host Responses in Tissue Repair and Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 241–276.
- Da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 2009, 20, 419–427.
- Yap, C.; Mieremet, A.; de Vries, C.J.M.; Micha, D.; de Waard, V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Kruppel-Like Factor 4). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2693–2707.
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702.
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. MiR-145 and MiR-143 Regulate Smooth Muscle Cell Fate and Plasticity. Nature 2009, 460, 705–710.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22.
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215.
- Parfejevs, V.; Antunes, A.T.; Sommer, L. Injury and Stress Responses of Adult Neural Crest-Derived Cells. Dev. Biol. 2018, 444, S356–S365.
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313.
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation 1974, 17, 331–340.
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. MBoC 2002, 13, 4279–4295.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228.
- Sabatini, F.; Petecchia, L.; Tavian, M.; de Villeroché, V.J.; Rossi, G.A.; Brouty-Boyé, D. Human Bronchial Fibroblasts Exhibit a Mesenchymal Stem Cell Phenotype and Multilineage Differentiating Potentialities. Lab. Investig. 2005, 85, 962–971.
- Bari, C.D.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942.
- Kordes, C.; Sawitza, I.; Götze, S.; Herebian, D.; Häussinger, D. Stellate Cells Are Mesenchymal Stem Cells. Eur. J. Med. Res. 2014, 19, S6.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812.
- Seo, B.-M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Gehron Robey, P.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155.
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79.
- Morsczeck, C.; Moehl, C.; Götz, W.; Heredia, A.; Schäffer, T.E.; Eckstein, N.; Sippel, C.; Hoffmann, K.H. In Vitro Differentiation of Human Dental Follicle Cells with Dexamethasone and Insulin. Cell Biol. Int. 2005, 29, 567–575.
- Hutson, E.L.; Boyer, S.; Genever, P.G. Rapid Isolation, Expansion, and Differentiation of Osteoprogenitors from Full-Term Umbilical Cord Blood. Tissue Eng. 2005, 11, 1407–1420.
- Fong, C.; Richards, M.; Manasi, N.; Biswas, A.; Bongso, A. Comparative Growth Behaviour and Characterization of Stem Cells from Human Wharton’s Jelly. Reprod. Biomed. Online 2007, 15, 708–718.
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of Amniotic Stem Cell Lines with Potential for Therapy. Nat. Biotechnol. 2007, 25, 100–106.
- Funk, P.E.; Stephan, R.P.; Witte, P.L. Vascular Cell Adhesion Molecule 1-Positive Reticular Cells Express Interleukin-7 and Stem Cell Factor in the Bone Marrow. Blood 1995, 86, 2661–2671.
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834.
- Schmitt-Gräff, A.; Skalli, O.; Gabbiani, G. α-Smooth Muscle Actin Is Expressed in a Subset of Bone Marrow Stromal Cells in Normal and Pathological Conditions. Virchows Arch. B Cell Pathol. 1989, 57, 291.
- Risbud, M.; Guttapalli, A.; Tsai, T.-T.; Lee, J.; Danielson, K.; Vaccaro, A.; Albert, T.; Gazit, Z.; Gazit, D.; Shapiro, I. Evidence for Skeletal Progenitor Cells in the Degenerate Human Intervertebral Disc. Spine 2007, 32, 2537–2544.
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333.
- Appaix, F. Brain Mesenchymal Stem Cells: The Other Stem Cells of the Brain? World J. Stem Cells 2014, 6, 134–143.
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-Cell Dissection of the Human Brain Vasculature. Nature 2022, 603, 893–899.
- Geerts, A. History, Heterogeneity, Developmental Biology, and Functions of Quiescent Hepatic Stellate Cells. Semin. Liver Dis. 2001, 21, 311–336.
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro-Oncology 2005, 7, 452–464.
- Shaw, I.; Rider, S.; Mullins, J.; Hughes, J.; Peault, B. Pericytes in the Renal Vasculature: Roles in Health and Disease. Nat. Rev. Nephrol. 2018, 14, 521–534.
- Hungerford, J.E.; Little, C.D. Developmental Biology of the Vascular Smooth Muscle Cell: Building a Multilayered Vessel Wall. J. Vasc. Res. 1999, 36, 2–27.
- Farrington-Rock, C.; Crofts, N.J.; Doherty, M.J.; Ashton, B.A.; Griffin-Jones, C.; Canfield, A.E. Chondrogenic and Adipogenic Potential of Microvascular Pericytes. Circulation 2004, 110, 2226–2232.
- Vanlandewijck, M.; He, L.; Mae, M.A.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature. Nature 2018, 554, 475–480.
- Rajan, A.M.; Ma, R.C.; Kocha, K.M.; Zhang, D.J.; Huang, P. Dual Function of Perivascular Fibroblasts in Vascular Stabilization in Zebrafish. PLoS Genet. 2020, 16, e1008800.
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258.
- Etchevers, H.C.; Vincent, C.; Le Douarin, M.; Couly, G.F. The Cephalic Neural Crest Provides Pericytes and Smooth Muscle Cells to All Blood Vessels of the Face and Forebrain. Development 2001, 128, 1059–1068.
- Joseph, N.M.; Mukouyama, Y.S.; Mosher, J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.L.; Lee, K.F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural Crest Stem Cells Undergo Multilineage Differentiation in Developing Peripheral Nerves to Generate Endoneurial Fibroblasts in Addition to Schwann Cells. Development 2004, 131, 5599–5612.
- Yamazaki, T.; Nalbandian, A.; Uchida, Y.; Li, W.; Arnold, T.D.; Kubota, Y.; Yamamoto, S.; Ema, M.; Mukouyama, Y. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-Beta Signaling in Developing Skin Vasculature. Cell Rep. 2017, 18, 2991–3004.
- Yamamoto, S.; Muramatsu, M.; Azuma, E.; Ikutani, M.; Nagai, Y.; Sagara, H.; Koo, B.-N.; Kita, S.; O’Donnell, E.; Osawa, T.; et al. A Subset of Cerebrovascular Pericytes Originates from Mature Macrophages in the Very Early Phase of Vascular Development in CNS. Sci. Rep. 2017, 7, 3855.
- Jiang, X.B.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the Mammalian Cardiac Neural Crest. Development 2000, 127, 1607–1616.
- Zachariah, M.A.; Cyster, J.G. Neural Crest-Derived Pericytes Promote Egress of Mature Thymocytes at the Corticomedullary Junction. Science 2010, 328, 1129–1135.
- Wilm, B.; Ipenberg, A.; Hastie, N.D.; Burch, J.B.E.; Bader, D.M. The Serosal Mesothelium Is a Major Source of Smooth Muscle Cells of the Gut Vasculature. Development 2005, 132, 5317–5328.
- Que, J.; Wilm, B.; Hasegawa, H.; Wang, F.; Bader, D.; Hogan, B.L.M. Mesothelium Contributes to Vascular Smooth Muscle and Mesenchyme during Lung Development. Proc. Natl. Acad. Sci. USA 2008, 105, 16626–16630.
- Cassiman, D.; Libbrecht, L.; Desmet, V.; Denef, C.; Roskams, T. Hepatic Stellate Cell/Myofibroblast Subpopulations in Fibrotic Human and Rat Livers. J. Hepatol. 2002, 36, 200–209.
- Asahina, K.; Zhou, B.; Pu, W.T.; Tsukamoto, H. Septum Transversum-Derived Mesothelium Gives Rise to Hepatic Stellate Cells and Perivascular Mesenchymal Cells in Developing Mouse Liver. Hepatology 2011, 53, 983–995.
- Kruger, G.M.; Mosher, J.T.; Bixby, S.; Joseph, N.; Iwashita, T.; Morrison, S.J. Neural Crest Stem Cells Persist in the Adult Gut but Undergo Changes in Self-Renewal, Neuronal Subtype Potential, and Factor Responsiveness. Neuron 2002, 35, 657–669.
- Kobayashi, A.; Mugford, J.W.; Krautzberger, A.M.; Naiman, N.; Liao, J.; McMahon, A.P. Identification of a Multipotent Self-Renewing Stromal Progenitor Population during Mammalian Kidney Organogenesis. Stem Cell Rep. 2014, 3, 650–662.
- Dettman, R.W.; Denetclaw, W.; Ordahl, C.P.; Bristow, J. Common Epicardial Origin of Coronary Vascular Smooth Muscle, Perivascular Fibroblasts, and Intermyocardial Fibroblasts in the Avian Heart. Dev. Biol. 1998, 193, 169–181.
- Hellstrom, M.; Kalen, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-Beta in Recruitment of Vascular Smooth Muscle Cells and Pericytes during Embryonic Blood Vessel Formation in the Mouse. Development 1999, 126, 3047–3055.
- Orange, D.E.; Yao, V.; Sawicka, K.; Fak, J.; Frank, M.O.; Parveen, S.; Blachere, N.E.; Hale, C.; Zhang, F.; Raychaudhuri, S.; et al. RNA Identification of PRIME Cells Predicting Rheumatoid Arthritis Flares. N. Engl. J. Med. 2020, 383, 218–228.
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Okawa, A.; Takeda, S.; Whetstone, H.; Nadesan, P.; et al. Mesenchymal Tumors Can Derive from Ng2/Cspg4-Expressing Pericytes with Beta-Catenin Modulating the Neoplastic Phenotype. Cell Rep. 2016, 16, 917–927.
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Müller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann Cell Precursors from Nerve Innervation Are a Cellular Origin of Melanocytes in Skin. Cell 2009, 139, 366–379.
- Di Carlo, S.E.; Peduto, L. The Perivascular Origin of Pathological Fibroblasts. J. Clin. Investig. 2018, 128, 54–63.
- Ehninger, A.; Trumpp, A. The Bone Marrow Stem Cell Niche Grows up: Mesenchymal Stem Cells and Macrophages Move In. J. Exp. Med. 2011, 208, 421–428.
- Ergün, S.; Tilki, D.; Klein, D. Vascular Wall as a Reservoir for Different Types of Stem and Progenitor Cells. Antioxid. Redox Signal. 2010, 15, 981–995.
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822.
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory Mechanisms of Mesenchymal Stem and Stromal Cells in Inflammatory Diseases. Nat. Rev. Nephrol. 2018, 14, 493–507.
- Le Blanc, K.; Mougiakakos, D. Multipotent Mesenchymal Stromal Cells and the Innate Immune System. Nat. Rev. Immunol. 2012, 12, 383–396.
- Le Blanc, K.; Davies, L.C. Mesenchymal Stromal Cells and the Innate Immune Response. Immunol. Lett. 2015, 168, 140–146.
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of Mesenchymal Stem/Stromal Cell Function. Stem Cell Res. Ther. 2016, 7, 125.
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736.
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal Stem Cells Inhibit Generation and Function of Both CD34+-Derived and Monocyte-Derived Dendritic Cells. J. Immunol. 2006, 177, 2080–2087.
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human Mesenchymal Stem Cells Inhibit Neutrophil Apoptosis: A Model for Neutrophil Preservation in the Bone Marrow Niche. Stem Cells 2008, 26, 151–162.
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal Stem Cell-Natural Killer Cell Interactions: Evidence That Activated NK Cells Are Capable of Killing MSCs, Whereas MSCs Can Inhibit IL-2-Induced NK-Cell Proliferation. Blood 2006, 107, 1484–1490.
- Galland, S.; Vuille, J.; Martin, P.; Letovanec, I.; Caignard, A.; Fregni, G.; Stamenkovic, I. Tumor-Derived Mesenchymal Stem Cells Use Distinct Mechanisms to Block the Activity of Natural Killer Cell Subsets. Cell Rep. 2017, 20, 2891–2905.
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone Marrow Mesenchymal Stem Cells Inhibit the Response of Naive and Memory Antigen-Specific T Cells to Their Cognate Peptide. Blood 2003, 101, 3722–3729.
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions Between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells 2006, 24, 74–85.
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone Marrow Stromal Cells Attenuate Sepsis via Prostaglandin E-2-Dependent Reprogramming of Host Macrophages to Increase Their Interleukin-10 Production. Nat. Med. 2009, 15, 42–49.
- Tsyb, A.F.; Petrov, V.N.; Konoplyannikov, A.G.; Saypina, E.V.; Lepechina, L.A.; Kalsina, S.S.H.; Semenkova, I.V.; Agaeva, E.V. In Vitro Inhibitory Effect of Mesenchymal Stem Cells on Zymosan-Induced Production of Reactive Oxygen Species. Bull. Exp. Biol. Med. 2008, 146, 158–164.
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal Stem Cells Program Macrophage Plasticity by Altering Their Metabolic Status via a PGE 2 -Dependent Mechanism. Sci. Rep. 2016, 6, 38308.
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone Marrow Mesenchymal Progenitor Cells Inhibit Lymphocyte Proliferation by Activation of the Programmed Death 1 Pathway. Eur. J. Immunol. 2005, 35, 1482–1490.
- Engela, A.U.; Baan, C.C.; Litjens, N.H.R.; Franquesa, M.; Betjes, M.G.H.; Weimar, W.; Hoogduijn, M.J. Mesenchymal Stem Cells Control Alloreactive CD8+CD28− T Cells. Clin. Exp. Immunol. 2013, 174, 449–458.
- De Mare-Bredemeijer, E.L.D.; Mancham, S.; Verstegen, M.M.A.; de Ruiter, P.E.; van Gent, R.; O’Neill, D.; Tilanus, H.W.; Metselaar, H.J.; de Jonge, J.; Kwekkeboom, J.; et al. Human Graft-Derived Mesenchymal Stromal Cells Potently Suppress Alloreactive T-Cell Responses. Stem Cells Dev. 2015, 24, 1436–1447.
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372.
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R.; et al. Mesenchymal Stem Cells Effectively Modulate Pathogenic Immune Response in Experimental Autoimmune Encephalomyelitis. Ann. Neurol. 2007, 61, 219–227.
- Lu, D.; Ma, T.; Zhou, X.; Jiang, Y.; Han, Y.; Li, H. B Lymphocytes Are the Target of Mesenchymal Stem Cells Immunoregulatory Effect in a Murine Graft-versus-Host Disease Model. Cell Transplant. 2019, 28, 1279–1288.
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal Cells Recruit and Regulate T Regulatory Cells. Exp. Hematol. 2008, 36, 309–318.
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human Leukocyte Antigen-G5 Secretion by Human Mesenchymal Stem Cells Is Required to Suppress T Lymphocyte and Natural Killer Function and to Induce CD4+CD25highFOXP3+ Regulatory T Cells. Stem Cells 2008, 26, 212–222.
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes Originating from MSCs Stimulated with TGF-β and IFN-γ Promote Treg Differentiation. J. Cell. Physiol. 2018, 233, 6832–6840.
- Rashedi, I.; Gómez-Aristizábal, A.; Wang, X.-H.; Viswanathan, S.; Keating, A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells 2017, 35, 265–275.
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-Stem-Cell-Induced Immunoregulation Involves FAS-Ligand-/FAS-Mediated T Cell Apoptosis. Cell Stem Cell 2012, 10, 544–555.
- Delorme, B.; Ringe, J.; Gallay, N.; Le Vern, Y.; Kerboeuf, D.; Jorgensen, C.; Rosset, P.; Sensebe, L.; Layrolle, P.; Haeupl, T.; et al. Specific Plasma Membrane Protein Phenotype of Culture-Amplified and Native Human Bone Marrow Mesenchymal Stem Cells. Blood 2008, 111, 2631–2635.
- Giunti, D.; Parodi, B.; Usai, C.; Vergani, L.; Casazza, S.; Bruzzone, S.; Mancardi, G.; Uccelli, A. Mesenchymal Stem Cells Shape Microglia Effector Functions Through the Release of CX3CL1. Stem Cells 2012, 30, 2044–2053.
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, Q.; Guo, S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front. Immunol. 2020, 11, 1076.
- Zhao, Y.; Su, G.; Wang, Q.; Wang, R.; Zhang, M. The CD200/CD200R Mechanism in Mesenchymal Stem Cells’ Regulation of Dendritic Cells. Am. J. Transl. Res. 2021, 13, 9607–9613.
- Tatara, R.; Ozaki, K.; Kikuchi, Y.; Hatanaka, K.; Oh, I.; Meguro, A.; Matsu, H.; Sato, K.; Ozawa, K. Mesenchymal Stromal Cells Inhibit Th17 but Not Regulatory T-Cell Differentiation. Cytotherapy 2011, 13, 686–694.
- Adamo, A.; Brandi, J.; Caligola, S.; Delfino, P.; Bazzoni, R.; Carusone, R.; Cecconi, D.; Giugno, R.; Manfredi, M.; Robotti, E.; et al. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front. Immunol. 2019, 10, 446.
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of MiRNAs. PLoS ONE 2010, 5, e11803.
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.-H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent Human Stromal Cells Improve Cardiac Function after Myocardial Infarction in Mice without Long-Term Engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706.
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The MicroRNA Regulatory Landscape of MSC-Derived Exosomes: A Systems View. Sci. Rep. 2018, 8, 1419.
- Katsuda, T.; Ochiya, T. Molecular Signatures of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Mediated Tissue Repair. Stem Cell Res. Ther. 2015, 6, 212.
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett. 2015, 589, 1257–1265.
- Kishore, R.; Khan, M. More than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ. Res. 2016, 118, 330–343.
- Collino, F.; Bruno, S.; Incarnato, D.; Dettori, D.; Neri, F.; Provero, P.; Pomatto, M.; Oliviero, S.; Tetta, C.; Quesenberry, P.J.; et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J. Am. Soc. Nephrol. 2015, 26, 2349–2360.
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular Vesicles from Human Cardiac Progenitor Cells Inhibit Cardiomyocyte Apoptosis and Improve Cardiac Function after Myocardial Infarction. Cardiovasc. Res. 2014, 103, 530–541.
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084.
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal Stem Cells within Tumour Stroma Promote Breast Cancer Metastasis. Nature 2007, 449, 557–563.
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.W.; Takashi, S.; Baik, G.H.; Shibata, W.; DiPrete, B.; Betz, K.S.; et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell 2011, 19, 257–272.
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992.
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013, 5, 167sr1.
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170, 1807–1816.
- Wynn, T.A. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210.
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428.
- Zeisberg, M.; Kalluri, R. Cellular Mechanisms of Tissue Fibrosis. 1. Common and Organ-Specific Mechanisms Associated with Tissue Fibrosis. Am. J. Physiol. Cell Physiol. 2012, 304, C216–C225.
- Duffield, J.S. Cellular and Molecular Mechanisms in Kidney Fibrosis. J. Clin. Investig. 2014, 124, 2299–2306.
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238–242.
- Humphreys, B.D.; Lin, S.-L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate Tracing Reveals the Pericyte and Not Epithelial Origin of Myofibroblasts in Kidney Fibrosis. Am. J. Pathol. 2010, 176, 85–97.
- Lin, S.-L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and Perivascular Fibroblasts Are the Primary Source of Collagen-Producing Cells in Obstructive Fibrosis of the Kidney. Am. J. Pathol. 2008, 173, 1617–1627.
- Schrimpf, C.; Duffield, J.S. Mechanisms of Fibrosis: The Role of the Pericyte. Curr. Opin. Nephrol. Hypertens. 2011, 20, 297–305.
- Smith, S.W.; Eardley, K.S.; Croft, A.P.; Nwosu, J.; Howie, A.J.; Cockwell, P.; Isacke, C.M.; Buckley, C.D.; Savage, C.O.S. CD248+ Stromal Cells Are Associated with Progressive Chronic Kidney Disease. Kidney Int. 2011, 80, 199–207.
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular Gli1+ Progenitors Are Key Contributors to Injury-Induced Organ Fibrosis. Cell Stem Cell 2015, 16, 51–66.
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823.
- Xie, T.; Liang, J.; Liu, N.; Huan, C.; Zhang, Y.; Liu, W.; Kumar, M.; Xiao, R.; D’Armiento, J.; Metzger, D.; et al. Transcription Factor TBX4 Regulates Myofibroblast Accumulation and Lung Fibrosis. J. Clin. Investig. 2016, 126, 3063–3079.
- Carlson, S.; Trial, J.; Soeller, C.; Entman, M.L. Cardiac Mesenchymal Stem Cells Contribute to Scar Formation after Myocardial Infarction. Cardiovasc. Res. 2011, 91, 99–107.
- Schneider, R.K.; Mullally, A.; Dugourd, A.; Peisker, F.; Hoogenboezem, R.; Van Strien, P.M.H.; Bindels, E.M.; Heckl, D.; Büsche, G.; Fleck, D.; et al. Gli1+ Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell 2017, 20, 785–800.
- Baker, A.H.; Peault, B. A Gli(1)Ttering Role for Perivascular Stem Cells in Blood Vessel Remodeling. Cell Stem Cell 2016, 19, 563–565.
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular Calcification: Pathobiological Mechanisms and Clinical Implications. Circ. Res. 2006, 99, 1044–1059.
- Sartore, S.; Chiavegato, A.; Faggin, E.; Franch, R.; Puato, M.; Ausoni, S.; Pauletto, P. Contribution of Adventitial Fibroblasts to Neointima Formation and Vascular Remodeling: From Innocent Bystander to Active Participant. Circ. Res. 2001, 89, 1111–1121.
- Kramann, R.; Goettsch, C.; Wongboonsin, J.; Iwata, H.; Schneider, R.K.; Kuppe, C.; Kaesler, N.; Chang-Panesso, M.; Machado, F.G.; Gratwohl, S.; et al. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell 2016, 19, 628–642.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
28 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




