Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stanislav Kotlyarov | -- | 2761 | 2022-07-26 09:06:24 | | | |
| 2 | Lindsay Dong | -24 word(s) | 2737 | 2022-07-27 03:32:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kotlyarov, S. Role of Short-Chain Fatty Acids in COPD. Encyclopedia. Available online: https://encyclopedia.pub/entry/25511 (accessed on 07 February 2026).
Kotlyarov S. Role of Short-Chain Fatty Acids in COPD. Encyclopedia. Available at: https://encyclopedia.pub/entry/25511. Accessed February 07, 2026.
Kotlyarov, Stanislav. "Role of Short-Chain Fatty Acids in COPD" Encyclopedia, https://encyclopedia.pub/entry/25511 (accessed February 07, 2026).
Kotlyarov, S. (2022, July 26). Role of Short-Chain Fatty Acids in COPD. In Encyclopedia. https://encyclopedia.pub/entry/25511
Kotlyarov, Stanislav. "Role of Short-Chain Fatty Acids in COPD." Encyclopedia. Web. 26 July, 2022.
Copy Citation
Chronic obstructive pulmonary disease (COPD) is a widespread socially significant disease. The development of COPD involves the innate immune system. The regulation of the innate lung immune system is related to the gut microbiota. This connection is due to the production by gut microorganisms of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate. Nutritional disturbances and changes in the structure of the intestinal microbiota lead to a decrease in SCFAs production and their effect on pulmonary immunity. The presence of a metabolic and immune axis linking the lungs and gut plays an important role in the pathogenesis of COPD.
COPD
gut microbiota
short-chain fatty acids
1. Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common respiratory diseases of social significance [1]. It is an important cause of health care seeking, hospitalizations, and disability [2][3]. Epidemiological studies show unfavorable trends in the prevalence of COPD in many countries [4][5]. The urgency of the problem is also increased by the fact that COPD is among the leading causes of death in the world. Moreover, treatment and rehabilitation of patients remain an unsolved problem in many respects, which requires new more detailed data on pathophysiological mechanisms of disease development and on those parts of pathogenesis, which can be effectively influenced for therapeutic purposes.
The development and progression of the disease is associated with exposure to tobacco smoke components and exogenous aeropollutants [6][7][8]. Inflammation in the bronchial tree is known to underlie the pathogenesis of COPD and is characterised by the involvement of many cells, including macrophages and neutrophils [9]. Prolonged persistent inflammation is associated with impaired immune mechanisms that provide a balance between maintaining and resolving inflammation [10].
It is known that COPD is a disease characterized by both pulmonary and extrapulmonary clinical heterogeneity [11][12]. Moreover, some features of the clinical picture and phenotypes of COPD are related to nutrition. Body mass index (BMI) is considered as one of the important markers in assessing the prognosis of COPD [13]. Lower body weight is associated with a greater risk of adverse outcome, whereas excess body weight has an even better prognosis [14]. This phenomenon has been called the “obesity paradox” [15][16][17]. The course of COPD, in which higher energy requirements lead to loss of fat and muscle tissue, is thought to be prognostically unfavorable [18][19]. The increased energy requirement may be related both to respiration and to the maintenance of inflammation in the bronchi. Dietary modification is considered an important tool that can improve the clinical course of COPD [20]. It is suggested that the nature of nutrition may be related to the pathophysiological mechanisms of COPD not only through the influence on energy and metabolic processes, but also through the regulation of some immune mechanisms [21].
A growing body of evidence supports a metabolic and immune axis linking the gut and lungs [22][23]. These links are bidirectional, and the gut microbiome is one of the central links in this interaction. The gut is the site of localization of much of the commensal bacterial mass [24][25]. This microbiome is metabolically active, participating in the production of many substances, such as, short-chain fatty acids (SCFAs) [26]. Accumulating evidence suggests that SCFAs can be considered as a leading link in the metabolic and immune axis between the gut and lungs. Indeed, SCFAs exhibit a variety of functions in immune defence, making them a potentially important target for clinical and experimental research.
2. Short-Chain Fatty Acids in the Pathogenesis of COPD
The presence of SCFAs in the sputum confirms the connection between the lungs and the intestine [27]. SCFAs are thought to contribute to the maintenance of lung immunometabolic tone [28]. As already noted, SCFAs can act on various parts of the innate immune defenses of the lungs. In addition to the described anti-inflammatory effects, the protective role of butyrate and propionate may include the restoration and maintenance of the barrier function of damaged airway epithelium by increasing the expression of ZO-1 dense contact proteins [29]. This seems important given the development of airway epithelial barrier dysfunction and impaired tight cell contacts in smoking and COPD [30]. In this regard, the regulation of barrier function under the influence of SCFAs may have some clinical significance [29].
The results showed differences in the composition of the gut microbiome in COPD and in healthy individuals. This is consistent with lower overall levels of SCFAs in the stage III-IV COPD group than in patients with stage I-II COPD and in healthy individuals [31]. Experimental data indicate that transplantation of fecal microbiota to mice led to inflammation in the lungs and increased IL-1β and TNF-α in plasma. At the same time, additional exposure to smoke from the biomass led to an accelerated decrease in lung function, emphysematous changes, airway remodeling, and mucus hypersecretion. These changes were accompanied by higher levels of claudin 1, α smooth-muscle actin (α-SMA), neutrophil elastase (NE) and matrix metalloproteinase 2 (MMP-2), and MUC5AC. In addition, gut microbiota obtained from stage III-IV COPD patients decreased body weight in recipient mice [31].
Thus, SCFAs are an important part of the immunological axis linking the gut microbiota and the lungs, which has implications in the pathogenesis of COPD (Figure 1).
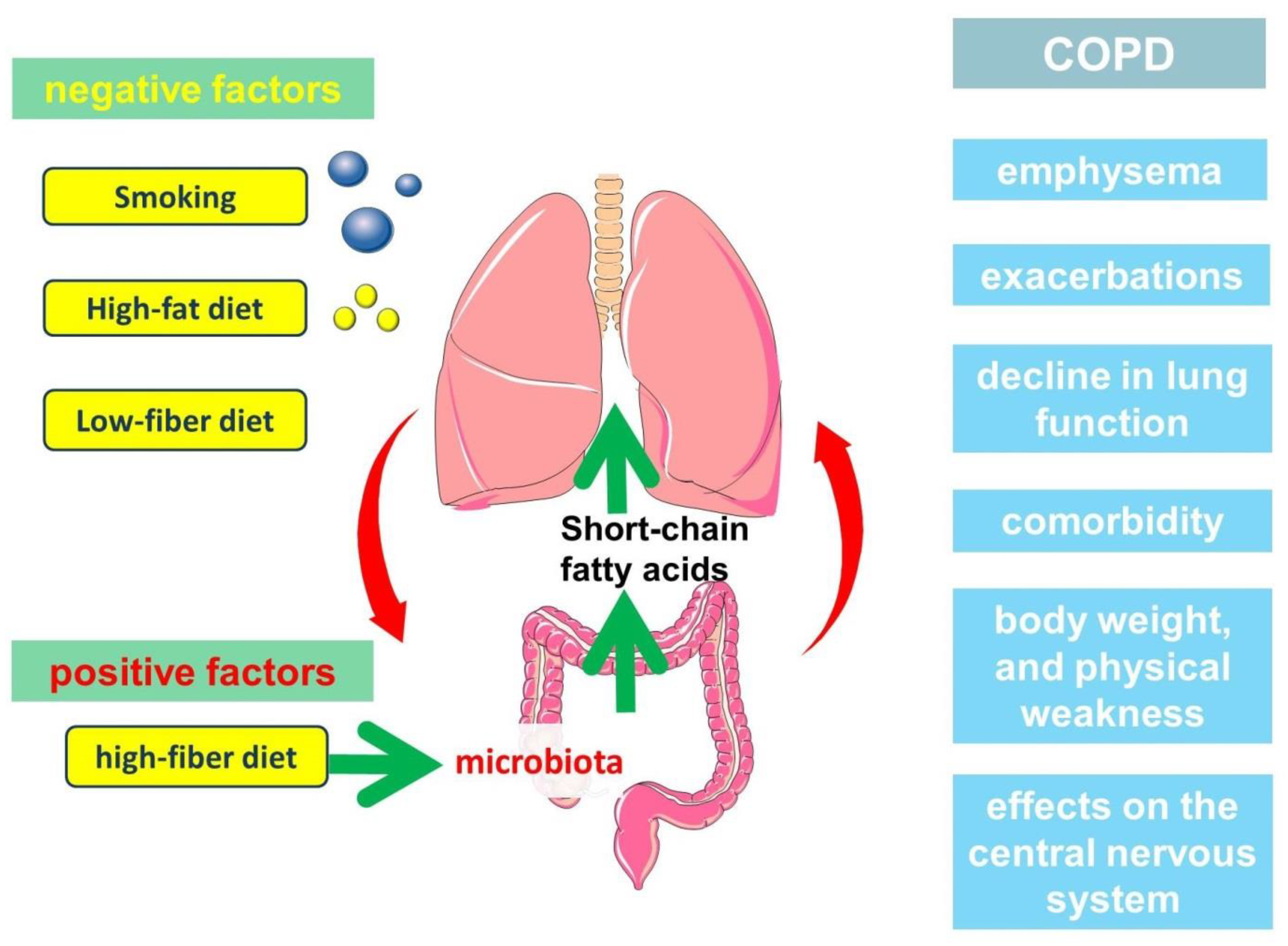
Figure 1. The participation of short-chain fatty acids in the pathogenesis of the clinically heterogeneous course of COPD.
2.1. Role of Short-Chain Fatty Acids in the Development of Emphysema
Emphysema is an important clinical phenotype of COPD. Numerous studies are devoted to the analysis of pathogenetic mechanisms of its development, among which of interest are the works studying the relationship of emphysema with nutrition. It is important to know about the development of emphysema during prolonged starvation, which was shown in the study of Warsaw ghetto prisoners, as well as observations in patients with anorexia nervosa [32][33]. These data, reinforced by experimental animal models, confirmed the link between nutrient deficiency and alveolar tissue destruction that leads to emphysema [34]. There are various explanations that justify these connections. Interestingly, anorexia nervosa is characterized by a decrease in intestinal microbial diversity, which is associated with the production of SCFAs, including butyrate and propionate [35][36].
A high-fiber diet has been shown to help prevent the progression of emphysema caused by cigarette smoke exposure. This is because a high-fiber diet changes the composition of the gut microbial community, resulting in increased production of SCFAs (acetate, propionate, and butyrate). SCFAs attenuated the pathological changes associated with the progression of emphysema and influenced the inflammatory response caused by cigarette smoke exposure [37]. Mice with emphysema that received fermentable fiber (pectin) were shown to have less inflammation than mice with emphysema that received nonfermentable fiber such as cellulose. This corresponded to lower concentrations of acetate, propionate, and butyrate in the emphysema group compared with the other groups. At the same time, macrophage and neutrophil counts were lower in the high fiber (cellulose and pectin) group than in the emphysema group [37]. In addition, inflammatory cytokine levels were lower in the high-fiber (cellulose and pectin) diet. In another study, butyrate was shown to reduce hypoxia-induced accumulation of alveolar (mainly CD68+) and interstitial (CD68+ and CD163+) macrophages in rat lungs [38].
The key events leading to emphysema are considered to be interalveolar septal avascularization, which is associated with the apoptosis of endothelial cells and alveolar epithelial cells [39]. In this regard, the information about butyrate participation in endothelial cell function is of interest. The anti-inflammatory properties of butyrate are in part related to its effect on the activation of NF-kB and PPARalpha and the associated expression of VCAM-1 and ICAM-1 [38][40]. In addition, butyrate inhibited the activation of endothelial NRLP3 inflammasome in endothelial cells [41]. Butyrate has also been shown to be involved in the inhibition of angiogenesis through HIF-1α as well as through the inhibition of vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2) [38][42][43]. Butyrate has also been shown to enhance the expression of tight junction proteins in pulmonary microvascular endothelial cells, affecting endothelial barrier function [38].
The effects of butyrate in endothelial cells are also related to NO production [44]. It has been shown that butyrate and acetate, can improve AngII-induced endothelial dysfunction through increased NO bioavailability [45]. The reduction of reactive oxygen species (ROS) levels in the vascular wall and the subsequent prevention of NO inactivation represent a key mechanism through which SCFAs act on endothelial function [45]. These findings are of interest given the frequent association of COPD with atherosclerosis.
Thus, the production of SCFAs by the intestinal microbiota may be one possible mechanism in the prevention of emphysema. It was found that a serum peptide-based enteral diet can suppress elastase-induced emphysema in mice by altering SCFAs levels in the cecum [46]. Modulation of the gut microbiota by prebiotics and transplantation of fecal microbiota from a high-fiber diet altered the composition of the gut microbiota, attenuating smoking-induced emphysema [47]. This was associated with a decrease in local and systemic inflammation through production of SCFAs, which protect against alveolar destruction and cellular apoptosis. The levels of IL-6 and IFN-γ in bronchoalveolar lavage fluid (BALF) were lowest in the high-fiber diet group, and the local concentration of SCFAs was markedly higher in mice with emphysema following fecal microbiota transplantation and the high-fiber diet than in mice with emphysema [47]. And the high-fiber diet had a more protective effect against emphysema than the high-protein diet [47].
2.2. Role of Short-Chain Fatty Acids in COPD Exacerbations
An important characteristic of the natural course of COPD is the frequency and severity of exacerbations. COPD exacerbations make a significant contribution to the clinical picture of COPD. The results of numerous studies suggest that the frequency of exacerbations is associated with a more rapid decrease in FEV1 and an unfavorable prognosis [48][49][50]. Given these facts, some scholars propose to consider high exacerbation frequency as a separate phenotype [49][51][52]. Infectious exacerbations of COPD are associated with disturbances in the structure of microbiota in the bronchi [53]. Colonization of the bronchi by microorganisms is necessary to maintain the immunological tone of the lungs. The available data suggest certain links between the intestinal and lung microbiome [54]. The respiratory tract microbiome can be supplemented with microorganisms from the gastrointestinal tract, which is important.
It is important to note that diet can affect not only the gut microflora, but also the respiratory tract microbiota [54][55]. SCFAs can have a direct effect on microorganisms as well as affecting their virulence [56]. Interestingly, high concentrations of SCFAs caused significant inhibition of Pseudomonas aeruginosa growth, which was enhanced at lower pH. At the same time, low concentrations of SCFAs resulted in enhanced bacterial growth [27]. Meanwhile, the administration of prebiotics in the form of oligosaccharides can modulate the immune and inflammatory response and outcome of pulmonary Pseudomonas aeruginosa infection in C57BL/6 mice through effects on the gut microbiota [57].
2.3. Role of Short-Chain Fatty Acids in the Decline of Lung Function
The rate at which lung function decreases is important in assessing the prognosis of patients with COPD. Given that bronchial obstruction is irreversible, progressive decline in lung function is associated with multiple systemic effects and the increased severity of comorbid conditions. Rapid progressive decline in lung function in COPD is suggested by some scholars to be a separate phenotype, given its relationship with the prognosis of the disease. Moreover, the rate of decline in lung function is associated with many factors, including the frequency of exacerbations. Interestingly, nutrition can also influence lung function. The high intake of sweets, oils, fat, and coffee has been shown to be negatively associated with lung function, including FEV1/FVC, and has been associated with an increased prevalence of COPD in men [20][58][59][60][61]. In contrast, high intake of fruits and vegetables, fatty fish, and low-fat foods was negatively associated with a diagnosis of COPD [62][63][64].
2.4. Short-Chain Fatty Acids, Body Weight, and Physical Frailty Phenotype
The role of diet as an important factor modifying the course of COPD has been the subject of numerous studies. Many of these studies have focused on the role of polyunsaturated fatty acids (PUFAs), especially ω-3 fatty acids [65][66]. Their influence on disease progression and prognosis has been analyzed, which is related to the involvement of both PUFAs themselves and their metabolites in the regulation of inflammation and resolution of inflammation.
Analysis of the role of dietary fiber is another important area that has been shown to be clearly related to the course of COPD. A population-based prospective cohort of 35 339 Swedish women evaluated the association between baseline and long-term dietary fiber intake and COPD risk. High fiber intake is an important modifiable factor that may reduce the risk of COPD primarily in current and former smokers [67].
2.5. Short-Chain Fatty Acids, the Central Nervous System, and the COPD Emotional Fragility Phenotype
Emotional lability is increasingly recognized as a phenotype of COPD because of its significant impact on treatment efficacy. Anxiety and depression are known to be associated with decreased quality of life, as well as increased hospitalizations and mortality.
Of great interest is the evidence of the influence of gut microbiota on the central nervous system through SCFAS, due to their several neuroactive properties. The exact mechanisms of these connections are still a subject for research. SCFAs have been shown to affect several neurological and mental diseases and behavioral processes. Their involvement in neuronal development, microglia maturation and the release of synaptic neurotransmitters is also known [68][69][70][71][72]. The involvement of SCFAs in neuroimmune processes in neurodegenerative diseases is important [72].
Short-chain fatty acids are involved in the onset of depression, which has been shown in macaques [73]. And it was found that in addition to plasma concentrations, some SCFAs (acetic acid, propanedioic acid, and butyric acid) are also impaired in macaque cerebrospinal fluid in a natural depression model. Butyrate levels differ significantly in both serum and liquor samples from these macaques [73]. These results are consistent with evidence of lower fecal SCFAs concentrations in depressed patients than in controls [74].
3. Conclusion
COPD is a clinically heterogeneous disease characterized by the development and progressive restriction of airflow due to chronic inflammation in the bronchi. Despite the known etiological factor, cigarette smoking, many aspects of COPD pathogenesis are still unknown. The causes of heterogeneity of the COPD course, which is associated with individual trajectories of progression and prognosis, remain a subject of discussion. Nutrition has a marked effect on the course of the disease. Indeed, patients who are severely underweight are at greatest risk for adverse COPD outcomes. The development of emphysema is also related to dietary patterns. Non-digestible carbohydrates, such as fiber, have been shown to favorably influence prognosis. Although these fibers are inaccessible to human digestive enzymes, they are actively metabolized by intestinal microflora. The products of enzymatic activity are SCFAs, which are an important link in the gut-lung immune axis. Short-chain fatty acids demonstrate a variety of functions in the regulation of inflammation and may play an important role in the clinical picture of COPD. SCFA production depends on the nature of the diet and the structure of the microflora. In this regard, dietary modification to include non-digestible fibers in the diet is seen as an important therapeutic tool that can affect not only the course of the disease, but also its outcome.
It should be noted that many of the effects of a diet containing dietary fiber may be related not only to the microbial fermentation of this fiber in the gut and SCFAs production, but also to various other dietary components, including micronutrients and vitamins. These findings are reflected in numerous studies that emphasize the importance of individual nutritional components [75][76][77]. Given the contribution of other nutritional components, it would not be very correct to link the clinical features of the course of COPD solely with SCFAs. It can be stated that the problems of diet in the natural history of COPD are far from being solved and require new research. In addition, individual trajectories of the natural history of COPD are shaped by many external and internal factors, a simplified understanding of which will not contribute to the interpretation of research results and improve approaches to the management of patients.
Indeed, many questions concerning both the pathophysiology of COPD and the involvement of SCFAs in these processes remain unanswered to date. Importantly, COPD patients are not a clinically homogeneous group, which requires a differentiated approach in the evaluation of research findings. The molecular mechanisms exhibited by SCFAs require new experimental and clinical confirmations. In this regard, investigation of the role of SCFAs in the clinically heterogeneous course of COPD may be a promising area for future research. These data will help to broaden the understanding of the pathophysiological mechanisms and their impairments that are associated with COPD phenotypes. A better understanding of these mechanisms will enhance the development of more effective therapeutic intervention programs that will be better adapted to individual disease course trajectories.
References
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 25 February 2022).
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596.
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prevalence, Characteristics, and Prognosis of Early Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2020, 201, 671–680.
- Hashemi, S.Y.; Momenabadi, V.; Faramarzi, A.; Kiani, A. Trends in burden of chronic obstructive pulmonary disease in Iran, 1995–2015: Findings from the global burden of disease study. Arch. Public Health 2020, 78, 45.
- Blanco, I.; Diego, I.; Bueno, P.; Casas-Maldonado, F.; Miravitlles, M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur. Respir. J. 2019, 54, 1900610.
- Kotaki, K.; Ikeda, H.; Fukuda, T.; Yuhei, K.; Yuki, F.; Kawasaki, M.; Wakamatsu, K.; Sugahara, K. Trends in the prevalence of COPD in elderly individuals in an air-polluted city in Japan: A cross-sectional study. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 791–798.
- Ni, L.; Chuang, C.-C.; Zuo, L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015, 6.
- Ding, Q.; Li, J.; Xu, S.; Gao, Y.; Guo, Y.; Xie, B.; Li, H.; Wei, X. Different Smoking Statuses on Survival and Emphysema in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 505–515.
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of inflammatory cells in airway remodeling in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3341–3348.
- Yang, A.; Wu, Y.; Yu, G.; Wang, H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: Mechanisms and development. Respir. Res. 2021, 22, 204.
- Agustí, A.; Vogelmeier, C.; Faner, R. COPD 2020: Changes and challenges. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L879–L883.
- Agusti, A.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Edwards, L.D.; Lomas, D.A.; MacNee, W.; Miller, B.E.; Rennard, S.; Silverman, E.K.; et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 2010, 11, 122.
- Kim, E.K.; Singh, D.; Park, J.H.; Park, Y.B.; Kim, S.I.; Park, B.; Park, J.; Kim, J.; Kim, M.A.; Lee, J.H.; et al. Impact of Body Mass Index Change on the Prognosis of Chronic Obstructive Pulmonary Disease. Respiration 2020, 99, 943–953.
- Guo, Y.; Zhang, T.; Wang, Z.; Yu, F.; Xu, Q.; Guo, W.; Wu, C.; He, J. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine 2016, 95, e4225.
- Iyer, A.S.; Dransfield, M.T. The “Obesity Paradox” in Chronic Obstructive Pulmonary Disease: Can It Be Resolved? Ann. Am. Thorac. Soc. 2018, 15, 158–159.
- Spelta, F.; Fratta Pasini, A.M.; Cazzoletti, L.; Ferrari, M. Body weight and mortality in COPD: Focus on the obesity paradox. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2018, 23, 15–22.
- Collins, P.F.; Stratton, R.J.; Kurukulaaratchy, R.; Elia, M. S163 The ‘Obesity Paradox’ in chronic obstructive pulmonary disease. Thorax 2010, 65, A73–A74.
- Barreiro, E.; Jaitovich, A. Muscle atrophy in chronic obstructive pulmonary disease: Molecular basis and potential therapeutic targets. J. Thorac. Dis. 2018, 10, S1415–S1424.
- Wüst, R.C.I.; Degens, H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 289–300.
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357.
- Kotlyarov, S.; Kotlyarova, A. Anti-Inflammatory Function of Fatty Acids and Involvement of Their Metabolites in the Resolution of Inflammation in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2021, 22, 12803.
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9.
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147.
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82.
- Vaughan, A.; Frazer, Z.A.; Hansbro, P.M.; Yang, I.A. COPD and the gut-lung axis: The therapeutic potential of fibre. J. Thorac. Dis. 2019, 11, S2173–S2180.
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455.
- Ghorbani, P.; Santhakumar, P.; Hu, Q.; Djiadeu, P.; Wolever, T.M.; Palaniyar, N.; Grasemann, H. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur. Respir. J. 2015, 46, 1033–1045.
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L65–L78.
- Richards, L.B.; Li, M.; Folkerts, G.; Henricks, P.A.J.; Garssen, J.; van Esch, B.C.A.M. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int. J. Mol. Sci. 2020, 22, 65.
- Tatsuta, M.; Kan-o, K.; Ishii, Y.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Ogawa, A.; Fujita, A.; Nakanishi, Y.; Matsumoto, K. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: Protective role of cathelicidin LL-37. Respir. Res. 2019, 20, 251.
- Li, N.; Dai, Z.; Wang, Z.; Deng, Z.; Zhang, J.; Pu, J.; Cao, W.; Pan, T.; Zhou, Y.; Yang, Z.; et al. Gut microbiota dysbiosis contributes to the development of chronic obstructive pulmonary disease. Respir. Res. 2021, 22, 274.
- Coxson, H.O.; Chan, I.H.T.; Mayo, J.R.; Hlynsky, J.; Nakano, Y.; Birmingham, C.L. Early Emphysema in Patients with Anorexia Nervosa. Am. J. Respir. Crit. Care Med. 2004, 170, 748–752.
- Fliederbaum, J.; Heller, A.; Zweibaun, K.; Zarchi, J. Clinical aspects of hunger disease in adults. Curr. Concepts Nutr. 1979, 7, 11–43.
- Wright, J.L.; Cosio, M.; Churg, A. Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L1–L15.
- Reed, K.K.; Abbaspour, A.; Bulik, C.M.; Carroll, I.M. The intestinal microbiota and anorexia nervosa: Cause or consequence of nutrient deprivation. Curr. Opin. Endocr. Metab. Res. 2021, 19, 46–51.
- Mack, I.; Cuntz, U.; Grämer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752.
- Jang, Y.O.; Kim, O.-H.; Kim, S.J.; Lee, S.H.; Yun, S.; Lim, S.E.; Yoo, H.J.; Shin, Y.; Lee, S.W. High-fiber diets attenuate emphysema development via modulation of gut microbiota and metabolism. Sci. Rep. 2021, 11, 7008.
- Karoor, V.; Strassheim, D.; Sullivan, T.; Verin, A.; Umapathy, N.S.; Dempsey, E.C.; Frank, D.N.; Stenmark, K.R.; Gerasimovskaya, E. The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 9916.
- Petrache, I.; Petrusca, D.N. The involvement of sphingolipids in chronic obstructive pulmonary diseases. Handb. Exp. Pharmacol. 2013, 216, 247–264.
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kołodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 2004, 15, 220–228.
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31.
- Ogawa, H.; Rafiee, P.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Binion, D.G. Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett. 2003, 554, 88–94.
- Kim, S.H.; Kim, K.W.; Jeong, J.W. Inhibition of hypoxia-induced angiogenesis by sodium butyrate, a histone deacetylase inhibitor, through hypoxia-inducible factor-1alpha suppression. Oncol. Rep. 2007, 17, 793–797.
- Morikawa, A.; Sugiyama, T.; Koide, N.; Mori, I.; Mu, M.M.; Yoshida, T.; Hassan, F.; Islam, S.; Yokochi, T. Butyrate enhances the production of nitric oxide in mouse vascular endothelial cells in response to gamma interferon. J. Endotoxin. Res. 2004, 10, 32–38.
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Aguilera-Sánchez, N.; Redondo, J.M.; Duarte, J. Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Front. Physiol. 2020, 11, 277.
- Tomoda, K.; Kubo, K.; Dairiki, K.; Yamaji, T.; Yamamoto, Y.; Nishii, Y.; Nakamura, A.; Yoshikawa, M.; Hamada, K.; Kimura, H. Whey peptide-based enteral diet attenuated elastase-induced emphysema with increase in short chain fatty acids in mice. BMC Pulm. Med. 2015, 15, 64.
- Jang, Y.O.; Lee, S.H.; Choi, J.J.; Kim, D.-H.; Choi, J.-M.; Kang, M.-J.; Oh, Y.-M.; Park, Y.-J.; Shin, Y.; Lee, S.W. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp. Mol. Med. 2020, 52, 1128–1139.
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H.; et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 324–330.
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2010, 363, 1128–1138.
- Sadatsafavi, M.; Xie, H.; Etminan, M.; Johnson, K.; FitzGerald, J.M.; Network, C.R.R. The association between previous and future severe exacerbations of chronic obstructive pulmonary disease: Updating the literature using robust statistical methodology. PLoS ONE 2018, 13, e0191243.
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256.
- Han, M.K.; Quibrera, P.M.; Carretta, E.E.; Barr, R.G.; Bleecker, E.R.; Bowler, R.P.; Cooper, C.B.; Comellas, A.; Couper, D.J.; Curtis, J.L.; et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 619–626.
- Kotlyarov, S.; Kotlyarova, A. Molecular Mechanisms of Lipid Metabolism Disorders in Infectious Exacerbations of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2021, 22, 7634.
- Madan, J.C.; Koestler, D.C.; Stanton, B.A.; Davidson, L.; Moulton, L.A.; Housman, M.L.; Moore, J.H.; Guill, M.F.; Morrison, H.G.; Sogin, M.L.; et al. Serial Analysis of the Gut and Respiratory Microbiome in Cystic Fibrosis in Infancy: Interaction between Intestinal and Respiratory Tracts and Impact of Nutritional Exposures. MBio 2012, 3, e00251-12.
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166.
- Machado, M.G.; Sencio, V.; Trottein, F.; Bäumler, A.J. Short-Chain Fatty Acids as a Potential Treatment for Infections: A Closer Look at the Lungs. Infect. Immun. 2021, 89, e00188-21.
- Bernard, H.; Desseyn, J.L.; Gottrand, F.; Stahl, B.; Bartke, N.; Husson, M.O. Pectin-Derived Acidic Oligosaccharides Improve the Outcome of Pseudomonas aeruginosa Lung Infection in C57BL/6 Mice. PLoS ONE 2015, 10, e0139686.
- Shin, M.-K.; Kwak, S.H.; Park, Y.; Jung, J.Y.; Kim, Y.S.; Kang, Y.A. Association between Dietary Patterns and Chronic Obstructive Pulmonary Disease in Korean Adults: The Korean Genome and Epidemiology Study. Nutrients 2021, 13, 4348.
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821.
- Wood, L.G.; Attia, J.; McElduff, P.; McEvoy, M.; Gibson, P.G. Assessment of dietary fat intake and innate immune activation as risk factors for impaired lung function. Eur. J. Clin. Nutr. 2010, 64, 818–825.
- Brigham, E.P.; Steffen, L.M.; London, S.J.; Boyce, D.; Diette, G.B.; Hansel, N.N.; Rice, J.; McCormack, M.C. Diet Pattern and Respiratory Morbidity in the Atherosclerosis Risk in Communities Study. Ann. Am. Thorac. Soc. 2018, 15, 675–682.
- Varraso, R.; Fung, T.T.; Hu, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax 2007, 62, 786–791.
- Varraso, R.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.; Camargo, C.A., Jr. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am. J. Clin. Nutr. 2007, 86, 488–495.
- Zheng, P.F.; Shu, L.; Si, C.J.; Zhang, X.Y.; Yu, X.L.; Gao, W. Dietary Patterns and Chronic Obstructive Pulmonary Disease: A Meta-analysis. COPD: J. Chronic Obstr. Pulm. Dis. 2016, 13, 515–522.
- Wood, L.G. Omega-3 polyunsaturated fatty acids and chronic obstructive pulmonary disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 128–132.
- Dirjayanto, V.J. Evidence on the Efficacy of Omega-3 Polyunsaturated Fatty Acids as an Adjunct Therapy for Chronic Obstructive Pulmonary Disease. J. Asian Med. Stud. Assoc. 2021, 9, 1.
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2020, 59, 1869–1879.
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478.
- SSzczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016, 19, 279–283.
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503.
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017, 11, 490.
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932.
- Deng, F.-L.; Pan, J.-X.; Zheng, P.; Xia, J.-J.; Yin, B.-M.; Liang, W.-W.; Li, Y.-F.; Wu, J.; Xu, F.; Wu, Q.-Y.; et al. Metabonomics reveals peripheral and central short-chain fatty acid and amino acid dysfunction in a naturally occurring depressive model of macaques. Neuropsychiatr. Dis. Treat. 2019, 15, 1077–1088.
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939.
- Zhai, T.; Li, S.; Hu, W.; Li, D.; Leng, S. Potential Micronutrients and Phytochemicals against the Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Nutrients 2018, 10, 813.
- Pearson, P.; Britton, J.; McKeever, T.; Lewis, S.A.; Weiss, S.; Pavord, I.; Fogarty, A. Lung function and blood levels of copper, selenium, vitamin C and vitamin E in the general population. Eur. J. Clin. Nutr. 2005, 59, 1043–1048.
- Mekal, D.; Czerw, A.; Deptala, A. Dietary Behaviour and Nutrition in Patients with COPD Treated with Long-Term Oxygen Therapy. Int. J. Environ. Res. Public Health 2021, 18, 12793.
More
Information
Subjects:
Respiratory System
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
27 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




