
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dhanya C R | -- | 2663 | 2022-07-21 11:01:12 | | | |
| 2 | Catherine Yang | Meta information modification | 2663 | 2022-07-27 07:56:57 | | | | |
| 3 | Catherine Yang | + 1 word(s) | 2664 | 2022-07-27 07:58:35 | | |
Video Upload Options
Pregnancy, alone, being associated with a state of immune alterations, exposes the maternal immune system to many challenges. Pregnant women, being a highly vulnerable group, need to be administered vaccines as early as possible; however, there is a lot of vaccine hesitancy among the population regarding immunization of pregnant women, who are avoided in the initial phases of most clinical trials. A look at available studies on the mechanisms of immune response in pregnant women and further, the reports of vaccine efficacy and outcomes among pregnant women against COVID-19 and EVD would definitely yield many insights that could be useful in the surveillance and planning of vaccination strategies for pregnant women against impending pathogenic RNA viruses.
1. Impact of COVID-19 and EVD on Pregnancy
2. Maternal and Neonatal Outcomes Associated with COVID-19 and EVD
| (a) | |||||||||||||
| Year of Study | Country | Study Approach | Case Type | Sample Size | Pre-Existing Medical Conditions | Need for Hospitalization | Maternal Mortality | Obstetric Complication | Perinatal Outcome | Reference | |||
| 2020 | 22 countries | Retrospective cohort study | Pregnant women with confirmed SARS-CoV-2 infection | 388 | Not available | ICU admission (11.1%) Mechanical Ventilation (9.3%) | 0.80% | Miscarriage (19.4%) Termination of pregnancy (1.1%) | Termination of pregnancy (1.1%) Pre-term birth (26.3%) Still birth (2.3%) Neonatal death (2%) SARS-CoV-2 positive (0.4%) | [10] | |||
| 2020 | United Kingdom | Prospective observational cohort study | pregnant women with confirmed SARS-CoV-2 infection | 427 | Asthma (7%) Hypertension (3%), Diabetes (3%) | Critical care (10%) | 1% | Pregnancy loss (1%) | Stillbirth (1%) Neonatal death (1%) Loss of Pregnancy (1%) SARS-CoV-2 positive (2%) | [11] | |||
| 2020 | Singapore | Prospective Cohort Study | Pregnant women with diagnosis of COVID-19 | 16 | Asthma (12.5%) HCV carriers (6.25%) | ICU admission (6.25%) | NIL | Spontaneous miscarriage (22.2%) | Neonatal death (6.25%) | [12] | |||
| 2020 | China | Retrospective Cohort study | Pregnant women who gave a single live birth between January 13 and March 18, 2020 | 65 | Not available | Higher need for Caesarean section (80%) | NIL | Gestational diabetes (3%) Gestational hypertension (11%) Pre-eclampsia (1%) | Pre-term birth (14%) Diarrhea (1.7%) Fever (5.17%) | [13] | |||
| 2020 | Iran | Prospective Cohort Study | Pregnant women with diagnosis of COVID-19 | 56 | Diabetes (16.1%) Hypertension (10.7%) Hypothyroidy (19.6%) | ICU admission (10.7%) Mechanical Ventilation (6.15%) Higher need for Caesarean section (67.3%) | NIL | Pre-eclampsia | Pre-term birth (34.5%) Perinatal death (3.6%) | [14] | |||
| 2020 | France | Retrospective Cohort study | Pregnant women with diagnosis of COVID-19 having a code for hospitalization for COVID-19 | 874 | Diabetes (1.3%) Hypertension (1.9%) | ICU admission (5.9%) Higher need for Caesarean section (33%) | 0.20% | Pre-eclampsia (4.8%) Gestational hypertension (2.3%) Postpartum hemorrhage (10%) | Pre-term birth (11.3%) | [15] | |||
| 2020 | Democratic Republic of the Congo | Case Study | Pregnant woman with confirmed SARS-CoV-2 infection | 1 | NIL | Caesarean section | NIL | Thrombotic vasculopathy in the placenta, Inflammatory appearance in the pelvic organs | SARS-CoV-2-infected Neonate, Perinatal death | [16] | |||
| 2020 | China | Retrospective Case Control study | Pregnant woman with confirmed SARS-CoV-2 infection, pregnant women with suspected infection and Control groups | 11 | Gestational diabetes (18.75%) Gestational hypertension (18.75%) Hypothyroidism (12.5%) | Caesarean section (87.5%) | NIL | Pre-eclampsia (6.25%) | Pre-term birth (18.8%), Low birth weight (17.6%) | [17] | |||
| 2020 | China | Case Study | Pregnant woman who was exposed to SARS-CoV-2 | 1 | NIL | Hospitalization Caesarean section | NIL | NIL | SARS-CoV-2-infected Neonate | [18] | |||
| 2020 | USA | Case Series | Pregnant women with suspected COVID-19 infection | 92 | NIL | Hospitalization (1.1%) | NIL | low morbidity | One fetal demise, but not sure whether it is due to COVID-19 | [19] | |||
| 2020 | Sweden | Case Series | Critically ill pregnant or newly delivered women positive for COVID-19 | 5 | Gestational diabetes (2 out of 5) Gestational Hypothyroidism (1 out of 5) Situs Inversus (1 out of 5) | Hospitalization for an average of 20 days (4 out of 5) Intubation (4 out of 5) | NIL | Severe respiratory distress syndromeCardiac arrest (1 out of 5) | NIL | [20] | |||
| 2020 | USA | Retrospective cohort study | Possible exposure or infection and positive COVID-19 test | 1609 | Chronic pulmonary disease (12.6%) Cardiac arrhythmia (10.4%) Hypertension (6.5%) Hypothyroidism (5%) Diabetes (3%) | Hospitalization (60.5%) | 0.20% | Not available | NIL | [21] | |||
| 2020 | USA | Retrospective cohort study | Pregnant and post-partum patients with SARS-CoV-2 infection | 2352 | Chronic pulmonary disease (12%) Hypertension (6.9%) Thyroid disease (3.9%) Diabetes (3.8%) | ICU admission (3.7%) | 0.20% | Post-partum hemorrhage (2.6%) Other infections (2.3%) Hypertensive disorders of pregnancy (10.1%) | Fetal/neonatal death (2.5%) Miscarriage (1.2%) Stillbirth (0.5%) Preterm birth (17.7%) | [22] | |||
| 2020 | USA | Observational Cohort study | Women who delivered and had SARS-CoV-2 infection during pregnancy | 252 | Gestational diabetes (3%) Chronic hypertension (5%) | Hospitalization (6%) | NIL | Pre-eclampsia (11%) Chorioamnionitis (10%) Excessive blood loss (7%) | Neonatal SARS-CoV-2 infection (3%) | [23] | |||
| 2020 | Iran | Retrospective case Control study | Pregnant women with COVID-19 positive test and a positive chest X-ray result | 110 | Hypertension (5.45%) Diabetes (9.09%) Asthma (5.45%) | ICU admission (6.9%) Requirement for invasive ventilation (1.7%) | NIL | Abortion (21.42%) Post-partum hemorrhage (5%) Pre-term birth (25%) | Still birth (5%) Fetal distress (10%) Low birth weight (10%) NICU admission (10%) | [24] | |||
| 2021 | 18 countries | Cohort study | Pregnant women with diagnosis of COVID-19 | 706 | Hypertension (3.7%), Diabetes (4.7%), Chronic respiratory disease (3.5%), Endocrine dysfunction (10.6%) | ICU admission (8.4%) | 1.60% | Hypertension Pre-eclampsia Anemia Infections | Pre-term birth (22.5%) Low birth weight (20.5%) SARS-CoV-2 positive (57.1%) | [25] | |||
| (b) | |||||||||||||
| Year of Study | Country | Study Approach | Case Type | Sample Size | Maternal Age | Gestational Age of Infection | Comorbidity | Clinical Presentation | Need for Hospitalization/ICU Admission | Maternal Mortality | Obstetric Morbidity | Perinatal Outcome | Reference |
| 1995 | Kikwit | Cohort Study | Ebola positive Pregnant women | 15 | 24–38 (mean age 32) | First trimester (27%), second trimester (40%) and third trimester (33%) | Not available | Fever (100%), asthenia (100%), abdominal pain (100%), conjunctivitis (100%), anorexia (100%), diarrhea (100%), arthralgia (100%), dysphagia (100%), headache (100%) | Admitted to General Hospital | 95.5% death | Genital bleeding (100%) | Abortion (67%), curettage performed due to incomplete abortion (20%), still birth (6.7%) | [26] |
| 2000 | North Uganda | Case study | Ebola positive Pregnant women | 1 | 31 | 28 weeks | Placenta had a moderate amount of malarial parasite pigment | Conjunctival injection, diffuse abdominal tenderness, and slight pulmonary rales | Admitted to ETU | Maternal survival | Placenta had mild subchorionitis | Still birth | [27] |
| 2012 | Congo | Case study | Ebola positive Pregnant women | 1 | 29 | 7 months | Not available | Fever, vomiting, dysphagia and diarrhea, drowsiness and wheezing, Dyspnea, coma stage 1b, light exophthalmos, cold limbs and sub icterus | Admitted to ETU | Maternal death | Dystocia | Death of neonate | [28] |
| 2014 | Liberia | Case Study | Ebola positive Pregnant women | 1 | 31 | Third trimester | Not available | vomiting, diarrhea, bleeding, and semi consciousness | Admitted to ETU | Maternal death | Not available | Intrauterine fetal death | [29] |
| 2014 | Guinea | Case Study | Ebola positive Pregnant woman | 1 | 40 | 4th month | Not available | abdominal pain, diarrhea and fever | Admitted to ETU | Maternal survival | Vaginal bleeding | Still birth | [30] |
| 2014 | Southern Guinea | Case study | Ebola positive Pregnant women | 2 | 20’s | 7 months | Malaria (50%) | Asthenia, fever, and vomiting, Anasarca (50%) | Admitted to ETU | Maternal survival (100%) | Absence of uterine contraction, cervical dilation (50%) and fetal heartbeat, hypertonic uterus (50%), post-partum hemorrhage (50%), suspected chorioamnionitis (50%) | Still birth (100%) | [31] |
| 2014 | Sierra Leone | Case study | Ebola positive Pregnant women | 1 | 34 | 36 | Not available | Headache, cough, and arthralgia | Admitted to ETU | Maternal survival | Hydropic Placenta | Still birth | [32] |
| 2014 | Sierra Leone | Cohort Study | Ebola positive Pregnant women | 55 | Mean age 25 | Not available | Not available | Fever (86.8%), fatigue or weakness (81.1%), nausea or vomiting (64.2%), headache (66%), muscle or joint pain (58.5%), vaginal bleeding (32.1%), unexplained bleeding (20.8%), and sore throat (13.2%) | Admitted to ETU | Not available | Vaginal bleeding (32%) | Not available | [33] |
| 2014 | Sierra Leone | Cohort Study | Ebola positive Pregnant women | 67 | Mean age 23 | 28–37 weeks | Not available | Fever (86.8%), abdominal pain (75.5%), fatigue (81.1%), nausea (64.2%) | Admitted to ETU | Maternal death (79%) | Vaginal bleeding (32%), obstetric hemorrhage (29.8%) and eclampsia (1.5%) | Spontaneous abortion (20.9%), Fetal death (5 out of 6), Still birth (8) | [34] |
| 2014 | Sierra Leone | Case study | qPCR negative, IgG positive | 1 | 19 | 36 weeks | Sickle cell anemia | Symptom free | Admitted to ETU | Maternal survival | Not available | Intrauterine fetal death, heavily macerated baby | [35] |
| 2014–15 | Liberia and Sierra Leone | Retrospective Cohort study | Ebola positive Pregnant women | 13 | 20-32 | Not available | Not available | Abdominal pain (85%) and nausea/vomiting (69%), Bleeding (30%), Hiccups (8%) and non-hemorrhagic rash (8%) | Admitted to ETU | 46% death | Not available | Preterm delivery (15%), Perinatal death (15%), Abortion (15%), Termination of pregnancy (7.6%), | [36] |
| 2014–2015 | Sierra Leone | Case series | Ebola positive Pregnant women (83.3%), Ebola survivor (16.6%) | 6 | 18-38 | Third trimester | Not available | Muscle pain (16.6%), headache (16.6%), diarrhea (16.6%), vomiting (16.6%) | Admitted to ETU | Maternal death (66.6%) | Postpartum hemorrhage (50%), hypovolemic shock (16.6%) | Neonate death (83.3%), still birth (16.6%) | [37] |
| 2015 | Sierra Leone | Case Study | Ebola positive Pregnant woman | 1 | 22 | 5 months | Not available | Anorexia, muscle pain, and joint pain | Admitted to ETU | Maternal survival | Leaking fluid | Intrauterine fetal death | [30] |
| 2015 | Sierra Leone | Case Study | IgG, IgM positive | 1 | 20 | Not available | Severe back pain, loss of appetite, and intense fatigue | Delivery attended by village traditional birth attendant | Maternal survival | Leakage of bloody fluid from vagina | Still birth | [38] | |
| 2016 | Guinea | Case Study | Ebola positive Pregnant women | 1 | 25 | 28th week | Not available | Hyperthermia, asthenia, and conjunctival infection | Admitted to ETU | Maternal death post delivery | Severe vaginal bleeding with signs of coagulopathy | Survived after treatment | [39] |
3. Effects of COVID-19 and EVD on Placenta and Vertical Transmission
4. Alterations in Immune Response in Pregnant Women with SARS-CoV-2 and EBOV Infections
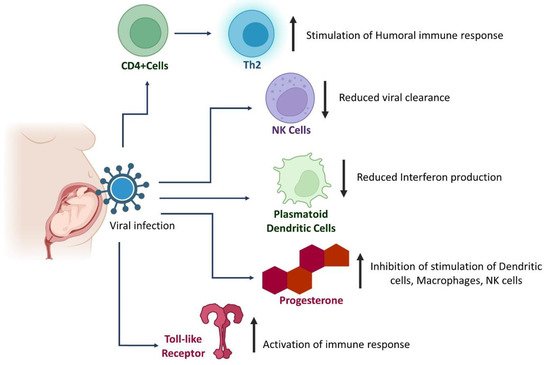
This entry is adapted from 10.3390/pathogens11070800
References
- Paixao, E.S.; Harron, K.; Campbell, O.; Teixeira, M.G.; Costa, M.D.C.N.; Barreto, M.L.; Rodrigues, L.C. Dengue in pregnancy and maternal mortality: A cohort analysis using routine data. Sci. Rep. 2018, 8, 9938.
- Susich, M.; Packer, C.H.; Chaiken, S.R.; Garg, B.; Caughey, A.B. 1115 A comparison of maternal and neonatal outcomes in acute and chronic hepatitis B. Am. J. Obstet. Gynecol. 2021, 224, S687.
- Kreitchmann, R.; Li, S.X.; Melo, V.H.; Coelho, D.F.; Watts, D.H.; João, E.; Coutinho, C.M.; Alarcon, J.O.; Siberry, G.K. Predictors of adverse pregnancy outcomes in women infected with HIV in Latin America and the Caribbean: A cohort study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1501–1508. Available online: https://obgyn.onlinelibrary.wiley.com/doi/10.1111/1471-0528.12680 (accessed on 26 May 2022).
- Foeller, M.E.; Valle, C.C.R.D.; Foeller, T.M.; Oladapo, O.T.; Roos, E.; Thorson, A.E. Pregnancy and breastfeeding in the context of Ebola: A systematic review. Lancet Infect. Dis. 2020, 20, e149–e158.
- World Health Organization. Guidelines for the Management of Pregnant and Breastfeeding Women in the Context of Ebola Virus Disease; World Health Organization: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/handle/10665/330851 (accessed on 26 May 2022).
- Kotlar, B.; Gerson, E.; Petrillo, S.; Langer, A.; Tiemeier, H. The impact of the COVID-19 pandemic on maternal and perinatal health: A scoping review. Reprod. Health 2021, 18, 10.
- Lokken, E.M.; Taylor, G.G.; Huebner, E.M.; Vanderhoeven, J.; Hendrickson, S.; Coler, B.; Sheng, J.S.; Walker, C.L.; McCartney, S.A.; Kretzer, N.M.; et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am. J. Obstet. Gynecol. 2021, 225, 75.e1–75.e16.
- Sutton, D.; Fuchs, K.; D’Alton, M.; Goffman, D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N. Engl. J. Med. 2020, 382, 2163–2164.
- Liu, H.; Wang, L.-L.; Zhao, S.-J.; Kwak-Kim, J.; Mor, G.; Liao, A.-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020, 139, 103122.
- Maternal and Perinatal Outcomes of Pregnant Women with SARS-CoV-2 Infection—2021—Ultrasound in Obstetrics & Gynecology—Wiley Online Library. Available online: https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.23107 (accessed on 26 May 2022).
- Characteristics and Outcomes of Pregnant Women Admitted to Hospital with Confirmed SARS-CoV-2 infection in UK: National Population Based Cohort Study | The BMJ. Available online: https://www.bmj.com/content/369/bmj.m2107 (accessed on 26 May 2022).
- Mattar, C.N.; Kalimuddin, S.; Sadarangani, S.P.; Tagore, S.; Thain, S.; Thoon, K.C.; Hong, E.Y.; Kanneganti, A.; Ku, C.W.; Chan, G.M.; et al. Pregnancy Outcomes in COVID-19: A Prospective Cohort Study in Singapore. Ann. Acad. Med. Singapore 2020, 49, 857–869.
- Yang, R.; Mei, H.; Zheng, T.; Fu, Q.; Zhang, Y.; Buka, S.; Yao, X.; Tang, Z.; Zhang, X.; Qiu, L.; et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: A population-based cohort study in Wuhan, China. BMC Med. 2020, 18, 330.
- Abedzadeh-Kalahroudi, M.; Sehat, M.; Vahedpour, Z.; Talebian, P. Maternal and neonatal outcomes of pregnant patients with COVID-19: A prospective cohort study. Int. J. Gynecol. Obstet. 2021, 153, 449–456.
- Epelboin, S.; Labrosse, J.; De Mouzon, J.; Fauque, P.; Gervoise-Boyer, M.-J.; Levy, R.; Sermondade, N.; Hesters, L.; Bergère, M.; Devienne, C.; et al. Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: A national retrospective cohort study. PLoS Med. 2021, 18, e1003857.
- Birindwa, E.K.; Mulumeoderhwa, G.M.; Nyakio, O.; Mbale, G.-Q.M.; Mushamuka, S.Z.; Materanya, J.M.; Kahasha, P.M.; Bisimwa, Y.K.; Kampara, F.M.; Irenge, J.M.; et al. A case study of the first pregnant woman with COVID-19 in Bukavu, eastern Democratic Republic of the Congo. Matern. Health Neonatol. Perinatol. 2021, 7, 6.
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and Neonatal Outcomes of Pregnant Women with Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin. Infect. Dis. 2020, 71, 2035–2041.
- Long, R.; Wu, D.; Lin, X.; Lv, D.; Wang, R.; Jin, L.; Liao, S.; Liu, W.; Deng, D. COVID-19 and Pregnancy: A Case Study. Glob. Chall. 2021, 5, 2000074.
- Fox, N.S.; Melka, S. COVID-19 in Pregnant Women: Case Series from One Large New York City Obstetrical Practice. Am. J. Perinatol. 2020, 37, 1002–1004.
- A Case Series on Critically Ill Pregnant or Newly Delivered Patients with Covid-19, Treated at Karolinska University Hospital, Stockholm. Available online: https://www.hindawi.com/journals/criog/2021/8868822/ (accessed on 26 May 2022).
- Qeadan, F.; Mensah, N.A.; Tingey, B.; Stanford, J.B. The risk of clinical complications and death among pregnant women with COVID-19 in the Cerner COVID-19 cohort: A retrospective analysis. BMC Pregnancy Childbirth 2021, 21, 305.
- Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality from Obstetric Complications | Infectious Diseases | JAMA | JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/2788985 (accessed on 26 May 2022).
- Pregnancy Outcomes Among Women with and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection | Global Health | JAMA Network Open | JAMA Network. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2773105 (accessed on 26 May 2022).
- Taghavi, S.-A.; Heidari, S.; Jahanfar, S.; Amirjani, S.; Aji-Ramkani, A.; Azizi-Kutenaee, M.; Bazarganipour, F. Obstetric, maternal, and neonatal outcomes in COVID-19 compared to healthy pregnant women in Iran: A retrospective, case-control study. Middle East. Fertil. Soc. J. 2021, 26, 17.
- Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study | Neonatology | JAMA Pediatrics | JAMA Network. Available online: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2779182 (accessed on 26 May 2022).
- Mupapa, K.; Mukundu, W.; Bwaka, M.A.; Kipasa, M.; De Roo, A.; Kuvula, K.; Kibadi, K.; Massamba, M.; Ndaberey, D.; Colebunders, R.; et al. Ebola Hemorrhagic Fever and Pregnancy. J. Infect. Dis. 1999, 179, S11–S12.
- Muehlenbachs, A.; Vázquez, O.D.L.R.; Bausch, D.G.; Schafer, I.J.; Paddock, C.D.; Nyakio, J.P.; Lame, P.; Bergeron, E.; McCollum, A.M.; Goldsmith, C.S.; et al. Ebola Virus Disease in Pregnancy: Clinical, Histopathologic, and Immunohistochemical Findings. J. Infect. Dis. 2016, 215, 64–69.
- Kratz, T.; Roddy, P.; Oloma, A.T.; Jeffs, B.; Ciruelo, D.P.; De La Rosa, O.; Borchert, M. Ebola Virus Disease Outbreak in Isiro, Democratic Republic of the Congo, 2012: Signs and Symptoms, Management and Outcomes. PLoS ONE 2015, 10, e0129333.
- Akerlund, E.; Prescott, J.; Tampellini, L. Shedding of Ebola Virus in an Asymptomatic Pregnant Woman. N. Engl. J. Med. 2015, 372, 2467–2469.
- Dilemmas in Managing Pregnant Women with Ebola: 2 Case Reports | Clinical Infectious Diseases | Oxford Academic. Available online: https://academic.oup.com/cid/article/62/7/903/2462754 (accessed on 26 May 2022).
- Baggi, F.M.; Taybi, A.; Kurth, A.; Van Herp, M.; Di Caro, A.; Wölfel, R.; Günther, S.; Decroo, T.; Declerck, H.; Jonckheere, S. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Eurosurveillance 2014, 19, 20983.
- Oduyebo, T.; Pineda, D.; Lamin, M.; Leung, A.; Corbett, C.; Jamieson, D. A Pregnant Patient with Ebola Virus Disease. Obstet. Gynecol. 2015, 126, 1273–1275.
- Mpofu, J.J.; Soud, F.; Lyman, M.; Koroma, A.P.; Morof, D.; Ellington, S.; Kargbo, S.S.; Callaghan, W. Clinical presentation of pregnant women in isolation units for Ebola virus disease in Sierra Leone, 2014. Int. J. Gynecol. Obstet. 2019, 145, 76–82.
- Lyman, M.; Mpofu, J.J.; Soud, F.; Oduyebo, T.; Ellington, S.; Schlough, G.W.; Koroma, A.P.; McFadden, J.; Morof, D. Maternal and perinatal outcomes in pregnant women with suspected Ebola virus disease in Sierra Leone, 2014. Int. J. Gynecol. Obstet. 2018, 142, 71–77.
- Okoror, L.; Kamara, A.; Kargbo, B.; Bangura, J.; Lebby, M. Transplacental Transmission: A Rare Case of Ebola Virus Transmission. Infect. Dis. Rep. 2018, 10, 53–55.
- Henwood, P.C.; Bebell, L.M.; Roshania, R.; Wolfman, V.; Mallow, M.; Kalyanpur, A.; Levine, A.C. Ebola Virus Disease and Pregnancy: A Retrospective Cohort Study of Patients Managed at 5 Ebola Treatment Units in West Africa. Clin. Infect. Dis. 2017, 65, 292–299.
- Pavlin, B.I.; Hall, A.; Hajek, J.; Raja, M.A.; Sharma, V.; Ramadan, O.P.; Mishra, S.; Rangel, A.; Kitching, A.; Roper, K.; et al. Atypical clinical presentation of Ebola virus disease in pregnancy: Implications for clinical and public health management. Int. J. Infect. Dis. 2020, 97, 167–173.
- Bower, H.; Brault, A.; Chege, E.; Saffa, G.; Seneca, D.; Salzer, J.S.; Grass, J.E.; Stroeher, U.; Belser, J.; Decroo, T.; et al. Delivery of an Ebola Virus-Positive Stillborn Infant in a Rural Community Health Center, Sierra Leone, 2015. Am. J. Trop. Med. Hyg. 2016, 94, 417–419.
- First Newborn Baby to Receive Experimental Therapies Survives Ebola Virus Disease | The Journal of Infectious Diseases | Oxford Academic. Available online: https://academic.oup.com/jid/article/215/2/171/2877903 (accessed on 26 May 2022).
- Resta, L.; Vimercati, A.; Cazzato, G.; Mazzia, G.; Cicinelli, E.; Colagrande, A.; Fanelli, M.; Scarcella, S.; Ceci, O.; Rossi, R. SARS-CoV-2 and Placenta: New Insights and Perspectives. Viruses 2021, 13, 723.
- Linehan, L.; O’Donoghue, K.; Dineen, S.; White, J.; Higgins, J.R.; Fitzgerald, B. SARS-CoV-2 placentitis: An uncommon complication of maternal COVID-19. Placenta 2021, 104, 261–266.
- Huynh, A.; Sehn, J.K.; Goldfarb, I.T.; Watkins, J.; Torous, V.; Heerema-McKenney, A.; Roberts, D.J. SARS-CoV-2 Placentitis and Intraparenchymal Thrombohematomas Among COVID-19 Infections in Pregnancy. JAMA Netw. Open 2022, 5, e225345.
- Shook, L.L.; Brigida, S.; Regan, J.; Flynn, J.P.; Mohammadi, A.; Etemad, B.; Siegel, M.R.; Clapp, M.A.; Li, J.Z.; Roberts, D.J.; et al. SARS-CoV-2 Placentitis Associated with B.1.617.2 (Delta) Variant and Fetal Distress or Demise. J. Infect. Dis. 2022, 225, 754–758.
- Lu-Culligan, A.; Chavan, A.R.; Vijayakumar, P.; Irshaid, L.; Courchaine, E.M.; Milano, K.M.; Tang, Z.; Pope, S.D.; Song, E.; Vogels, C.B.; et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med 2021, 2, 591–610.e10.
- Bordt, E.A.; Shook, L.L.; Atyeo, C.; Pullen, K.M.; De Guzman, R.M.; Meinsohn, M.-C.; Chauvin, M.; Fischinger, S.; Yockey, L.J.; James, K.; et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 2021, 13.
- Bebell, L.M.; Oduyebo, T.; Riley, L.E. Ebola virus disease and pregnancy: A review of the current knowledge of Ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 2017, 109, 353–362. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/bdra.23558 (accessed on 25 May 2022).
- Cribiù, F.M.; Erra, R.; Pugni, L.; Rubio-Perez, C.; Alonso, L.; Simonetti, S.; Croci, G.A.; Serna, G.; Ronchi, A.; Pietrasanta, C.; et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J. Clin. Investig. 2021, 131, e145427.
- Olgun, N.S. Viral Infections in Pregnancy: A Focus on Ebola Virus. Curr. Pharm. Des. 2018, 24, 993–998.
- Kliman, H.J. From Trophoblast to Human Placenta; p. 23, 2006. Available online: https://medicine.yale.edu/obgyn/kliman/placenta/research/trophoblast%20to%20placenta%20eor_163163_284_18220_v1.pdf (accessed on 1 July 2022).
- Ouyang, Y.; Bagalkot, T.; Fitzgerald, W.; Sadovsky, E.; Chu, T.; Martínez-Marchal, A.; Brieño-Enríquez, M.; Su, E.J.; Margolis, L.; Sorkin, A.; et al. Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin. mSphere 2021, 6, e00250-21. Available online: https://journals.asm.org/doi/10.1128/mSphere.00250-21 (accessed on 26 May 2022).
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572.
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848.
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128.
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2020, 184, 628–642.e10.
- Yuan, J.; Qian, H.; Cao, S.; Dong, B.; Yan, X.; Luo, S.; Zhou, M.; Zhou, S.; Ning, B.; Zhao, L. Is there possibility of vertical transmission of COVID-19: A systematic review. Transl. Pediatr. 2021, 10, 423–434.
- Tolu, L.B.; Ezeh, A.; Feyissa, G.T. Vertical transmission of Severe Acute Respiratory Syndrome Coronavirus 2: A scoping review. PLoS ONE 2021, 16, e0250196.
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Belfort, M.B.; Pace, C.D.W.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv 2020.
- Krogstad, P.; Contreras, D.; Ng, H.; Tobin, N.; Chambers, C.D.; Bertrand, K.; Bode, L.; Aldrovandi, G.M. No infectious SARS-CoV-2 in breast milk from a cohort of 110 lactating women. Pediatr. Res. 2022, 1–6.
- Medina-Rivera, M.; Centeno-Tablante, E.; Finkelstein, J.L.; Rayco-Solon, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N.; Rogers, L.; Ridwan, P.; Martinez, S.S.; Andrade, J.; et al. Presence of Ebola virus in breast milk and risk of mother-to-child transmission: Synthesis of evidence. Ann. N. Y. Acad. Sci. 2020, 1488, 33–43.
- Nordenstedt, H.; Bah, E.I.; De La Vega, M.-A.; Barry, M.; N’Faly, M.; Barry, M.; Crahay, B.; DeCroo, T.; Van Herp, M.; Ingelbeen, B. Ebola Virus in Breast Milk in an Ebola Virus–Positive Mother with Twin Babies, Guinea, 2015. Emerg. Infect. Dis. 2016, 22, 759–760.
- Rapid Outbreak Sequencing of Ebola Virus in Sierra Leone Identifies Transmission Chains Linked to Sporadic Cases | Virus Evolution | Oxford Academic. Available online: https://academic.oup.com/ve/article/2/1/vew016/1753554 (accessed on 26 May 2022).
- Keita, A.K.; Vidal, N.; Toure, A.; Diallo, M.S.K.; Magassouba, N.; Baize, S.; Mateo, M.; Raoul, H.; Mely, S.; Subtil, F.; et al. A 40 months follow-up of Ebola virus disease survivors in Guinea (Postebogui) reveals longterm detection of Ebola viral RNA in semen and breast milk. Open Forum Infect. Dis. 2019, 6, ofz482.
- Ebola Virus Localization in the Macaque Reproductive Tract during Acute Ebola Virus Disease—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0002944017309501?via%3Dihub (accessed on 26 May 2022).
- Liu, W.J.; Sesay, F.R.; Coursier, A.; Knust, B.; Marrinan, J.E.; Whitmer, S.; McDonald, S.L.R.; Gaillard, P.; Liu, Y.; Su, Q.; et al. Comprehensive Clinical and Laboratory Follow-up of a Female Patient with Ebola Virus Disease: Sierra Leone Ebola Virus Persistence Study. Open Forum Infect. Dis. 2019, 6, ofz068.
- Khoiwal, K.; Kalita, D.; Kumari, R.; Dhundi, D.; Shankar, R.; Kumari, R.; Gaurav, A.; Bahadur, A.; Panda, P.K.; Tomy, A.; et al. Presence of SARS-CoV -2 in the lower genital tract of women with active COVID-19 infection: A prospective study. Int. J. Gynecol. Obstet. 2022, 157, 744–747.
- Barber, E.; Kovo, M.; Leytes, S.; Sagiv, R.; Weiner, E.; Schwartz, O.; Mashavi, M.; Holtzman, K.; Bar, J.; Engel, A.; et al. Evaluation of SARS-CoV-2 in the Vaginal Secretions of Women with COVID-19: A Prospective Study. J. Clin. Med. 2021, 10, 2735.
- Qiu, L.; Liu, X.; Xiao, M.; Xie, J.; Cao, W.; Liu, Z.; Morse, A.; Xie, Y.; Li, T.; Zhu, L. SARS-CoV-2 Is Not Detectable in the Vaginal Fluid of Women with Severe COVID-19 Infection. Clin. Infect. Dis. 2020, 71, 813–817.
- Frontiers | Cesarean Section or Vaginal Delivery to Prevent Possible Vertical Transmission from a Pregnant Mother Confirmed With COVID-19 to a Neonate: A Systematic Review | Medicine. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2021.634949/full (accessed on 26 May 2022).
- Lopian, M.; Kashani-Ligumsky, L.; Czeiger, S.; Cohen, R.; Schindler, Y.; Lubin, D.; Olteanu, I.; Neiger, R.; Lessing, J.B.; Somekh, E. Safety of vaginal delivery in women infected with COVID-19. Pediatr. Neonatol. 2020, 62, 90–96.
- Ferrazzi, E.; Frigerio, L.; Savasi, V.; Vergani, P.; Prefumo, F.; Barresi, S.; Bianchi, S.; Ciriello, E.; Facchinetti, F.; Gervasi, M.; et al. Vaginal Delivery in SARS-CoV-2-infected Pregnant Women in Northern Italy: A Retrospective Analysis. Obstet. Anesthesia Dig. 2021, 41, 82.
- Martínez-Perez, O.; Vouga, M.; Melguizo, S.C.; Acebal, L.F.; Panchaud, A.; Muñoz-Chápuli, M.; Baud, D. Association Between Mode of Delivery Among Pregnant Women with COVID-19 and Maternal and Neonatal Outcomes in Spain. JAMA 2020, 324, 296–299.
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433.
- Frontiers | Innate Immune Responses to Acute Viral Infection During Pregnancy | Immunology. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.572567/full (accessed on 26 May 2022).
- Frontiers | Immune Response to COVID-19 During Pregnancy | Immunology. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.675476/full (accessed on 26 May 2022).
- Seery, V.; Raiden, S.C.; Algieri, S.C.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lalla, S.; Cairoli, H.; Chiolo, M.J.; Meregalli, C.N.; et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. eBioMedicine 2021, 67, 103357.
- Maternal-Fetal Immune Responses in Pregnant Women Infected with SARS-CoV-2 | Nature Communications. Available online: https://www.nature.com/articles/s41467-021-27745-z (accessed on 26 May 2022).
- Andrikopoulou, M.; Madden, N.; Wen, T.; Aubey, J.J.; Aziz, A.; Baptiste, C.D.; Breslin, N.; D’Alton, M.E.; Fuchs, K.M.; Goffman, D.; et al. Symptoms and Critical Illness Among Obstetric Patients with Coronavirus Disease 2019 (COVID-19) Infection. Obstet. Gynecol. 2020, 136, 291–299.
- Juttukonda, L.J.; Wachman, E.M.; Boateng, J.; Jain, M.; Benarroch, Y.; Taglauer, E.S. Decidual immune response following COVID-19 during pregnancy varies by timing of maternal SARS-CoV-2 infection. J. Reprod. Immunol. 2022, 151, 103501.




